Abstract
Background and objective
Adhesions are the most common cause of chronic abdominal pain after surgery. Surgical adhesiolysis can relieve symptoms in selected patients, but many require other treatments. The aim of this study is to evaluate analgesic treatments other than abdominal surgery in chronic pain related to adhesions.
Database and data treatment
A search was conducted in PubMed, Embase, and Central. Studies with patients suffering from chronic postoperative pain related to adhesions and undergoing all types’ analgesic treatment were included. The primary outcome was the number of patients who improved in pain at long-term follow-up (at least 1 year). Secondary outcomes included improvement in pain at 3 months follow-up, quality of life, and physical functioning.
Results
Searches identified 3022 citations. Four studies were included, one trial, one cohort study, and two case reports. The primary outcome was not reported. In a small trial (n = 18) pregabalin tended to have a benefit over placebo improving pain at 3 months. In the cohort study, 17 patients with chronic pelvic pain underwent a trial of sacral nerve stimulation. Eight patients who responded positively received an implanted device for continuous modulation, reporting sustainable improvement during follow-up (range: 6–36 months). One case report described improved pain at 6 months with trans-abdominis plane stimulation. The second report described improvement of physical function with manual therapy at long-term follow-up.
Conclusions
Low level of evidence is available regarding analgesic treatments of chronic abdominal and pelvic pain related to adhesions. The benefit of pregabalin is doubtful; nerve modulation is promising in a selected group.
Adhesions are a frequent cause of chronic abdominal and pelvic pain after surgery.
Many patients are not good candidates for surgery (Adhesiolysis) or have relapses of pain.
There is an important knowledge gap regarding non-surgical analgesic treatment.
Analgesia in adhesion-related chronic abdominal pain after surgery.
Highlights
Introduction
Chronic abdominal and pelvic pain develops in as many as 10–20% of all patients undergoing peritoneal surgery, negatively impacting quality of life in millions of patients [Citation1–4]. Adhesions are the most prominent cause of chronic post-operative pain and are estimated to account for approximately 60% of cases [Citation5,Citation6].
Chronic pain from adhesions seems to be distinct from other causes of chronic abdominal and pelvic pain in a way that it can be resolved by surgery [Citation7]. Adhesiolysis (release of adhesions during a reoperation) combined with adhesion prevention in selected cases results in long-term pain relief in 80% of patients, which is prognostically much better than for many other conditions of chronic pain [Citation8]. However, a significant number of patients with adhesions are not eligible for adhesiolysis, because of increased risk of iatrogenic injuries or unfitness for reoperation. Furthermore, there is a 20% risk of recurrence of adhesion-related symptoms despite optimal secondary prevention. These patients continue to be dependent on chronic pain medication, often opioids, or undergo alternative therapies, for example, neuromodulation, nerve blockades, physiotherapy, or behavioral therapy.
The effects of treatments other than adhesiolysis are not evident. Many studies on analgesic treatments of chronic abdominal and pelvic pain include a substantial amount of patients with adhesion-related pain, however, most do not report efficacy for this subgroup separately [Citation9,Citation10]. Moreover, some of these studies report on therapies unlikely to affect adhesion-related pain, for example, hormonal therapy for patients with pelvic pain, which primarily targets endometriosis.
Another strategy often utilized to manage chronic abdominal pain that might be amendable to patients with adhesions is the use of neuropathic pain medication [Citation11,Citation12]. This strategy is frequently promoted to substitute or avoid the chronic use of opioids. Neuropathic pain components have been described in relation to adhesions [Citation1]. The hypothesis that adhesion-related pain has a neuropathic origin is supported by histological evidence of ingrowth of neuron fibers in adhesions, and the role of neuromodulating molecules like neurokinin-1, substance P, and Calcitonin gene-related peptide in the adhesion formation cascade [Citation13–15]. Conversely, non-neuropathic origins of pain related to adhesions have also been hypothesized stating that adhesions cause pain by mechanical traction to organs adhered together or to the abdominal wall, or from organ dysfunction secondary to adhesions [Citation16]. Adhesive partial bowel obstruction with predominant chronic pain is an example of such a form of organ dysfunction [Citation16,Citation17].
There is a need for advising on effective non-surgical, non-opioid, analgesic treatment of adhesions related to chronic abdominal and pelvic pain to patients who are not eligible or have failed adhesiolysis [Citation8,Citation18]. To this need, we systematically reviewed the literature regarding the efficacy of analgesic treatments other than adhesiolysis in patients suffering from chronic abdominal and pelvic pain related to post-surgical adhesions.
Materials and methods
All studies evaluating patients with chronic postoperative abdominal and pelvic pain related to adhesions were eligible. Inclusion criteria were persistent pain likely caused by adhesions for at least 3 months after surgery and a follow-up of pain outcomes for at least 3 months after the start of treatment. Pain is considered to be caused by the adhesions when the pain has developed after a surgical procedure and diagnosis of adhesions was either confirmed by previous surgery or suggested on imaging studies, or other etiologies of pain were excluded.
Studies that did not contain original data or included patients with chronic abdominal or pelvic pain without prior surgery were excluded. Studies that included patients with adhesions more likely related to other etiologies, for example, endometriosis, inflammatory disease were only considered if data for the subgroup of patients with adhesions after surgery could be extracted separately, or the prevalence of adhesions as the most likely cause of the pain was more than 70%. There was no restriction in language, date of publications, and study method. The full protocol was registered with Prospero as part of a larger project evaluating the efficacy of different treatments including surgery for adhesion-related pain (registration number CRD 42015024902). A detailed evaluation of the efficacy of adhesiolysis has been published elsewhere [Citation18]. The Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines were followed in reporting the results.
Search strategy
Searches were performed in PubMed, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL). Searches included the following Mesh-terms descriptors, ‘abdominal pain’, ‘pelvic pain’, ‘postoperative pain’, ‘post-surgical pain’, ‘visceral pain’, ‘neuropathic pain’, ‘tissue adhesion’, and ‘pain management’. A detailed description of our search technique can be found in Supplementary 1. No language or date restriction was applied. Only human studies were included. The search was performed on the 15thof October 2019. A reference list of identified studies was additionally searched to identify additional trials. Supplemental searches were performed to find relevant grey literature and unpublished trials (Supplementary 1).
Outcomes
The primary outcome was the number of patients with pain relief at long-term follow-up measured by self-reporting. Long-term was defined as 1 year or longer after the start of treatment. Secondary outcome measures were improvement in quality of life, the number of patients who reported a lower pain score at midterm follow-up (3 months to 1 year), improvement in mean pain score, physical functioning, sleep and mood disorders, and healthcare utilization.
Risk of systematic error (bias)
For quality assessment for randomized trials, we used Cochrane Collaboration’s tool for bias risk assessment [Citation19]. For quality assessment of non-randomized trials and case-control studies, we used the revised Newcastle–Ottawa score for cohort studies and case-control studies, with a maximum score of five stars [Citation20]. Five stars is considered high quality, three or four stars is considered intermediate quality, and one or two stars is considered low quality (shown in and ).
Table 1. Cochrane Collaboration’s tool for bias risk assessment.
Table 2. Newcastle–Ottawa score for cohort studies and case-control studies.
Data extraction
Two reviewers independently extracted the data. For relevant articles we extracted information on study design, therapy given, follow-up period, the number of participants, and outcomes reported.
Outcome measures extracted from the literature were graded according to clinical relevance (‘critical for decision-making’, ‘important for decision-making’, or ‘of limited importance’) as suggested by the Grading of Recommendation, Assessment, Development and Evaluation (GRADE) working group [Citation21]. Efficacy outcomes defined as ‘critical for decision-making’ were pain relief at long-term follow-up (1 year or longer), and improved quality of life measured by a validated score at long-term follow-up (1 year or longer). Efficacy outcomes graded as ‘important for decision-making’ were improvement of physical functioning including mood and sleep disorder and pain at any follow-up (3 months or longer), improved quality of life at any follow-up (3 months or longer), pain scores at any follow-up (3 months or longer) and return to daily activity or work. The efficacy outcome ‘of limited importance’ was healthcare utilization.
When outcome measures were registered during several time intervals within the same study, we used the data from the longest follow-up period.
Statistical analysis
The results from this systematic review were not suited for the pooling of data. Therefore, descriptive statistics are used.
Results
Search results
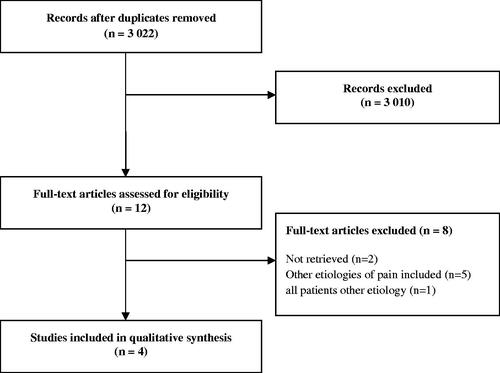
shows the results of the search and selection procedure. Our search yielded 3022 unique articles. Despite upcoming techniques in anesthesiology and pain management, only 29 studies were identified regarding various techniques of regional analgesia by electric stimuli or local anesthesia to alleviate chronic peritoneal pain. After the screening of all 3022 titles and summaries, 12 articles were considered potentially relevant. After reviewing the full text, 8 articles were excluded. In total four studies with a total of 37 participants were included, one pain medication study, two studies on electric nerve modulation, and one study on manual therapy.
Characteristics of included studies
Characteristics of included studies are listed in . One was a randomized controlled trial, one a prospective cohort study, and two were case reports. In all studies, all patients had a history of previous peritoneal surgery and clinical assessment had shown no other causes of pain. Adhesions were described to be the most likely cause in all studies, although the work-up to rule out other causes was not fully described. Specific imaging for adhesions was not reported. 79–100% of patients were female.
Table 3. Characteristics of included studies.
Outcomes of studies
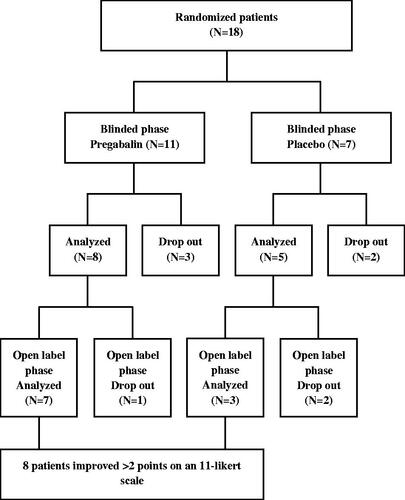
In the trial of Silverman et al., 18 patients were randomized between pregabalin and placebo [Citation22]. The trial consisted of two phases of respectively 8 and 4 weeks. The first phase was a blinded phase in which patients were randomized between pregabalin 150 mg (n = 11) and placebo (n = 7) during 8 weeks. The second phase was an open-label period of 4 weeks in which all patients received 300 mg pregabalin. The primary outcome of the study was the reduction in adhesion-related abdominal pain on an 11-point Likert scale. Secondary outcomes were daily sleep interference scores and adverse events related to pregabalin and placebo. The study had a high risk of bias as assessed by the Cochrane’s Collaboration tool; high risk of bias was recorded on the domains ‘blinding of outcome assessment’, ‘incomplete outcome data’, and ‘other (i.e. slow accrual leading to premature stop)’ ().
The study ended prematurely because of the slow accrual of patients. During the study, dropout was high with seven patients (39%). In the second phase, two patients who were previously allocated to the placebo group discontinued treatment because of the side effects of pregabalin.
The second phase started at 8 weeks before the mid-term follow-up was reached. Pain results and quality of life at long-term follow-up were not reported. 10 patients (seven pregabalin, three placebo groups) completed the open-label phase. Eight of these patients reached the predefined criteria for success, which was defined as a decrease in pain score of two-point or more on the 11-point Likert scale. At the end of the first phase, 8 of 11 patients were analyzed in the pregabalin treated group, the other three patients discontinued because of side effects of the drug. Seven (88%) patients reported improvement of pain after 8 weeks reaching the endpoint of at least 2-point decrease on the 11-point Likert scale. In the placebo group, two patients discontinued during the first phase. Two of five (40%) patients analyzed in the placebo group showed a decrease in pain after the first phase. The pain scores at the end of the first phase were significantly lower in the pregabalin group compared to the placebo group (p = 0.0024). In the open-label phase, seven patients were analyzed who were previously randomized to the drug group, all patients reported improvement of pain. Three patients who were previously randomized to placebo were analyzed in the open-label phase. The other two discontinued in the open-label phase. One patient already reported improvement of pain with a placebo. The other two patients reported significant improvement in pain using pregabalin during the open-label phase (p = 0.043) ().
Improvement in sleep tended to be larger in the drug group (n = 7) but the difference was not significant in the first phase. In the open-label phase, the sleep disturbance score continued to improve in the drug group. A large improvement in sleep disturbance score was found in the open-label phase in the group previously randomized to placebo. The difference in change of score during the open-label phase was significantly higher in the placebo group compared to the pregabalin group (p = 0.024). By the end of the open-label phase, all of the ten remaining patients (seven pregabalin, three placebo groups) improved in sleep. Seven patients improve more than 2 points from baseline on a daily sleep interference score. Physical functioning and healthcare utilization were not reported.
In the cohort study of Martellucci et al., 17 patients were enrolled with chronic pelvic pain and a history of abdominal or pelvic surgery for benign disease [Citation23]. A trial of sacral nerve stimulation by an extracorporeal electrode was performed in all patients. The criteria to continue implanting a medical device for continuous sacral nerve stimulation were defined as a decrease in pain score of five points on a 0–10 scale or a pain score below three after 4 weeks of stimulation. The quality calculated by the Newcastle–Ottawa scale was four, corresponding with a high risk of bias (). The population of interest was a selected group of patients with abdominal pain after surgery. All outcomes were based on written self-reporting data. Not all patients completed the primary outcome of 12 months follow-up, but the drop-out percentage was below 20%.
Prior to the start, all patients used pain medication, three received tramadol. At the start of the study, all pain medication was stopped. No patients reported complications during the trial. Eight patients (47%) reached the criteria for implanting the medical device. Seven patients (88%) improved in pain score, quality of life at long-term follow-up was not reported. At 6-month follow-up pain score significantly decreased in eight patients compared to baseline (1.9 ± 1.2compared to 8.2 ± 0.9, p < 0.001). At 12 months follow-up pain scores were 2.0 ± 1.3 in seven patients. Quality of life as measured by SF-36 was reported to have significantly improved at 6 months of follow-up without presenting details. Physical functioning, sleep and mood disturbances, and healthcare utilization were not reported.
Gupta et al. described the positive effects of peripheral abdominal wall nerve stimulation in a case report [Citation24]. A trans-abdominis plane stimulator was applied in a patient with chronic post-operative abdominal pain related to adhesions with a score of six on a ten-point scale. In a test period of 2 weeks, pain was completely relieved after the device was implanted. The use of analgesic medication was largely reduced after 3 months. At 3 months opioid use dropped from 15–20 mg oxycodone daily to 5 mg daily. The remaining opioid use was primarily related to persistent knee complaints. At 6 months follow-up pain scores remained low with a maximum of two points. Long-term follow-up results were not reported. Physical functioning in daily activities had reportedly improved, although this was not quantified. Quality of life and sleep and mood disorders were not reported.
Wong et al., described manual massage therapy called ‘soft tissue mobilization’ [Citation25]. A soft tissue mobilization is a form of massage therapy by which the physical therapists palpate the visible and painful areas manually and by a metal blade to the maximal tolerable pain level in several sessions. The therapy was applied to a woman with five previous abdominal surgical procedures of which one was an adhesiolysis for chronic pain without improvement of pain. Other causes of pain were ruled out by additional computed tomography and plain X-rays of the hip and pelvis, which showed no abnormalities. After 1 year follow-up, the patient reported persistent pain relief, further not quantified. Physical functioning was reported to have improved fully passing a military physical readiness test at 6 and 12 months. Prior to treatment, the patient had failed this test based on the items ‘running’ and ‘curl-ups’. Quality of life, sleep and mood disorders, and healthcare utilization were not reported.
Discussion
Summary of evidence
There is scant low-level evidence on the efficacy of treatments for adhesion-related chronic pain, other than adhesiolysis. There are only a few studies regarding neuropathic pain medication and neuromodulation using electric nerve stimulation. By its design, it is difficult to draw firm conclusions on the efficacy of pregabalin from the trial from Silverman et al. on the mid-term outcomes. Nevertheless, pregabalin seems to improve both pain and sleep interference, with a trend towards higher efficacy when compared to placebo. After the first phase, there was a significant improvement in pain relief compared to placebo, however, the blinded period ended before 3 months which was taken as our secondary endpoint. The open-label phase improvement was seen in the placebo group after starting pregabalin. A large number of patients discontinue using pregabalin before the end of the study. Important reasons for discontinuation were side effects. Implantation of an abdominal or sacral nerve modulator showed a strong reduction of pain and a decrease in the use of analgesics in a small and selected group of patients [Citation23,Citation24]. In these studies, no complications related to nerve modulation were reported, however, the case number was low. In one case report complete and long-term pain relief was reported with the improvement of physical function during specific manual therapy.
Strength and limitations of the review
We included all studies without any limitations to the type of study, languages, and date, or the region of pain (pelvic pain vs. other parts of the abdomen). Despite these broad criteria, the yield of studies was very low, indicating that adhesions are often not considered a distinct pain problem outside the large body of surgical and gynecological literature on adhesiolysis [Citation18]. We chose a follow-up of 1 year for the primary outcome to rule out a placebo effect or spontaneous improvement. Placebo effects in pain studies tend to be large but the effect duration is not known [Citation26–28]. Although in other pharmacological treatment trials maximal placebo effects are reported in the first few months, placebo effects lasting for a year or more have been reported [Citation29–31]. Only a few patients in the four studies were followed for 1 year. When looking at our minimum follow-up time of 3 months, it is important to realize that results might still be clouded by a placebo effect. The pregabalin trial had a very short randomized blinded phase of fewer than 3 months, it is therefore difficult to conclude that the effects can be attributed to treatment with pregabalin.
The low number of studies and patients included is in sharp contrast with the high incidence of adhesion-related pain and the many chronic abdominals and pelvic pain studies that have been reported [Citation32] In a systematic review of 13 non-surgical trials including 750 women suffering from chronic pelvic pain, adhesions were considered the pain caused in 180 patients but results were not specified for this group. In another 247 patients, the cause of pain was unspecified. One possible explanation for not distinguishing adhesions from other causes of chronic abdominal pain is the lack of non-operative methods diagnosing adhesions. Until recently, invasive methods such as laparoscopy were needed to establish this diagnosis. Surgeons and physicians have been reluctant to use such invasive diagnostics because of a high rate of negative laparoscopies (20–30%) and a significant burden from iatrogenic injuries if unexpected extensive adhesions had to be lysed [Citation18]. Non-invasive techniques to evaluate visceral slides such as CineMRI or ultrasound have now been available for more than a decade [Citation33,Citation34], however, these techniques are not broadly adopted. No study in this review reported the use of specific non-invasive imaging for the detection of adhesions. With experience, diagnostic accuracies of 90% or more can be achieved using such methods [Citation35]. We expect that implementing CineMRI or ultrasound in the diagnostic process will increase the number of patients diagnosed with adhesion-related pain and provide the opportunity for proper treatment.
Comparison with other literature
Previous reviews on the treatment of adhesion-related pain included studies assessing the efficacy of operative treatment, for example, adhesiolysis [Citation18,Citation36]. Reports of adhesiolysis are conflicting, with high heterogeneity in patient selection, surgical technique, the use of barriers, and outcomes reported. A recent study showed that the addition of non-invasive imaging techniques can assist in selecting patients that might benefit from adhesiolysis. In a cohort of 106 patients, CineMRI was used to diagnose adhesions non-invasively. Based on the result of CineMRI 50 patients were selected for surgery, of these patients 80% had sustainable improvement at 1.5 years follow-up. However, 40% of patients with adhesions did not undergo surgery mainly due to estimated high operative risk and about 20% of operated patients had pain recurrence in the long term. These findings emphasize that there is a continued need for alternative efficacious treatments, other than adhesiolysis [Citation8].
Clinical and research implications
Based on this review, no recommendations can be made as to what is the best treatment for adhesion-related pain in those not eligible for adhesiolysis. The possibilities of nerve stimulation are effective in selected groups of patients and have been performed under controlled research conditions. The adequate patient selection seems to increase the effect of non-surgical treatment. Given the complexity of chronic abdominal and pelvic pain, it is necessary to implement a multidisciplinary approach towards adhesion-related pain as we have previously reported [Citation8]. Currently, a large portion of patients with adhesion-related pain is opioid-dependent [Citation8,Citation37]. Given the growing body of literature on the negative consequences of chronic opioid use, it is important to develop novel strategies that reduce opioid need [Citation38]. In part, such strategies might be sought in existing concepts for the treatment of complex and chronic pain. The multimodal approach addressing the broad spectrum of behavior, environmental and psychological factors is frequently advocated. Strong evidence for multimodal treatment however is absent in conditions related to abdominal pain, such as pancreatitis [Citation12]. Another upcoming treatment modality that might be of interest is virtual reality therapy [Citation39,Citation40]. However, evidence is predominantly regarding procedural and acute pain and not chronic pain.
More insight into the mechanisms through which adhesions cause pain could also help developing new efficacious treatments. Molecular mediators that might link adhesion formation to nociception are the proinflammatory peptide substance P and Neurokinin-1 which are up-regulated in other conditions of chronic pain [Citation15]. Currently, in a prospective cohort (NCT03938168) we are comparing histopathology and molecular features of adhesions from patients with chronic pain and adhesions in patients without pain symptoms. Features we aim to investigate include expression of Transient Receptor Potential Vanilloid 1, Substance P, neurokinin-1, and the presence of nerve fibers.
Conclusion
Scant research with a low level of evidence is available regarding analgesic treatments of chronic abdominal and pelvic pain related to adhesions. Pregabalin might improve midterm results of pain, quality of life measured, and sleep disturbance. Nerve modulation is a safe analgesic treatment improving adhesion-related chronic abdominal pain and lowering opioid use in selected groups.
Author contributions
BAWvdB: Study design, search and strategy, data extraction, data analysis, drafting of manuscript, final approval. RdR: search, data extraction, data analysis, drafting of manuscript, final approval. SvdW: strategy, critical review of the manuscript, final approval. HvG: Study design, critical review of manuscript, final approval. RPGtB: Study design, supervision of search strategy and data extraction, data interpretation, supervision of drafting of the manuscript, final approval. All authors discussed the results and commented on the manuscript.
Supplemental Material
Download PDF (204.4 KB)Disclosure statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Bruce J, Krukowski ZH, Al-Khairy G, et al. Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. Br J Surg. 2001;88(9):1157–1168.
- Sperber AD, Morris CB, Greemberg L, et al. Development of abdominal pain and IBS following gynecological surgery: a prospective, controlled study. Gastroenterology. 2008;134(1):75–84.
- Strik C, van den Beukel B, van Rijckevorsel D, et al. Risk of pain and gastrointestinal complaints at 6 months after elective abdominal surgery. J Pain. 2019;20(1):38–46.
- ten Broek RP, Issa Y, van Santbrink EJ, et al. Burden of adhesions in abdominal and pelvic surgery: systematic review and met-analysis. BMJ. 2013;347(1):f5588.
- Krielen P, van den Beukel BA, Stommel MWJ, et al. In-hospital costs of an admission for adhesive small bowel obstruction. World J Emerg Surg. 2016;11(1):49.
- Howard FM, El-Minawi AM, Sanchez RA. Conscious pain mapping by laparoscopy in women with chronic pelvic pain. Obstet Gynecol. 2000;96:934–939.
- Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev. 2014;3:Cd008797.
- van den Beukel BAW, Stommel MWJ, van Leuven S, et al. A shared decision approach to chronic abdominal pain based on cine-MRI: a prospective cohort study. Am J Gastroenterol. 2018;113(8):1229–1237.
- Sator-Katzenschlager SM, Scharbert G, Kress HG, et al. Chronic pelvic pain treated with gabapentin and amitriptyline: a randomized controlled pilot study. Wien Klin Wochenschr. 2005;117(21–22):761–768.
- Engel CC, Jr., Walker EA, Engel AL, et al. A randomized, double-blind crossover trial of sertraline in women with chronic pelvic pain. J Psychosom Res. 1998;44(2):203–207.
- Bouwense SA, Olesen SS, Drewes AM, et al. Effects of pregabalin on central sensitization in patients with chronic pancreatitis in a randomized, controlled trial. PLoS One. 2012;7(8):e42096.
- Drewes AM, Bouwense SAW, Campbell CM, et al. Guidelines for the understanding and management of pain in chronic pancreatitis. Pancreatology. 2017;17(5):720–731.
- Herrick SE, Mutsaers SE, Ozua P, et al. Human peritoneal adhesions are highly cellular, innervated, and vascularized. J Pathol. 2000;192(1):67–72.
- Sulaiman H, Gabella G, Davis C, et al. Growth of nerve fibres into murine peritoneal adhesions. J Pathol. 2000;192(3):396–403.
- Reed KL, Fruin AB, Bishop-Bartolomei KK, et al. Neurokinin-1 receptor and substance P messenger RNA levels increase during intraabdominal adhesion formation. J Surg Res. 2002;108(1):165–172.
- Demco L. Pain mapping of adhesions. J Am Assoc Gynecol Laparosc. 2004;11(2):181–183.
- Jarrar A, Church J. Treating symptomatic adhesions to the sigmoid colon: colectomy improves quality of life. Int J Colorectal Dis. 2013;28(10):1407–1411.
- van den Beukel BA, de Ree R, van Leuven S, et al. Surgical treatment of adhesion-related chronic abdominal and pelvic pain after gynaecological and general surgery: a systematic review and meta-analysis. Hum Reprod Update. 2017;23:276–288.
- Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(2):d5928.
- Wells GS, O’Connell D, Peterson J, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. http://www.ohrica/programs/clinical_epidemiology/oxfordasp Language english
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926.]
- Silverman A, Samuels Q, Gikas H, et al. Pregabalin for the treatment of abdominal adhesion pain: a randomized, double-blind, placebo-controlled trial. Am J Ther. 2012;19(6):419–428.
- Martellucci J, Naldini G, Del Popolo G, et al. Sacral nerve modulation in the treatment of chronic pain after pelvic surgery. Colorectal Dis. 2012;14(4):502–507.
- Gupta M, Goodson R. Transverse abdominal plane neurostimulation for chronic abdominal pain: a novel technique. Pain Phys. 2014;17:E619–E622.
- Wong YY, Smith RW, Koppenhaver S. Soft tissue mobilization to resolve chronic pain and dysfunction associated with postoperative abdominal and pelvic adhesions: a case report. J Orthop Sports Phys Ther. 2015;45(12):1006–1016.
- Finniss DG, Kaptchuk TJ, Miller F, et al. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010;375(9715):686–695.
- Kaptchuk TJ, Goldman P, Stone DA, et al. Do medical devices have enhanced placebo effects? J Clin Epidemiol. 2000;53(8):786–792.
- Kaptchuk TJ, Stason WB, Davis RB, et al. Sham device v inert pill: randomised controlled trial of two placebo treatments. BMJ. 2006;332(7538):391–397.
- Hansen BJ, Meyhoff HH, Nordling J, et al. Placebo effects in the pharmacological treatment of uncomplicated benign prostatic hyperplasia. The ALFECH Study Group. Scand J Urol Nephrol. 1996;30(5):373–377.
- Elsenbruch S, Enck P. Placebo effects and their determinants in gastrointestinal disorders. Nat Rev Gastroenterol Hepatol. 2015;12(8):472–485.
- Kosek E, Rosen A, Carville S, et al. Lower placebo responses after long-term exposure to fibromyalgia pain. J Pain. 2017;18(7):835–843.
- van Rijckevorsel DC, de Vries M, Schreuder LT, et al. Risk factors for chronic postsurgical abdominal and pelvic pain. Pain Manag. 2015;5(2):107–116.
- Zinther NB, Fedder J, Friis-Andersen H. Noninvasive detection and mapping of intraabdominal adhesions: a review of the current literature. Surg Endosc. 2010;24(11):2681–2686.
- Lienemann A, Sprenger D, Steitz HO, et al. Detection and mapping of intraabdominal adhesions by using functional cine MR imaging: preliminary results. Radiology. 2000;217(2):421–425.
- Gerner-Rasmussen J, Donatsky AM, Bjerrum F. The role of non-invasive imaging techniques in detecting intra-abdominal adhesions: a systematic review. Langenbecks Arch Surg. 2019;404(6):653–661.
- Gerner-Rasmussen J, Burcharth J, Gögenur I. The efficacy of adhesiolysis on chronic abdominal pain: a systematic review. Langenbecks Arch Surg. 2015;400(5):567–576.
- Wang D. Opioid medications in the management of chronic abdominal pain. Curr Pain Headache Rep. 2017;21(9):40.
- Vadivelu N, Kai AM, Kodumudi V, et al. The opioid crisis: a comprehensive overview. Curr Pain Headache Rep. 2018;22(3):16.
- Jones T, Moore T, Choo J. The impact of virtual reality on chronic pain. PLoS One. 2016;11(12):e0167523.
- Mallari B, Spaeth EK, Goh H, et al. Virtual reality as an analgesic for acute and chronic pain in adults: a systematic review and meta-analysis. J Pain Res. 2019;12:2053–2085.