Abstract
Objectives
To provide a critical update identifying the knowledge gaps and controversies in medication-related osteonecrosis of the jaw (MRONJ) within the Belgian healthcare context and outline opportunities for improvement and research in these areas.
Methods
A literature review was performed to identify guidelines from international clinical societies in oncology or oral and maxillofacial surgery on diagnosing, preventing, and treating MRONJ. The recommendations were critically assessed in light of recent developments in the field and confronted with the clinical experience of experts.
Results
Despite progress in the diagnostic criteria of MRONJ, the continued need for an 8-week timeout period should be reconsidered. Furthermore, 3D imaging techniques should be introduced to improve diagnosis and staging. The staging system remains ambiguous regarding Stage 0 MRONJ, and ongoing confusion exists regarding the term non-exposed MRONJ. The prevention of MRONJ should be tailored, considering the individual patient’s risk of MRONJ, frailty, and life expectancy. More research seems needed into the efficacy and safety of drug holidays, considering the risks of rebound remodeling on fractures. With renewed interest in surgical and adjunct management techniques, adequately designed clinical studies are needed to help translate trial outcomes into universally applicable treatment guidelines taking into account individual patient characteristics.
Conclusions
Important knowledge gaps remain and hamper the development of clinical guidelines. Several controversies were identified where consensus is lacking, and further harmonization between stakeholders is necessary. Finally, the need for randomized controlled comparative clinical trials in MRONJ resonates harder than ever to identify the best treatment for individual patients.
Introduction
Bone modifying agents (BMA), such as bisphosphonates and denosumab are prescribed for skeletal disorders, including osteoporosis, bone metastases, and multiple myeloma [Citation1,Citation2]. Medication-related osteonecrosis of the jaw (MRONJ) associated with their use has a relatively recent history, with the first reports emerging in the early 2000s. The initial cases were observed in cancer patients receiving high-dose intravenous bisphosphonate therapy. Subsequent reports identified MRONJ cases in patients receiving bisphosphonates for non-cancerous conditions such as osteoporosis, albeit with a considerably lower risk [Citation3].
Although MRONJ is considered an infrequent condition, the potential impact on patients’ quality of life and oral health necessitates a comprehensive approach to its diagnosis and management and effective preventive strategies. The recommendations for diagnosing and treating MRONJ have shifted over the years, but some controversies and knowledge gaps remain. A state of equipoise in the various treatment options for MRONJ still exists due to the lack of good-quality comparative clinical trials. In addition to the optimal initial approach, the sequencing of available treatments also remains uncertain. As a result, clinical decision-making is often based on the preference and expertise of the treating healthcare professionals.
This review provides a critical update identifying open issues and controversies in MRONJ within the Belgian healthcare context. Furthermore, we will outline opportunities for improvement and research in these areas, highlighting the gaps in knowledge and practice.
Epidemiology and pathophysiology
MRONJ risk with high-dose BMA treatment in cancer
Randomized controlled clinical trials with high-dose BMAs in advanced cancer patients show an incidence of MRONJ (adjusted for patient-year exposure) of 1.1% in the first year, 3.7% in the second year, and 4.6% after that, highlighting the increased MRONJ risk with more prolonged exposure [Citation4]. This risk was slightly higher in multiple myeloma patients, with an incidence of 2.0% during the first year of treatment, 5.0% in the second year, and 4.5% per year thereafter [Citation5].
Data from Belgium confirms the higher MRONJ risk with increasing cumulative exposure, with a 6% incidence beyond two years of treatment [Citation6]. However, subgroups with higher MRONJ susceptibility may exist, as a higher MRONJ risk of 11% was reported in a cohort of renal cell cancer patients treated with both BMA and anti-angiogenic agents [Citation7]. Similarly, sequential treatment with bisphosphonates and denosumab conferred a slightly higher risk of MRONJ early after switching agents compared to patients remaining on bisphosphonates [Citation8].
MRONJ risk with low-dose BMA treatment in osteoporosis
With low-dose treatment for osteoporosis, the incidence of MRONJ in randomized controlled trials was considerably lower, with 0.04% at 3 years, 0.06% at 5 years, and 0.44% at 10 years [Citation9]. While no specific data is available for Belgium, a recent large cohort study in Switzerland reported MRONJ in 0.55% [Citation10]. In the same study, previous bisphosphonate therapy before switching to denosumab was an additional risk factor for ONJ development, similar to the findings with high-dose treatment.
Patient risk factors for MRONJ
The risk of MRONJ is associated with cumulative exposure to BMAs. In contrast, the route of administration (IV versus PO) of the BMA is no longer considered a risk factor for MRONJ [Citation11]. Focusing on modifiable risk factors in light of preventing MRONJ, poor periodontal and dental health, ill-fitting dentures, uncontrolled diabetes mellitus, and tobacco use deserve special attention. Data from Belgian cohorts have consistently demonstrated that the risk of developing MRONJ more than doubles in active smokers compared to never or former smokers, underscoring the need for smoking cessation [Citation6,Citation7,Citation12]. Whether risk factors differ between low-dose and high-dose treatment has not been established.
Impact of the COVID-19 pandemic
The COVID-19 pandemic significantly impacted oncology care due to efforts to minimize exposure to the virus. In an international online survey conducted over the summer of 2020, 94.6% and 78.8% of respondents reported a decreased administration of bisphosphonates and denosumab, respectively [Citation13]. While efforts were undertaken to minimize the adverse patient impact by switching to home administration or oral treatment routes, BMAs have likely been underused over the last two years. As a result, an artificially lower incidence of MRONJ may present in the coming years, but prevention should continue unchanged as the use of these agents is restored to pre-pandemic levels.
Understanding MRONJ pathophysiology
The pathophysiology of MRONJ is considered multifactorial but remains incompletely understood [Citation14]. All BMAs share the ability to suppress osteoclast activity, reducing bone turnover that can affect bone-healing capacity. In MRONJ, the prolonged suppression of osteoclast function is hypothesized to result in an accumulation of microdamage. This compromised bone healing capacity and the subsequent accumulation of unrepaired microcracks has been shown to contribute to the development of MRONJ in preclinical models [Citation15].
Preclinical studies have confirmed the role of (chronic) trauma and infection in the development and progression of MRONJ [Citation16]. Dental infections can stress the local defense mechanisms of the jaw and initiate a cascade leading to osteonecrosis. Trauma, including tooth extractions or chronic irritation by ill-fitting dental prostheses, subsequently promotes the development of MRONJ. In addition, the impairment of wound healing caused by anti-angiogenic drugs further contributes to MRONJ [Citation17].
Interestingly, post-COVID-19-related osteonecrosis of the jaw was recently reported. This condition only affects the maxilla, and most patients have comorbidities, including diabetes, or were treated with corticosteroids or other immune modulators [Citation18]. Like MRONJ, the pathophysiologic mechanism remains unknown, but SARS-CoV-2 promotes a hyperinflammatory state, with elevated cytokines and immune dysregulation, in addition to microvascular thromboses and a hypercoagulability state [Citation18]. These findings suggest that mucosal immune homeostasis and osteoimmunity may be more important than previously recognized.
Diagnosis and staging
Diagnostic criteria of MRONJ
Early case definitions were put forward by the American Association of Oral and Maxillofacial Surgeons (AAOMS) () [Citation19], the American Society for Bone and Mineral Research (ASBMR) [Citation20], and later also by the Task Force on Osteonecrosis of the Jaw (TFONJ) [Citation21]. An arbitrary timeout period of eight weeks is included to rule out other conditions that can be expected to heal within this timeframe to avoid overdiagnosis. Notably, the 8-week interval only starts at the time that exposed bone is first documented by a healthcare provider [Citation19,Citation20].
Table 1. Case definition of MRONJ according to the AAOMS [Citation25].
However, some patients presented with non-specific symptoms or suspicious radiographic changes without exposed bone and did not fit these criteria [Citation22]. With the introduction of Stage 0 in 2009, the AAOMS attempted to fit patients without exposed bone but with non-specific symptoms or (imaging) findings within the staging system of MRONJ [Citation23]. Somewhat confusingly, Stage 0 MRONJ continues to be interpreted as a potential prodromal stage of MRONJ by the AAOMS and ITFONJ, even though it is listed as an actual stage of MRONJ, but not included in the actual MRONJ case definition [Citation24,Citation25]. Another major change occurred with the 2014 update, adding bone that can be probed through an intraoral or extraoral fistula to the diagnostic criteria. In addition, treatment with an anti-angiogenic agent was added to the list of culprit medications. In 2022, immune modulators were included [Citation26]. However, it has become ambiguous whether using an anti-angiogenic agent alone remains sufficient to meet the diagnosis of MRONJ under the new AAOMS definition [Citation25].
Staging system for MRONJ
Three staging systems have been frequently used in the literature, put forward by the AAOMS () [Citation25], the TFONJ [Citation20], and by Weitzman et al. [Citation27]. The latter was designed to be compatible with the Common Toxicity Criteria (CTC) adverse event reporting system from the National Cancer Institute (NCI). Other clinical societies have aligned with the AAOMS system [Citation20,Citation28].
Table 2. AAOMS staging system for MRONJ [Citation25].
Difficulties emerged with the AAOMS system in classifying patients presenting with chronic sinus tracts without evidence of exposed bone at the initial visit. The term non-exposed MRONJ was coined to describe this phenotype [Citation29]. According to some authors, up to 25% of cases may have been missed in clinical trials up to that time, where adjudicators strictly adhered to the AAOMS definition [Citation30]. Bagan et al. proposed to include patients with an oral fistula and without apparent bone exposure in Stage 1 [Citation31]. In contrast, Woo et al. created a new Stage 0 MRONJ, defined as patients without exposed bone but with sinus tracts or localized deep periodontal pockets [Citation32]. The 2014 update of the AAOMS staging system addressed this open issue by adding ‘a fistula that probes to the bone’ to the description of exposed bone in Stages 1–3. In addition, this update clarified that Stage 0 is considered a prodromal and non-exposed state [Citation26]. However, this interpretation is markedly different from that used earlier in the literature, where the non-exposed variants were not considered prodromal but actual clinical manifestations similar to Stages 1–3 and requiring treatment.
Remarkably, the case definitions for MRONJ used by the ASBMR and the TFONJ have not mirrored these changes. In particular, the TFONJ has not broadened the stage definition to include fistulas that probe to bone and does not recognize a preclinical variant of MRONJ [Citation33]. Similarly, the MASC/ISOO/ASCO considers Stage 0 only as an indicator of increased MRONJ risk, but not true MRONJ [Citation28]. Their main concern is that Stage 0 terminology may lead to MRONJ overdiagnosing because these same presenting symptoms may ultimately lead to an alternative diagnosis [Citation24]. A harmonization effort is needed to resolve these conflicting views ().
Table 3. Controversies and challenges in the diagnosis and staging of MRONJ.
Imaging criteria
The radiographic changes in MRONJ include a persisting alveolar socket, osteosclerosis, osteolysis, thickening of the lamina dura, narrowing of the mandibular canal, widening of the periodontal ligament space, and sequestrum formation. Unfortunately, only a limited role is attributed to imaging to improve the early diagnosis, staging and management of MRONJ in current guidelines, despite evidence suggesting that imaging can contribute in these areas. Indeed, cone beam computed tomography (CBCT) could contribute to a more specific differential diagnosis between conditions that can mimic MRONJ while providing more detailed information on disease extent in patients with overt disease [Citation34]. Despite these potential advantages, some clinical societies do not support including CBCT findings, out of concern that this may lead to overdiagnosis [Citation28,Citation35].
Prevention
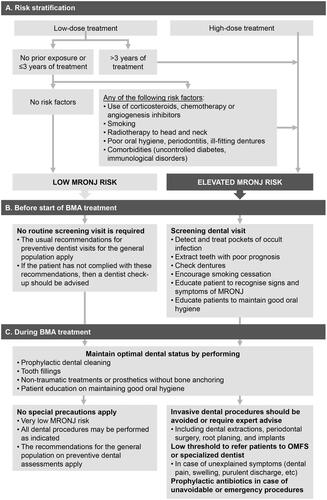
Multidisciplinary collaboration among healthcare providers is fundamental in identifying high-risk individuals (), educating patients, and implementing preventive measures [Citation36]. Educational leaflets, websites with dedicated patient information (e.g. www.kaaknecrose.be), or other educational materials can contribute to these goals.
Before the start of BMA
Preventive dental care in patients starting high-dose BMA treatment reduces the risk of MRONJ by 68–77% [Citation37]. In other words, one needs to send approximately 25 patients for preventive dental care to avoid one MRONJ case in this group. Broad consensus exists that a dental and periodontal examination should be performed, including panoramic or intraoral radiographs, before starting BMA therapy () [Citation28]. However, it is currently unclear how aggressive preventive dental care should be and how each intervention reduces the MRONJ risk (). Moreover, there seems to be a need for individually tailoring prevention, as all medically indicated dental care might not be feasible, considering the patient’s frailty, the limited life expectancy, and the urgency of prompt initiation of a BMA (). When dental extractions are performed, BMA therapy can be started after mucosal coverage has occurred [Citation28].
Table 4. Controversies and challenges in the prevention of MRONJ.
Table 5. Descriptions of complete, partial and minimal dental evaluation and treatment protocols.
In patients starting low-dose BMA therapy, in the absence of other risk factors or history of treatment with another BMA, the MRONJ risk is so limited that universal prevention measures are more difficult to justify, as the number needed to submit to prevention would increase to 334 to avoid one case of MRONJ on a 10-year horizon. Nevertheless, an annual dental check-up is advised for every patient, as in the general population, to maintain and improve oral hygiene [Citation36]. If a patient has not complied with this general advice, a preventive dental visit is still recommended.
During treatment with a BMA
Restrictions apply only to patients treated with a high-dose BMA, after prolonged therapy (>3 years) with a low-dose BMA, or in the presence of other risk factors. In these patients, elective invasive dental procedures should be avoided (such as tooth extractions, periodontal surgery, scaling and root planing, or placement of implants) (). For acute problems, an endodontic approach or a root submergence technique is preferred, and, in case surgery is deemed unavoidable, the prophylactic use of antibiotics is indicated. In contrast, extractions and dental implant placement can be carried out for patients without risk factors, receiving short-term low-dose therapy and adhering to regular dental visits [Citation33]. Routine dental care is allowed in all patients and should be performed without soft tissue injury.
Drug holiday
Despite convincing preclinical evidence of the benefit of a drug holiday, the clinical evidence is contradictory [Citation38]. Even though a recent retrospective Belgian study suggested a relative reduction in MRONJ risk by 17% for every month of drug holiday with high-dose treatment, this effect was not observed in the low-dose treatment cohort [Citation12]. These effects were similar for the different BMAs. On the other hand, a recent meta-analysis concluded that drug holidays do not minimize the risk of MRONJ [Citation39].
Importantly, treatment with a BMA should never be interrupted, even temporarily, without consulting the prescribing physician. Indeed, a rapid rebound of bone resorption may occur and can put patients at an increased fracture risk, particularly after denosumab treatment, due to its quickly reversible effects [Citation40]. Currently, the uncertainty regarding the efficacy and safety of a drug holiday is reflected in the different guidelines, leaving the option to interrupt the BMA to the treating physician [Citation25,Citation28,Citation33].
Bone turnover markers
An equally contentious issue remains the use of bone turnover markers reflecting the process of bone remodeling. In particular, C-terminal telopeptide (CTX) is one of the resorption markers, reflecting the activity of osteoclasts. It was hypothesized that low CTX values (<0.10 ng/mL) would indicate a highly suppressed bone turnover and, thus, an elevated risk of MRONJ [Citation41]. Despite the appeal of having a simple blood test for predicting MRONJ risk, subsequent studies have failed to show sufficient predictive value of CTX and its use is not recommended, regardless of the dosing of BMA treatment [Citation33].
Dental care access
Lastly, patient access to high-quality, affordable dental care within an acceptable timeframe is essential for MRONJ prevention. In a recent report, Belgium scores average in Europe on many metrics relating to financing, access, and provision of oral healthcare [Citation42]. However, some trends may cause barriers for patients to maintain optimal oral health and obtain preventive dental care when starting BMAs. In some regions, patient waiting times can be long due to a local shortage of dentists. In addition, given the partial statutory coverage system of dental care in Belgium, interventions, such as periodontal treatment, prosthodontics, dental implants, or imaging have limited or no reimbursement and require significant co-payment from the patient. Estimates show that in 2019 out-of-pocket payments represented 65% of the total expenditure for dental care in Belgium. Moreover, the unmet need for dental care has doubled over the last decade, rising to approximately 8% among the lowest quintile incomes [Citation42]. Policymakers must safeguard access to affordable preventive dental care, particularly for the often frail population at risk of MRONJ. In the meantime, establishing dedicated referral pathways within the hospital can help to improve the time to preventive dental care and avoid treatment delays.
Treatment
Almost 20 years after the first reports of MRONJ were published, the condition remains difficult to treat, and there is still no defined treatment algorithm (). Consensus exists that the treatment of MRONJ should be tailored to the individual patient in a multidisciplinary care setting, and clear treatment goals should be agreed upon to facilitate communication, improve patient adherence, and avoid disappointment [Citation43].
Table 6. Controversies and challenges in the treatment of MRONJ.
Setting individual treatment goals
Patients with MRONJ can present with a broad spectrum of signs and symptoms, ranging from a small asymptomatic area of exposed bone to uncontrolled infection and severe tissue loss. Similarly, the patient’s prognosis – independent of the presence of MRONJ – can be considerably different depending on the underlying condition. At one end of the spectrum, treatment should focus on symptom control in frail patients with limited life expectancy or deemed unfit for more aggressive surgical approaches. Conversely, young and fit patients may be candidates for treatment approaches aiming for superior cosmetic and functional outcomes.
Importantly, the different treatment goals have not yet translated into a consensus on appropriate endpoints in clinical trials with MRONJ. Currently, there is a strong focus on achieving mucosal closure as the primary outcome measure of efficacy. Little attention has been paid to patient symptoms, quality of life, functioning and well-being during treatment, even though the resolution of MRONJ symptoms and limiting treatment-related adverse events may be equally important to patients. Ideally, patient organizations and advocacy groups should be involved to align endpoints with meaningful outcomes.
Conservative treatment
The focus is the control of symptoms and infection through maintaining optimum oral hygiene, eliminating active dental and periodontal disease, and using topical antibacterial mouth rinses and systemic antibiotic therapy [Citation28]. In combination, limited surgical debridement and contouring of MRONJ lesions are typically performed to remove sharp edges. While well tolerated, it can take a long time before a sequester forms and can be removed. After that, mucosal coverage of the bone defect usually occurs. However, patients need to be aware of the slow healing rate, and pertinent questions on the effects of the long-term use of antibiotics have not been well studied. In addition, there is considerable heterogeneity regarding the reported outcomes with conservative treatment [Citation44].
Minimally invasive surgery
Building on the backbone of conservative treatment, a minimally invasive approach using the local application of leukocyte and platelet-rich plasma (LPRF) membranes to achieve primary wound closure has shown promising results in treating MRONJ. A Belgian cohort study using this approach showed healing of the MRONJ lesion in 64–67% of patients with Stage 1 or 2 diseases [Citation45]. However, the value of LPRF remains challenging to assess, given the lack of randomized clinical trials. Further clinical research is therefore needed to confirm the therapeutic signal detected in these preliminary studies [Citation46].
Primary surgical resection
Expanding insights into the role of infection and selecting appropriate surgical techniques have resulted in improved outcomes using this approach. In particular, it was recognized that adequate antibiotic treatment should precede the surgical procedure to eliminate indolent infection to the maximum extent possible before any invasive local treatment is performed [Citation47].
Marginal resection of the MRONJ lesion is performed by elevating a full-thickness mucoperiosteal flap which is extended to reveal the entire area of exposed bone and beyond to disease-free margins; resection of the affected bone to reach healthy-appearing, bleeding bone; and finally achieving primary soft tissue closure after smoothing of sharp edges [Citation43]. However, heterogeneous outcomes with surgery across all stages of MRONJ have been reported [Citation44].
Salvage surgical treatment
In advanced stages, uncontrolled symptoms, or disease progression despite the abovementioned treatments, segmental mandibulectomy and bony free flap reconstruction may be offered as a last-resort option. In particular, when the disease involves full-thickness mandibular destruction, pathologic fracture, and fistulization, with chronic pain and infection. A recent systematic review found that for MRONJ stage III patients, extensive bony resection up to the viable bleeding margins, with or without a microvascular flap reconstruction provided the best outcomes [Citation48]. Nevertheless, chronic infection and underlying medical comorbidities may predispose to substantial perioperative complications [Citation49].
Adjuvant treatments
Various adjuvant therapies have been investigated in MRONJ. These include hyperbaric oxygen therapy, low-level laser therapy, topical ozone, and teriparatide (parathyroid hormone) treatment. However, the evidence regarding their efficacy in MRONJ management remains limited or anecdotal, and further research is needed to establish their role and effectiveness.
Treatment selection
Despite the various available treatment modalities, the lack of randomized controlled comparisons between these treatments severely hamper interpreting the results and ultimately choosing the best and most appropriate treatment for patients with MRONJ [Citation43]. Nevertheless, according to ClinicalTrials.gov (accessed 01/08/2023), only four interventional trials comparing MRONJ treatments are currently active. Of these studies, the BETCON trial (BEst Treatment Choice of OsteoNecrosis of the jaw; ClinicalTrials.gov: NCT04512638; www.betcon.be) running in several Flemish hospitals is the only study comparing the available standard-of-care treatments for MRONJ in a randomized way to establish their effectiveness, both regarding mucosal healing and humanistic outcomes.
Treatment response assessment
The change in the AAOMS stage or mucosal healing is commonly used to report treatment outcomes. However, more granular response assessment criteria may be required to capture clinical outcomes, including radiographic changes. The MASCC/ISOO/ASCO expert panel has proposed a 4-level response system incorporating clinical signs, symptoms, and radiographic changes (). Nevertheless, patients with healed MRONJ lesions can still suffer from significant sequellae due to tissue loss or problems with dentition.
Table 7. Proposed response assessment criteria for treatment of MRONJ.
Conclusion
MRONJ poses significant challenges in clinical practice, and knowledge gaps hamper its diagnosis, prevention, and treatment. Concerning diagnosis and staging, we propose reconsidering the mandatory diagnostic 8-week window, resolving the uncertainty regarding the conflicting interpretation of stage 0 MRONJ, harmonizing the definition of non-exposed MRONJ, and incorporating advanced imaging tools. In addition, the prevention of MRONJ could benefit from standardized risk stratification protocols, risk-based preventive dental screening protocols, the identification of subgroups that may benefit from drug holidays, and policies to safeguard access to dental care. Improvements in MRONJ management require defining individual treatment goals, treatment selection guidelines considering patient characteristics, selecting relevant treatment response criteria, and identifying meaningful trial endpoints. Lastly, further prospective randomized comparative trials in MRONJ are urgently needed to generate high-quality evidence to support these goals.
Disclosure statement
This research is funded by a grant from Kom op tegen Kanker (Stand up to Cancer), the Flemish Cancer Society, awarded to the Antwerp University Hospital (2018/11482/1). The authors declare no other conflicts of interest.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Coleman R, Hadji P, Body JJ, et al. Bone health in cancer: ESMO clinical practice guidelines. Ann Oncol. 2020;31(12):1650–1663. doi: 10.1016/j.annonc.2020.07.019.
- Kanis JA, Cooper C, Rizzoli R, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30(1):3–44. doi: 10.1007/s00198-018-4704-5.
- Yarom N, Yahalom R, Shoshani Y, et al. Osteonecrosis of the jaw induced by orally administered bisphosphonates: incidence, clinical features, predisposing factors and treatment outcome. Osteoporos Int. 2007;18(10):1363–1370. doi: 10.1007/s00198-007-0384-2.
- Stopeck AT, Fizazi K, Body JJ, et al. Safety of long-term denosumab therapy: results from the open label extension phase of two phase 3 studies in patients with metastatic breast and prostate cancer. Support Care Cancer. 2016;24(1):447–455. doi: 10.1007/s00520-015-2904-5.
- Raje N, Terpos E, Willenbacher W, et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: an international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018;19(3):370–381. doi: 10.1016/S1470-2045(18)30072-X.
- Van den Wyngaert T, Delforge M, Doyen C, et al. Prospective observational study of treatment pattern, effectiveness and safety of zoledronic acid therapy beyond 24 months in patients with multiple myeloma or bone metastases from solid tumors. Support Care Cancer. 2013;21(12):3483–3490. doi: 10.1007/s00520-013-1934-0.
- van Cann T, Loyson T, Verbiest A, et al. Incidence of medication-related osteonecrosis of the jaw in patients treated with both bone resorption inhibitors and vascular endothelial growth factor receptor tyrosine kinase inhibitors. Support Care Cancer. 2018;26(3):869–878. doi: 10.1007/s00520-017-3903-5.
- Loyson T, Van Cann T, Schoffski P, et al. Incidence of osteonecrosis of the jaw in patients with bone metastases treated sequentially with bisphosphonates and denosumab. Acta Clin Belg. 2018;73(2):100–109. doi: 10.1080/17843286.2017.1348001.
- Bone HG, Wagman RB, Brandi ML, et al. 10 Years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5(7):513–523. doi: 10.1016/S2213-8587(17)30138-9.
- Everts-Graber J, Lehmann D, Burkard JP, et al. Risk of osteonecrosis of the jaw under denosumab compared to bisphosphonates in patients with osteoporosis. J Bone Miner Res. 2022;37(2):340–348. doi: 10.1002/jbmr.4472.
- Drudge-Coates L, Van den Wyngaert T, Schiodt M, et al. Preventing, identifying, and managing medication-related osteonecrosis of the jaw: a practical guide for nurses and other allied healthcare professionals. Support Care Cancer. 2020;28(9):4019–4029. doi: 10.1007/s00520-020-05440-x.
- Coropciuc R, Coopman R, Garip M, et al. Risk of medication-related osteonecrosis of the jaw after dental extractions in patients receiving antiresorptive agents - A retrospective study of 240 patients. Bone. 2023;170:116722. doi: 10.1016/j.bone.2023.116722.
- Brown JE, Wood SL, Confavreux C, et al. Management of bone metastasis and cancer treatment-induced bone loss during the COVID-19 pandemic: an international perspective and recommendations. J Bone Oncol. 2021;29:100375. doi: 10.1016/j.jbo.2021.100375.
- Migliorati CA, Brennan MT, Peterson DE. Medication-related osteonecrosis of the jaws. J Natl Cancer Inst Monogr. 2019;2019(53):lgz009. doi: 10.1093/jncimonographs/lgz009.
- Kim JW, Landayan ME, Lee JY, et al. Role of microcracks in the pathogenesis of bisphosphonate-related osteonecrosis of the jaw. Clin Oral Investig. 2016;20(8):2251–2258. doi: 10.1007/s00784-016-1718-2.
- Bolette A, Lecloux G, Rompen E, et al. Influence of induced infection in medication-related osteonecrosis of the jaw development after tooth extraction: a study in rats. J Craniomaxillofac Surg. 2019;47(2):349–356. doi: 10.1016/j.jcms.2018.08.011.
- Guarneri V, Miles D, Robert N, et al. Bevacizumab and osteonecrosis of the jaw: incidence and association with bisphosphonate therapy in three large prospective trials in advanced breast cancer. Breast Cancer Res Treat. 2010;122(1):181–188. doi: 10.1007/s10549-010-0866-3.
- Al-Mahalawy H, El-Mahallawy Y, Dessoky NY, et al. Post-COVID-19 related osteonecrosis of the jaw (PC-RONJ): an alarming morbidity in COVID-19 surviving patients. BMC Infect Dis. 2022;22(1):544. doi: 10.1186/s12879-022-07518-9.
- Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws, American Association of Oral and Maxillofacial Surgeons. American association of oral and maxillofacial surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2007;65(3):369–376.
- Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for bone and mineral research. J Bone Miner Res. 2007;22(10):1479–1491. doi: 10.1359/jbmr.0707onj.
- Khan AA, Sándor GKB, Dore E, et al. Bisphosphonate associated osteonecrosis of the jaw. J Rheumatol. 2009;36(3):478–490. doi: 10.3899/jrheum.080759.
- Arce K, Assael LA, Weissman JL, et al. Imaging findings in bisphosphonate-related osteonecrosis of jaws. J Oral Maxillofac Surg. 2009;67(5):75–84. doi: 10.1016/j.joms.2008.12.002.
- Ruggiero SL, Dodson TB, Assael LA, et al. Position paper on bisphosphonate-related osteonecrosis of the jaws–2009 update. J Oral Maxillofac Surg. 2009;67(5):2–12. doi: 10.1016/j.joms.2009.01.009.
- Khan AA, Morrison A, Hanley DA, et al. Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Miner Res. 2015;30(1):3–23. doi: 10.1002/jbmr.2405.
- Ruggiero SL, Dodson TB, Aghaloo T, et al. American Association of oral and maxillofacial surgeons’ position paper on Medication-related osteonecrosis of the jaws-2022 update. J Oral Maxillofac Surg. 2022;80(5):920–943. doi: 10.1016/j.joms.2022.02.008.
- Ruggiero SL, Dodson TB, Fantasia J, et al. Position paper on medication-related osteonecrosis of the jaw–2014 update. J Oral Maxillofac Surg. 2014;72(10):1938–1956. doi: 10.1016/j.joms.2014.04.031.
- Weitzman R, Sauter N, Eriksen EF, et al. Critical review: updated recommendations for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in cancer patients–may 2006. Crit Rev Oncol Hematol. 2007;62(2):148–152. doi: 10.1016/j.critrevonc.2006.12.005.
- Yarom N, Shapiro CL, Peterson DE, et al. Medication-Related osteonecrosis of the jaw: MASCC/ISOO/ASCO clinical practice guideline. J Clin Oncol. 2019;37(25):2270–2290. doi: 10.1200/JCO.19.01186.
- Junquera L, Gallego L. Nonexposed bisphosphonate-related osteonecrosis of the jaws: another clinical variant? J Oral Maxillofac Surg. 2008;66(7):1516–1517. doi: 10.1016/j.joms.2008.02.012.
- Fedele S, Bedogni G, Scoletta M, et al. Up to a quarter of patients with osteonecrosis of the jaw associated with antiresorptive agents remain undiagnosed. Br J Oral Maxillofac Surg. 2015;53(1):13–17. doi: 10.1016/j.bjoms.2014.09.001.
- Bagan JV, Jimenez Y, Diaz JM, et al. Osteonecrosis of the jaws in intravenous bisphosphonate use: proposal for a modification of the clinical classification. Oral Oncol. 2009;45(7):645–646. doi: 10.1016/j.oraloncology.2008.05.011.
- Woo SB, Mawardi H, Treister N. Comments on "osteonecrosis of the jaws in intravenous bisphosphonate use: proposal for a modification of the clinical classification. Oral Oncol. 2009;45(8):740. doi: 10.1016/j.oraloncology.2008.10.001.
- Khan AA, Morrison A, Kendler DL, et al. Case-Based review of osteonecrosis of the jaw (ONJ) and application of the international recommendations for management from the international task force on ONJ. J Clin Densitom. 2017;20(1):8–24. doi: 10.1016/j.jocd.2016.09.005.
- Gaêta-Araujo H, Vanderhaeghen O, Vasconcelos KdF, et al. Osteomyelitis, osteoradionecrosis, or medication-related osteonecrosis of the jaws? Can CBCT enhance radiographic diagnosis? Oral Dis. 2021;27(2):312–319. doi: 10.1111/odi.13534.
- Bedogni A, Fusco V, Agrillo A, et al. Learning from experience. Proposal of a refined definition and staging system for bisphosphonate-related osteonecrosis of the jaw (BRONJ). Oral Dis. 2012;18(6):621–623. doi: 10.1111/j.1601-0825.2012.01903.x.
- Nicolatou-Galitis O, Schiodt M, Mendes RA, et al. Medication-related osteonecrosis of the jaw: definition and best practice for prevention, diagnosis, and treatment. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;127(2):117–135. doi: 10.1016/j.oooo.2018.09.008.
- Karna H, Gonzalez J, Radia HS, et al. Risk-reductive dental strategies for medication related osteonecrosis of the jaw among cancer patients: a systematic review with meta-analyses. Oral Oncol. 2018;85:15–23. doi: 10.1016/j.oraloncology.2018.08.003.
- Otto S, Pautke C, Arens D, et al. A drug holiday reduces the frequency and severity of medication-related osteonecrosis of the jaw in a minipig model. J Bone Miner Res. 2020;35(11):2179–2192. doi: 10.1002/jbmr.4119.
- Aboalela AA, Farook FF, Alqahtani AS, et al. The effect of antiresorptive drug holidays on medication-related osteonecrosis of the jaw: a systematic review and Meta-Analysis. Cureus. 2022;14(10):e30485. doi: 10.7759/cureus.30485.
- Cosman F, Huang S, McDermott M, et al. Multiple vertebral fractures after denosumab discontinuation: FREEDOM and FREEDOM extension trials additional post hoc analyses. J Bone Miner Res. 2022;37(11):2112–2120. doi: 10.1002/jbmr.4705.
- Marx RE, Cillo JE, Jr., Ulloa JJ. Oral bisphosphonate-induced osteonecrosis: risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J Oral Maxillofac Surg. 2007;65(12):2397–2410. doi: 10.1016/j.joms.2007.08.003.
- Winkelmann J, Gómez Rossi J, van Ginneken E. Oral health care in Europe: financing, access and provision. Health Syst Transit. 2022;24(2):1–176.
- Otto S, Pautke C, Van den Wyngaert T, et al. Medication-related osteonecrosis of the jaw: prevention, diagnosis and management in patients with cancer and bone metastases. Cancer Treat Rev. 2018;69:177–187. doi: 10.1016/j.ctrv.2018.06.007.
- Ramaglia L, Guida A, Iorio-Siciliano V, et al. Stage-specific therapeutic strategies of medication-related osteonecrosis of the jaws: a systematic review and meta-analysis of the drug suspension protocol. Clin Oral Investig. 2018;22(2):597–615. doi: 10.1007/s00784-017-2325-6.
- Coropciuc RG, Grisar K, Aerden T, et al. Medication-related osteonecrosis of the jaw in oncological patients with skeletal metastases: conservative treatment is effective up to stage 2. Br J Oral Maxillofac Surg. 2017;55(8):787–792. doi: 10.1016/j.bjoms.2017.06.014.
- Lopez-Jornet P, Sanchez Perez A, Amaral Mendes R, et al. Medication-related osteonecrosis of the jaw: is autologous platelet concentrate application effective for prevention and treatment? A systematic review. J Craniomaxillofac Surg. 2016;44(8):1067–1072. doi: 10.1016/j.jcms.2016.05.004.
- Hoefert S, Yuan A, Munz A, et al. Clinical course and therapeutic outcomes of operatively and non-operatively managed patients with denosumab-related osteonecrosis of the jaw (DRONJ). J Craniomaxillofac Surg. 2017;45(4):570–578. doi: 10.1016/j.jcms.2017.01.013.
- Vanpoecke J, Verstraete L, Smeets M, et al. Medication-related osteonecrosis of the jaw (MRONJ) stage III: conservative and conservative surgical approaches versus an aggressive surgical intervention: a systematic review. J Craniomaxillofac Surg. 2020;48(4):435–443. doi: 10.1016/j.jcms.2020.02.017.
- Hanasono MM, Militsakh ON, Richmon JD, et al. Mandibulectomy and free flap reconstruction for bisphosphonate-related osteonecrosis of the jaws. JAMA Otolaryngol Head Neck Surg. 2013;139(11):1135–1142. doi: 10.1001/jamaoto.2013.4474.