Abstract
Purpose
The presence of lateral lymph nodes (LLNs) in patients with rectal cancer is not always acknowledged by the multidisciplinary team or treated in a standardized manner, and (inter)national guidelines concerning this topic are lacking. This study aimed to evaluate current practices regarding the assessment and treatment of LLNs in rectal cancer patients based on a survey among Dutch colorectal surgeons.
Methods
An online survey was sent to members of the Dutch Association of Coloproctology. The survey consisted of 16 questions addressing their views on diagnosis, restaging, and treatment approaches for suspicious LLNs.
Results
A total of 62 surgeons from 50 Dutch hospitals responded. For patients with a distal cT3/T4 rectal tumor; lateral lymph node compartments were routinely discussed during multidisciplinary meetings in only nine hospitals (18%). When defining what makes an LLN suspicious; the size threshold varied from >3 to >10 mm (median 7, SD 2), and MRI-based malignant features were mentioned by 29 surgeons (47%). Surgeons stated eight different treatment strategies as their designated treatment of suspicious LLNs. A total of 33 surgeons (53%) would add a radiotherapy boost to the neoadjuvant treatment. In cases of surgical resection; 12 surgeons (19%) would remove the suspicious LLN by ‘node-picking’ and 44 surgeons (71%) would perform a lateral lymph node dissection. The variation was not influenced by hospital type or surgeon's experience.
Conclusion
These results highlight the vast variation in the awareness, definition of suspicious LLNs in rectal cancer, and different treatment approaches. International guidelines based on further research are warranted.
1. Introduction
In the Netherlands, 4000 people are annually diagnosed with rectal cancer, of which ∼30% have a distal cT3/T4 rectal tumor [Citation1]. Tapering of the mesorectum and anatomical constraints make it difficult to create a clear resection margin (R0) for such distal tumors. Since the introduction of adequate staging based on high-quality MRI images, neoadjuvant (chemo)radiotherapy ((C)RT) and the total mesorectal excision (TME) technique, the positive resection margin rate (R1) has dropped dramatically and the local recurrence rate is currently 5–10% [Citation2–4]. When considering the current aspect of local recurrences, the location has shifted from central recurrences due to an R1 resection towards lateral local recurrences (LLR), with a higher relative contribution of lateral lymph node (LLN) metastases. Nowadays, ∼50% of the local recurrences of rectal cancer are situated in the lateral compartments [Citation5,Citation6].
LLN metastases occur mainly in distal cT3/T4 rectal tumors, as the lymphatic drainage of the distal part of the rectum follows the iliac vessels [Citation7]. However, there is no (inter)national consensus about the assessment and treatment of LLNs [Citation8]. In Eastern countries, a lateral lymph node dissection (LLND) is routinely performed in patients with a cT3/T4 rectal tumor situated below the peritoneal reflection. In Western countries, such as the Netherlands, LLNs are not always acknowledged by the radiologist or discussed during multidisciplinary meetings (MDTs). The presence of a suspicious LLN is sometimes considered as a distant metastatic disease and therefore thought to be incurable. Furthermore, Western physicians often believe that LLNs are adequately treated by (C)RT only. Similarly, some surgeons may opt for the removal of only the suspicious LLN (node-picking), hoping to limit the probability of morbidity associated with a formal LLND.
The lack of (inter)national guidelines has resulted in a wide range of diagnostics and treatment schedules, based on the perspectives, interpretations, and knowledge of the various clinicians involved. It is important to understand what these views are so that these can be properly addressed during future research and the production of guidelines. The current study aims to gain insight into the current opinions and preferences among Dutch colorectal surgeons regarding the assessment, prognostic implications, and treatment of lateral nodal disease in rectal cancer.
2. Methods
2.1. Survey
An online survey consisting of 16 questions was designed to evaluate the current practices regarding lateral nodal disease in rectal cancer. The survey contained questions regarding the definition of a suspicious LLN, the awareness and discussion of the LLN compartments in MDTs, the oncological meaning (metastatic vs. local disease), the designated treatment, and the surgeon’s own opinion on the uniformity of the current treatment of LLN in the Netherlands. This survey was sent to all members of the Dutch Association of Coloproctology (WCP) in the newsletter of July 2020 and again in October 2020. The Dutch Association has 246 members who are surgical fellows or surgeons (reference Citation27 January 2021). A personal reminder was sent in November 2020 and January 2021 to the contact of each hospital for the Dutch Colorectal Audit, a national register for colorectal cancer surgery. The complete survey can be found in the Appendix.
2.2. Definitions
Low rectal cancer was defined as a rectal adenocarcinoma requiring an abdominoperineal resection or a coloanal anastomosis. A formal LLND was defined as a resection of all the lymph node tissue within the internal iliac and obturator compartments. A node-picking procedure comprises removal of only the suspicious LLN, without formal resection of all lymphatic tissue through dissection along anatomical borders of the lateral compartments.
2.3. Data extraction and statistical analysis
The data were collected using an online survey tool. Statistical analyses were performed using the Statistical Package for the Social Sciences (IBM SPSS Statistics version 26). The results are presented through descriptive statistics; numerical data is reported as a median with a standard deviation (SD) and categorical variables are reported as number (n) and proportion in percentages. Sub-analyses were performed separately for the type of hospital (academic, teaching, and non-teaching) and surgeons experience in rectal cancer surgery (<10 or ≥10 years). The chi-square test was used for comparing categorical variables. For comparing continuous variables with a normal distribution, the independent t-test was used for comparing two groups and the One-way ANOVA test for more than two groups. For comparing continuous variables without a normal distribution, the Mann-Whitney U test and the Kruskal Wallis test were used for respectively two or more than two groups.
3. Results
3.1. Respondents’ characteristics
A total of 62 colorectal surgeons participated and represent 50 of the 68 Dutch hospitals performing rectal cancer surgery. The 50 Dutch hospitals consisted of six academic hospitals, 31 teaching hospitals, and 13 non-teaching hospitals. The median experience of the colorectal surgeons was 12 years (SD 7). An overview of the respondents’ characteristics can be found in .
Table 1. Respondents’ characteristics.
3.2. Diagnostics
According to 11 surgeons (18%), who represent nine hospitals (18%), the radiologist consequently discussed the lateral compartments during MDTs for every patient with a distal cT3/T4 rectal tumor. Sixteen surgeons (26%) answered ‘almost always’ and 35 surgeons (57%) answered ‘half of the time or less’. This variation was not significantly different between the different types of hospitals (p = 0.477) or between surgeons with less or more than 10 years of experience (p = 0.934). Furthermore, different definitions of a suspicious LLN were used. The size threshold for when an LLN is suspicious differed from >3 to >10 mm (median 7 mm, SD 2), and malignant features were included in the definition according to 29 surgeons (47%). Again, this was not significantly different between the types of hospitals (p = 0.291 and p = 0.193, respectively) and the experience of the surgeons (p = 0.799 and p = 0.474, respectively). Mesorectal lymph node criteria were mentioned by seven surgeons (11%) (>9 mm, or >5–9 mm with malignant features). When asked what percentage of patients with a distal cT3/T4 rectal tumor generally would have a suspicious LLN, the reported proportion varied from 2 to 60% (median 15%, SD 9). Three of the six academic hospitals (50%), 17 of the 31 teaching hospitals (55%), and seven of the 13 non-teaching hospitals (54%) reported the expected incidence of suspicious LLN to be <16%.
3.3. Treatment
When asked about the role of ‘node-picking’ of suspicious LLN, 16 surgeons (26%) answered that this is their routine practice, 27 surgeons (44%) perform ‘node-picking’ sometimes and 18 surgeons (29%) never resected suspicious LLN by ‘node-picking’. On the contrary, 27 surgeons (44%) had performed a formal LLND in the past five years. These surgeons represented five academic hospitals, 12 teaching hospitals, and four non-teaching hospitals. For those surgeons who had performed an LLND, the median number of LLND performed in the past 5 years was five (SD 9), varying from 1 to 38 LLNDs.
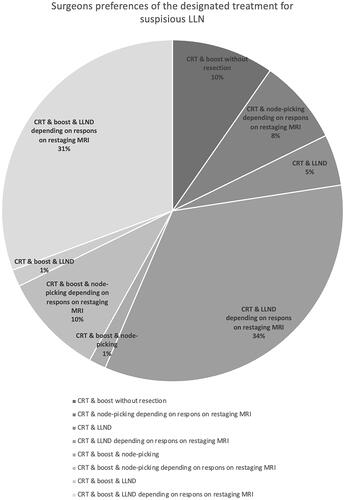
Eight different answers were given when the surgeons were asked what the designated treatment for suspicious LLN in rectal cancer should be. A boost added to the neoadjuvant treatment was preferred by 33 surgeons (53%). As for the surgical technique; 12 surgeons (19%) would choose node-picking and 44 surgeons (71%) would perform an LLND. Six surgeons (10%) would not perform a resection at all. The preference for either boosting or the type of surgical removal of the LLN(s) was not affected by the type of hospital (p = 0.775 and p = 0.159, respectively) or the years of experience of the surgeon (p = 0.807 and p = 0.374, respectively). The different preferences given are shown in . Different statements were presented to the surgeons of which the results are shown in .
Table 2. Answers to different statements in the survey.
4. Discussion
The outcomes of this survey, completed by 62 surgeons from 50 different Dutch hospitals, emphasize the current lack of uniformity in the assessment and treatment of LLNs in patients with low rectal cancer regardless of the type of hospital (academic, teaching, and non-teaching) and experience of the surgeon. In high-risk patients, LLNs are not always discussed during MDTs, and various definitions of a suspicious LLN are used. There is no uniformity in neoadjuvant treatment, neither in indication for surgery and type of surgical procedure among the responding surgeons of the Dutch Association of Coloproctology.
4.1. Lateral lymph node assessment
In half of the hospitals, the lateral compartments were only discussed in ∼50% of the distal cT3/T4 rectal cancer cases or less. A recent retrospective study including 1216 patients with a distal cT3/T4 tumor and treated with (C)RT + TME, has shown that an LLN ≥ 7 mm on primary MRI is associated with a high LLR rate of 19.5% [Citation9]. When the LLN in the internal iliac compartment was >4 mm or the LLN in the obturator compartment was >6 mm, the risk of an LLR further increased up to 52.3%, while the patients with sufficient response (≤4 mm in the internal iliac compartment and ≤6 mm in the obturator compartment) developed no LLR [Citation10]. When an LLND was performed in patients with insufficient response, the LLR rate was only 5.7%. It is therefore of the utmost importance to consistently address the lateral nodal area in high-risk patients (distal cT3/T4 tumor) in the MDT to ensure the appropriate treatment to prevent LLR.
Ogura et al. showed that 16% of the patients with a distal cT3/T4 had at least one suspicious LLN (≥7 mm). This corresponds with the median answer given by the responders in this survey (15%), but this varied widely between 2 and 60%. The estimation of the incidence of enlarged LLNs is likely influenced by the patient volume of the hospital where the participating surgeon works. Low-volume hospitals might see very few of these patients. However, the underestimation of incidence was also often present in high-volume centers.
Furthermore, suspicious LLNs are still sometimes interpreted as a sign of distant metastatic disease, although in this survey only six surgeons (10%) totally or partially agreed with this statement. Different studies have demonstrated that involved LLNs have no additional risk for distant metastases when compared to involved mesorectal lymph nodes, and should be considered as a locoregional disease that can be treated with curative intent [Citation9,Citation11].
4.2. Lateral lymph node criteria vs. mesorectal lymph node criteria
Many different answers were given for the definition of a suspicious LLN. This might be explained by the lack of a clear definition in the current guidelines. In the MRI Primary Rectal Cancer Staging Template v.2020 made by the rectal cancer Disease Focus Group (DFP) of the Society of Abdominal Radiology (SAR), the topic ‘extra-mesorectal lymph nodes’ is included in the list of characteristics that should be reported standardly [Citation12]. This recommendation was also added to the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) meeting [Citation13]. However, in contrast to reporting mesorectal lymph nodes, the template does not indicate what a suspicious LLN actually entails. Mesorectal lymph nodes have clear criteria and are suspicious if fulfilling criteria according to a certain size in combination with suspicious morphologic criteria. Often, criteria for mesorectal lymph nodes are also applied to LLNs in daily practice. However, recent literature has shown that, in contrast to mesorectal lymph nodes, suspicious morphologic criteria play a less accurate role in LLNs and it is predominantly the size of an LLN that predicts the presence of involved LLN [Citation9,Citation14,Citation15]. This distinction is important to be taken into account, otherwise, some LLN might go unreported if they lack suspicious morphologic criteria, but do have a size of ≥7 mm. That 47% of the surgeons mention these morphologic criteria as a prerequisite for defining a suspicious LLN, reiterates the necessity for increased awareness.
4.3. Treatment
When asking the surgeons about their designated treatment choice, almost half of the responders would like to give an additional radiotherapy boost dose to the LLNs. This is more common in gynecological malignancies, but there is very little evidence on the added value of a boost on LLNs from rectal cancer and is not a recommendation in the current national guideline [Citation16]. Personal communication with radiation oncologists in Dutch radiation centers reveals that in a few centers this is sometimes applied. A previous survey amongst US radiation oncologists showed that around 60% of the responders recommended a boost of the internal iliac and/or obturator LLNs [Citation17]. Two small retrospective studies investigating the outcomes after a boost for LLNs from rectal cancer suggested that a boost might be an effective treatment with minimal adverse events in patients who did not receive an LLND [Citation18,Citation19]. However, these retrospective studies included a small number of (heterogenic) patients and one study had a follow-up of only one year. Moreover, different criteria for a suspicious LLN were used, and also the exact location was unknown. Therefore, these results must be interpreted carefully. Adding a boost to the LLNs might create more response and therefore may reduce the need for an LLND, however functional effects of a boost are still unclear and in case there is still insufficient response, an additional LLND after boosting might be even more hazardous. Also, as only 22% of the more aggressive internal iliac LLNs in the Lateral Node Consortium study showed sufficient response, the effect of a boost might be limited [Citation10].
Twelve surgeons (20%) preferred node-picking over a formal LLND and 43 surgeons (70%) do this always or sometimes in cases of a suspicious LLN. There is very limited evidence of the oncological outcomes after node-picking. All previous prospective studies investigating the surgical treatment of LLNs performed an LLND [Citation20–22]. Only a small retrospective series of node-picking are available. The results of a recent study by Kim et al. showed no improvement of local control in patients treated with node-picking vs. no node-picking [Citation23]. The 5-years disease-free survival was 75.4% in patients with node-picking vs. 82.2% in patients without node-picking (p = 0.722). Furthermore, for five of the 30 included patients, no lymph node tissue was found in the pathology examination. The disadvantage of only resecting the suspicious LLN is that small undetected deposits in other lateral nodes may be left behind, or even that the wrong node is resected. In the Lateral Node Consortium study, 12 patients were treated with node-picking and 51% of these patients developed an LLR [Citation9]. Again the numbers are very small and this should be further investigated, but it suggests that node-picking is oncologically a less sound option compared to a formal LLND. Also, as there is no standardized procedure with medialization of the ureter together with the hypogastric nerve plexus, as it is done with the formal LLND, one could imagine there would be a significant risk of nerve damage after node picking, which could lead to worse functional outcomes.
A total of 27 surgeons (44%) had performed an LLND in the past five years, with a median of five LLNDs. It was not specified whether this was an LLND for a primary rectal tumor or if this was performed in case of a total exenteration or recurrent rectal cancer. Therefore, this number might not be representative of the actual number of LLND performed for primary rectal cancer. Furthermore, no details were asked about the technique and extent of the LLND, which might have differed. However, the number of surgeons performing this procure is high, while the indication for an LLND for a primary rectal tumor is expected to be only 50–100 patients a year in the Netherlands (if all correctly diagnosed). An LLND is a difficult procedure with an increased risk of bleeding and nerve damage, possibly resulting in sexual and/or bladder dysfunction [Citation24–27]. It is therefore important to gain consensus on the technique of the LLND and to nationally concentrate this difficult and infrequent procedure. During a meeting of the Dutch Association of Coloproctology (WCP), all surgeons present agreed unanimously that this procedure should preferably be performed by a selected group of surgeons to limit the risks accompanying this procedure.
4.4. Future research
The responders almost unanimously agreed that further research is needed regarding the optimal treatment of LLNs. It is important that the problem of the current variation in de diagnostic and treatment of LLNs is acknowledged and the optimal treatment is further prospectively investigated. A retrospective, cross-sectional collaborative research project, Snapshot rectal cancer 2016, is ongoing in 67 of the 69 hospitals in the Netherlands that performed rectal resections in 2016. Data regarding diagnostics, neoadjuvant therapy, surgical procedures, and long-term (oncological) outcomes will be collected for all patients. In addition, the MRIs and radiation target areas of the distal cT3/T4 tumors will be reassessed by the local radiologist and radiation oncologist to evaluate the variation in assessment and treatment of the LLNs. The main goal of this study is to evaluate practice, but also to raise awareness on LLNs.
Also, the prospective (inter)national registration study, the LaNoReC trial (Lateral Nodal Recurrence in Rectal Cancer), is currently recruiting patients. Radiologists trained in detecting lateral lymph nodes on MRI will assess the LLNs in the participating centers and after confirmation by a central review board, the patient can be included. The patient will receive standardized and quality controlled neoadjuvant CRT, ensuring that the enlarged LLNs receive adequate radiation and selective LLNDs will be offered by trained surgeons in expert hospitals to those with insufficient shrinkage of enlarged LLNs.
4.5. Limitations
A limitation of this survey study is that only colorectal surgeons were included. Rectal cancer requires a multidisciplinary approach in which also radiologists and radiotherapists have an important role. However, surgeons often play the lead role in the MDTs to determine the suitable treatment for the patients and this study’s main focus was the variation in surgical strategies in the presence of suspicious LLNs. Furthermore, a surgeon should also be able to assess an MRI of the rectum to fully be prepared for the surgery.
Approximately 25% of the colorectal surgeons [62/246 of the members of the Dutch Association of Coloproctology (WCP)] responded to the survey, which might raise concerns about the representativeness of the Dutch surgical colorectal community. However, surgeons from 50 of the 68 hospitals performing rectal cancer surgery responded and therefore represent 75% of the Dutch hospitals. It is expected that most surgeons who are interested and experienced in the treatment of LLNs have responded. So, it is expected that the variation would be even larger among other surgeons and hospitals. Moreover, some centers were represented by more than one surgeon in this survey, possibly causing bias in the results. However, even within the same hospital, the answers of the surgeons showed variation.
5. Conclusion
The results from this survey provide evidence for nationwide variability in the definitions and current treatment of suspicious LLNs. Consequent acknowledgement, clear definitions, and high-quality scientific evidence for the optimal treatment of suspicious LLNs are needed to establish (inter)national consensus and guidelines for the proper treatment of lateral nodal disease.
Author contributions
All mentioned authors and collaborators have made a substantial contribution and have approved the final version.
Geolocation information
The study was conducted in the Netherlands.
| Abbreviations | ||
| (C)RT | = | (chemo) radiotherapy |
| TME | = | total mesorectal excision |
| LLN | = | lateral lymph node |
| LLND | = | lateral lymph node dissection |
| LLR | = | lateral local recurrence |
| R1 | = | positive resection margin |
| MDT | = | multidisciplinary meeting |
Acknowledgements
All contributors are listed as collaborators.
Disclosure of interest
The authors report no conflict of interest.
References
- [cited 2021 Feb 1]. Available from: https://www.iknl.nl/nkr/cijfers-op-maat
- Kusters M, Marijnen CA, van de Velde CJ, et al. Patterns of local recurrence in rectal cancer; a study of the Dutch TME trial. Eur J Surg Oncol. 2010;36(5):470–476.
- Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345(9):638–646.
- Martling A, Holm T, Johansson H, et al. The Stockholm II trial on preoperative radiotherapy in rectal carcinoma: long-term follow-up of a population-based study. Cancer. 2001;92(4):896–902.
- Iversen H, Martling A, Johansson H, et al. Pelvic local recurrence from colorectal cancer: surgical challenge with changing preconditions. Colorectal Dis. 2018;20(5):399–406.
- Kim TH, Jeong SY, Choi DH, et al. Lateral lymph node metastasis is a major cause of locoregional recurrence in rectal cancer treated with preoperative chemoradiotherapy and curative resection. Ann Surg Oncol. 2008;15(3):729–737.
- Steup WH, Moriya Y, van de Velde CJ. Patterns of lymphatic spread in rectal cancer. A topographical analysis on lymph node metastases. Eur J Cancer. 2002;38(7):911–918.
- Kusters M, Beets GL, van de Velde CJ, et al. A comparison between the treatment of low rectal cancer in Japan and The Netherlands, focusing on the patterns of local recurrence. Ann Surg. 2009;249(2):229–235.
- Ogura A, Konishi T, Cunningham C, et al. Neoadjuvant (chemo)radiotherapy with total mesorectal excision only is not sufficient to prevent lateral local recurrence in enlarged nodes: results of the multicenter lateral node study of patients with low cT3/4 rectal cancer. J Clin Oncol. 2019;37(1):33–43.
- Ogura A, Konishi T, Beets GL, et al. Lateral nodal features on restaging magnetic resonance imaging associated with lateral local recurrence in low rectal cancer after neoadjuvant chemoradiotherapy or radiotherapy. JAMA Surg. 2019;154(9):e192172.
- Akiyoshi T, Watanabe T, Miyata S, et al. Results of a Japanese nationwide multi-institutional study on lateral pelvic lymph node metastasis in low rectal cancer: is it regional or distant disease? Ann Surg. 2012;255(6):1129–1134.
- Available from: https://cdn.ymaws.com/abdominalradiology.site-ym.com/resource/resmgr/education_dfp/rectal_cancer/sar_primary_rectal_cancer_st.pdf
- Beets-Tan RGH, Lambregts DMJ, Maas M, et al. Magnetic resonance imaging for clinical management of rectal cancer: updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2018;28(4):1465–1475.
- Kusters M, Slater A, Muirhead R, et al. What to do with lateral nodal disease in low locally advanced rectal cancer? A call for further reflection and research. Dis Colon Rectum. 2017;60(6):577–585.
- Schaap DP, Ogura A, Nederend J, et al. Prognostic implications of MRI-detected lateral nodal disease and extramural vascular invasion in rectal cancer. Br J Surg. 2018;105(13):1844–1852.
- Valentini V, Gambacorta MA, Barbaro B, et al. International consensus guidelines on clinical target volume delineation in rectal cancer. Radiother Oncol. 2016;120(2):195–201.
- Yahya JB, Herzig DO, Farrell MJ, et al. Does a fine line exist between regional and metastatic pelvic lymph nodes in rectal cancer-striking discordance between national guidelines and treatment recommendations by US radiation oncologists. J Gastrointest Oncol. 2018;9(3):441–447.
- Hartvigson PE, Apisarnthanarax S, Schaub S, et al. Radiation therapy dose escalation to clinically involved pelvic sidewall lymph nodes in locally advanced rectal cancer. Adv Radiat Oncol. 2019;4(3):478–486.
- Chen H, Nguyen KNB, Huang H, et al. Effect and safety of radiation therapy boost to extramesorectal lymph nodes in rectal cancer. Pract Radiat Oncol. 2020;10(5):e372–e377.
- Fujita S, Mizusawa J, Kanemitsu Y, et al. Mesorectal excision with or without lateral lymph node dissection for clinical stage II/III lower rectal cancer (JCOG0212): a multicenter, randomized controlled, noninferiority trial. Ann Surg. 2017;266(2):201–207.
- Wei M, Wu Q, Fan C, et al. Lateral pelvic lymph node dissection after neoadjuvant chemo-radiation for preoperative enlarged lateral nodes in advanced low rectal cancer: study protocol for a randomized controlled trial. Trials. 2016;17(1):561.
- Konishi T, Shinozaki E, Murofushi K, et al. Phase II trial of neoadjuvant chemotherapy, chemoradiotherapy, and laparoscopic surgery with selective lateral node dissection for poor-risk low rectal cancer. Ann Surg Oncol. 2019;26(8):2507–2513.
- Kim YI, Jang JK, Park IJ, Park SH, et al. Lateral lymph node and its association with distant recurrence in rectal cancer: a clue of systemic disease. Surgical Oncology. 2020;35:174–181.
- Yang X, Yang S, Hu T, et al. What is the role of lateral lymph node dissection in rectal cancer patients with clinically suspected lateral lymph node metastasis after preoperative chemoradiotherapy? A meta-analysis and systematic review. Cancer Med. 2020;9(13):4477–4489.
- Fujita S, Akasu T, Mizusawa J, et al. Postoperative morbidity and mortality after mesorectal excision with and without lateral lymph node dissection for clinical stage II or stage III lower rectal cancer (JCOG0212): results from a multicentre, randomised controlled, non-inferiority trial. Lancet Oncol. 2012;13(6):616–621.
- Ito M, Kobayashi A, Fujita S, et al. Urinary dysfunction after rectal cancer surgery: results from a randomized trial comparing mesorectal excision with and without lateral lymph node dissection for clinical stage II or III lower rectal cancer (Japan clinical oncology group study, JCOG0212). Eur J Surg Oncol. 2018;44(4):463–468.
- Saito S, Fujita S, Mizusawa J, et al. Male sexual dysfunction after rectal cancer surgery: results of a randomized trial comparing mesorectal excision with and without lateral lymph node dissection for patients with lower rectal cancer: Japan clinical oncology group study JCOG0212. Eur J Surg Oncol. 2016;42(12):1851–1858.
Appendix
Survey questions
In which hospital are you a practicing surgeon?
How many years experience do you have as a colorectal surgeon?
How many rectal resections do you perform annually?
How many rectal resections does your department perform annually?
How many low (APR of coloanal anastomosis) cT3/T4 tumors do you resect annually?
What percentage of patients with a low cT3/T4 tumor have suspicious lateral lymph nodes on the primary MRI?
Free text
What definition of a suspicious lateral lymph node is used in your hospital during multidisciplinary meetings?
Free text
How often is the presence of a lateral lymph node discussed by the radiologist during multidisciplinary meetings for patients with a low cT3/T4 tumor, regardless of their size?
1—Never
2
3
4
5—Always
In case of suspicious lateral lymph nodes on the primary MRI without distant metastasis, is this considered as metastatic disease?
1—Totally disagree
2
3
4
5—Totally agree
What is the designated treatment in case of suspicious lateral lymph nodes in rectal cancer?
Palliative treatment
Only node-picking without irradiation of the lateral compartment(s)
Only a lateral lymph node dissection without irradiation of the lateral compartment(s)
Chemoradiation without further resection of the lateral lymph node(s)
Chemoradiation with a boost on the suspicious lateral lymph node(s) without further resection of the lateral lymph node(s)
Chemoradiation with node-picking based on the primary MRI
Chemoradiation with node-picking based on the response on the restaging MRI
Chemoradiation with a lateral lymph node dissection based on the primary MRI
Chemoradiation with a lateral lymph node dissection based on the response on the restaging MRI
Chemoradiation with a boost and node-picking based on the primary MRI
Chemoradiation with a boost and node-picking based on the response on the restaging MRI
Chemoradiation with a boost and a lateral lymph node dissection based on the primary MRI
Chemoradiation with a boost and a lateral lymph node dissection based on the response on the restaging MRI
In case of suspicious lateral lymph nodes, do you resect them during the surgery via node-picking?
Always
Sometimes
Never
Have you ever performed a lateral lymph node dissection in the past 5 years (= resection of all lymph node tissue in the internal iliac and obturator compartment)?
Yes
No, these patients are referred to another hospital
No, we do not see an added value of this procedure
If yes, how many times did you perform a lateral lymph node dissection in the past five years?
Free text
The benefit of a lateral lymph node dissection (lower risk of a locoregional recurrence) does not outweigh the risks (complications, morbidity)
1—Totally disagree
2
3
4
5—Totally agree
Do you think that suspicious lateral lymph nodes are treated in a standardized manner?
1—Totally disagree
2
3
4
5—Totally agree
Do you think that more research is needed to investigate the optimal treatment of suspicious lateral lymph nodes in rectal cancer?
1—Totally disagree
2
3
4
5—Totally agree