Abstract
Background: The objective of the study was to assess the effect of prophylactic antibiotics on the outcome of bone augmentation and subsequent dental implant placement by combining the recommended quality assessment methods for systematic reviews and primary studies.
Materials and methods: This is a complex systematic review in which systematic reviews as well as primary studies are scrutinised. A search of Medline (OVID), The Cochrane Library (Wiley) and EMBASE, PubMed and Health technology assessment (HTA) organisations as-well as a complementary hand-search was carried out. Selected primary studies were assessed using GRADE. Each study was reviewed by three authors independently.
Results: Abstract screening yielded six potential systematic reviews allocated for full-text inspection. A total of ten primary studies were read in full-text. No relevant systematic reviews regarding the topic of this article were found. The quality assessment resulted in two primary studies with a moderate risk of bias. Of the two studies with a moderate risk of bias, one compared a single dose of clindamycin 600 mg preoperatively with the same preoperative dose followed by four doses of 300 mg every 6 h. The second study compared a single dose prophylaxis of two different types of antibiotic compounds.
Conclusion: In conclusion, the scientific evidence regarding the use of antibiotic prophylaxis for reducing the risk of infection in conjunction with bone augmentation procedures during dental implant placement is very limited. The infection rate as compared to nonusage of prophylactic antibiotics, selection of the most suitable compound, and the optimal duration of prophylactic treatment is still unknown.
Introduction
Antibiotic resistance is considered the largest threat to modern health care as many treatment options are dependent on effective antibiotics [Citation1]. Some parts of the world have entered the postantibiotic era where the efficacy of antibiotics can no longer be safely predicted [Citation2]. An important measure to fight this development is restrictive antibiotic utilisation [Citation3]. The stated correlation between increasing rates of antibiotic resistance and antibiotic consumption is unchallenged and well supported [Citation4–7]. The suggestion that short-term antibiotic treatments would pose a reduced risk for antibiotic resistance is not well established [Citation8]. Also a single dose of amoxicillin can select for resistant strains in the oral cavity [Citation9]. For each indication and patient, the potential risk with antibiotic prescription must be weighed against the putative benefits. The antibiotics prescribed in dentistry sum up to approximately 5–10% of the total usage in health care according to reports from Europe and the USA thus contributing to a substantial part of the consumption [Citation10–14].
Insertion of dental implants is a commonly used therapeutic option for replacement of missing teeth and displays excellent success rates [Citation15–24]. When the residual bone volume is insufficient it is common to perform bone augmentation procedures prior to, or in conjunction with, implant placement [Citation25–27].
Considering the large amount of patients subjected to this therapy, the antibiotic used during these procedures may significantly contribute to the overall usage especially if a prophylaxis regimen beyond the day of surgery is prescribed. However, the scientific evidence to support this routine is unclear. A literature review regarding antibiotic prophylaxis in surgery included over 600 references but could not find support for antibiotic prescription beyond the day of surgery for prevention of postoperative infections in any of the studied surgical fields [Citation28]. In the light of this, it is reasonable to question whether prolonging the antibiotics beyond the day of surgery during bone augmentation procedures is a motivated procedure.
The number of published systematic reviews has increased significantly in recent years [Citation29,Citation30]. This provides a valuable mean to synthesise current knowledge within a particular field. However, a poorly performed systematic review may be misleading giving the incorrect impression of sound conclusions. It is therefore of great importance that systematic reviews are performed according to high standards and subjected to independent quality control similar to the assessment of original research [Citation31,Citation32]. AMSTAR is a validated and reliable tool that is increasingly being used for evaluation of systematic reviews [Citation33–35]. It has been suggested that when reviewing literature pre-existing reviews should, in concert with the primary study, be incorporated into a complex systematic review [Citation36]. A strict predefined PICO (population, intervention, control and outcome/observation) is mandatory in this process, as well as quality assessment by independent reviewers, resulting in strict inclusion only of systematic reviews of high quality.
The aim of the current study was to assess the available scientific literature regarding the efficacy of prophylactic antibiotics at bone augmentation procedures and subsequent dental implant placement. Including both staged bone augmentation and bone augmentation with simultaneous implant placement.
Materials and methods
Objective
The objective of the study was to assess the effect of antibiotics on frequency of postoperative infections after bone augmentation in conjunction with dental implant placement, both staged bone augmentation and bone augmentation with simultaneous implant placement.
Criteria for considering studies
The predefined study population, intervention, the comparison of therapies, and outcome parameters for the eligible studies are summarised in . Additionally, inclusion and exclusion criteria for systematic reviews and primary studies are presented in .
Table 1. Parameters of interest regarding eligible studies including inclusion and exclusion criteria for both systematic reviews and primary studies.
Search strategies
Two of the authors (AK and ANA) and two information specialists (from Karolinska Institutet University library) performed the literature search. The following databases were searched until October 26, 2015: Medline (OVID), The Cochrane Library (Wiley) and EMBASE (embase.com), PubMed (nonindexed articles). No limitation regarding start year were used. The search was initially performed without any filters for the search of primary studies and then repeated once more with a filter for systematic reviews.
For the detection of recent publications, a complementary search was undertaken in PubMed on August 27, 2018. The additional search did not use any filters and all new findings from both primary studies as well as systematic reviews were screened.
The search terms used for the databases are summarised in . Search terms used were; e.g. alveolar ridge augmentation, alveolar bone grafting, dental implantation, implant-supported, sinus floor augmentation, sinus floor augmentation, bone graft augmentation, dental implants, antibiotic prophylaxis ().
Table 2. Search strategy.
Health technology assessment (HTA) organisations were searched regarding the effect of antibiotics versus no treatment or placebo on the outcome of bone augmentation in conjunction with dental implant installation until October 30 2015: National Institute for Health and Care Excellence (NICE), http://www.nice.org.uk/; CADTH, http://www.cadth.ca/; CRD database, http://www.crd.york.ac.uk/CRDWeb/; Kunnskapssenteret, http://www.kunnskapssenteret.no/home?language=english, and ASERNIP-S http://www.surgeons.org/for-health-professionals/audits-and-surgical-research/asernip-s/publications/. The reference lists of all the eligible studies were hand-searched for potential complementary studies. The search was not restricted by language. However, retrieved papers in a language other than English, German, French, or Swedish were excluded.
Study selection
Eligible studies were included in accordance with the inclusion/exclusion criteria. AK (first author) went over the retrieved list of publications and performed a crude exclusion of irrelevant publications based on their title. In case of uncertainty, a study remained included until the next selection step, which consisted of an examination of abstracts. The abstracts were read independently in duplicate by three reviewers, either BL, AK and ST or MH, ANA and BK. Selected primary studies and systematic reviews were read in full-text by three reviewers each, respectively. Any disagreement during the screening process was resolved by discussion in the project group.
Quality assessment
Systematic reviews
No systematic reviews were left for quality assessment, due to out of topic reason. Had there been any systematic reviews to review the quality of the studies would have been assessed according to Mejare 2015 [Citation37], based on AMSTAR assessment items [Citation33].
Primary studies
The quality of the included primary studies was assessed using a protocol for assessment of randomised studies [Citation38]. The quality assessment protocol focus on the risk of bias in individual studies and specific outcomes as well as the overall quality of evidence.
Quality of evidence
The scientific quality of the evidence in the primary studies was graded according to GRADE (GRADing quality of Evidence and strength of recommendations) and set as high, moderate, low, and very low [Citation39] (). GRADE have 4 steps of evidence grading. The system was developed by the GRADE working group. GRADE is used by e.g. World Health Organization (WHO), NICE, Cochrane Collaboration and British Medical Journal (BMJ).
Table 3. Significance of the four levels of evidence.
Data extraction
Systematic reviews
No systematic reviews remained for data extraction due to out of topic reason. Data extraction from the systematic reviews would have been the following: objectives, main results, authors’ estimated level of evidence, and knowledge gaps according to authors.
Primary studies
Data was extracted from the primary studies regarding number of included patients, age, gender distribution, length of follow-up, type of intervention, type of control treatment, and relevant treatment outcomes.
Results
Literature search and study selection
The search resulted in 1155 titles after deduplication. The results include both primary studies and systematic reviews (n = 150). Search strategy, presented for each database, is shown in . The search of HTA organisations did not yield any further studies. Flow-charts of the screening process for primary studies and systematic reviews are described in and , respectively.
Figure 1. Flow chart, primary studies. From Moher et al. [Citation31].
![Figure 1. Flow chart, primary studies. From Moher et al. [Citation31].](/cms/asset/daeaf832-d7eb-46ca-92a6-11bbc13defa0/iode_a_1656819_f0001_c.jpg)
Figure 2. Flow chart, systematic reviews. From Moher et al. [Citation31].
![Figure 2. Flow chart, systematic reviews. From Moher et al. [Citation31].](/cms/asset/92fde8af-d3f3-4367-8e01-1c051db2de3c/iode_a_1656819_f0002_c.jpg)
The additional search on August 27, 2018 added 372 new articles: all were screened for both systematic reviews and primary studies.
Systematic reviews
Abstract screening yielded six potential systematic reviews allocated for full-text inspection. The most common reason for exclusion was that the study was out of topic (n = 4) (Out of topic means that the study was not within our PICO, e.g. if the article did not mention bone augmentation in association with implants and antibiotics).The second most common reason for exclusion was that the study was not considered a systematic review (n = 2) [Citation40–45] ().
Table 4. Excluded systematic reviews.
Primary studies
A total of ten primary studies were read in full-text. At this stage another six studies were excluded, yielding four primary studies included for further analysis. Primary studies that were regarded as nonrelevant to the current systematic review were excluded at this stage and reason for exclusion were recorded () [Citation46–51]. Reason for exclusion could be e.g. language, not a randomized controlled trial (RCT), or a research question that was not correct.
Table 5. Excluded primary studies read in full text.
Quality assessment and data extraction
Systematic reviews
No systematic reviews were left for quality assessment and data extraction due to out of topic reason.
Primary studies
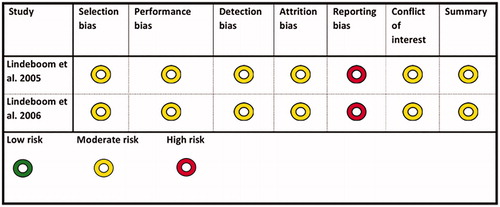
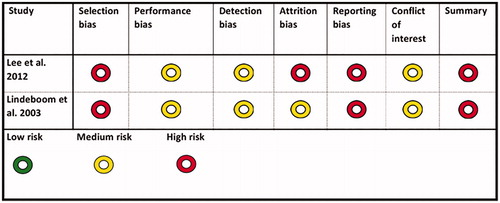
The quality assessment resulted in two studies with a moderate risk of bias, [Citation52,Citation53], and two studies with a high risk of bias [Citation54,Citation55]. The main reason for the two studies being regarded as having a high risk of bias was shortcomings and uncertainties in the randomisation process. The first of the two studies with a moderate risk of bias, Lindeboom et al. [Citation52], compared a single dose of clindamycin 600 mg preoperatively with the same preoperative dose followed by four doses of 300 mg every 6 h.
The second study, Lindeboom et al. [Citation53], compared a single dose prophylaxis of two different types of antibiotic compounds (). Type of intervention and study population characteristics for studies classified as being of low or moderate risk of bias are also shown in . Outcome of primary studies classified as being of low or moderate risk of bias are shown in . In the first study, Lindeboom et al. [Citation52], 2 of 62 patients in the intervention group (600 mg clindamycin 1 h preop.) developed a postoperative infection. In the control group (clindamycin 24 h) 3 of 62 patients developed a postoperative infection at receptor site. In the second study, Lindeboom et al. [Citation53], 4 of 75 patients in the intervention group (2 g penicillin 1 h preop.) developed a postoperative infection. In the control group (600 mg clindamycin 1 h preop.) 2 of 75 patients developed a postoperative infection at receptor site (). Due to few events, statistical analysis was not applicable. The two studies with a high risk of bias are presented in .
Table 6. Characteristics and quality assessment of included primary studies with low or moderate risk of bias.
Table 7. Outcome of included primary studies with low or moderate risk of bias.
Publication bias and heterogeneity
Not applicable due to too few studies available to make a meta-analysis.
Discussion
The results of the present systematic review illustrates that there is a lack of existing scientific evidence on antibiotic prophylaxis for oral bone augmentation procedures in the alveolar ridge and subsequent dental implant placement.
No data were available on implant placement with simultaneous bone augmentation. Since this procedure is frequently used in implant dentistry, the lack of data for this indication is observable and in need of further research on the subject.
No data was available on any of the other parameters in our PICO, in the included studies.
The study by Lindeboom et al. [Citation52] compared the risk of developing a postsurgical infection at the bone graft receptor site between patients given a single dose of antibiotic prophylaxis and patients receiving a more extended 24-h dose of clindamycin. Although the study showed that there was a difference in risk, albeit not a statistically significant one, the number of postsurgical infections was low in both groups. Therefore the outcome of a statistical comparison between the two groups may be hampered by insufficient power. In addition, Lindeboom et al. [Citation53] found no significant difference in the occurrence of postsurgical recipient site infection when comparing two different antibiotic compounds, penicillin and clindamycin used as a single dose of antibiotic prophylaxis. The result of both the included studies indicates that the wound infection rate when using a single dose of prophylactic antibiotics was low.
There is very limited available evidence on antibiotic prophylaxis on staged bone augmentation with intraoral donor sites and the complete lack of evidence on staged bone augmentation with extra-oral donor bone and implant placement.
Due to the limited number of studies eligible for inclusion in the present systematic review, we were not able to draw a definite conclusion regarding whether prolonged antibiotic prophylaxis is needed to reduce the risk of postoperative infection during bone grafting procedures or if a single dose is equally sufficient. Consequently, there is a gap in our knowledge concerning the efficacy of antibiotic prophylaxis, regardless of length, compared to no use of antibiotics for prevention of potential postoperative infections after bone grafting procedures. Only two primary studies fulfilling the inclusion criteria were considered to be of moderate risk of bias [Citation52,Citation53]. The other eligible primary studies (n = 2) were rejected due to a high risk of bias [Citation54,Citation55]. The main reasons for exclusion due to a high risk of bias were an unclear randomisation process and allocation concealment, insufficiently described blinding, and unclear outcome measures.
The core of the systematic review will always be the quality of the included primary studies. Therefore, it is of great importance that RCTs should be designed, performed, and reported in accordance with internationally recognised standards such as the CONSORT statement, in order for accurate conclusions to be drawn [Citation56]. Since the quality of reviews differs significantly, one needs to approach the assessment of systematic reviews in the same way as the current study has approached it [Citation36].
While there are many published systematic reviews on the use of antibiotics in dental implant surgery, we were not able to find any that fulfilled our inclusion criteria. For example, this was the case with a systematic review initially allocated, evaluating survival and success rates of implants placed into fresh extraction sockets, since data of prophylactic antibiotics given in bone grafting procedures was not separately compared [Citation43].
In addition, RCTs on the topic of antibiotics and bone augmentation procedures are lacking. Generally, large numbers of systematic reviews are seen in fields where primary studies are sparse and the results contradictory. This repeated effort to extract and summarise data is probably due to the desire to synthesise solid evidence despite divergent results and/or underpowered primary studies. The phenomenon as such indicates a further need for high-quality research. The results of the present study thus serve to emphasise the fact that one needs to be vigilant about drawing conclusions from such published systematic reviews.
Traditionally, antibiotic prophylaxis has been administered either pre-, peri-, or post-operative to prevent infection developing at the surgical wound site. A placebo-controlled double blind pilot study comparing the prophylactic use of phenethicillin with a placebo in buccal bone-grafting procedures found a high infection rate (40%) at the receptor site in the placebo group. This indicates that antibiotic prophylaxis maybe beneficial in these procedures [Citation53]. These results, however, should be interpreted with caution since the number of patients included in this pilot study was small. It is not known specifically how antibiotics are used in everyday practice. Empiric prophylactic and antibiotic usage varies by country, region, choice of antibiotic, and the duration of the prophylaxis. This is not unexpected considering the weak scientific evidence. Thus, while there is considerable disagreement as to the type and length of the antibiotic prophylaxis, the most common misuse of prophylactic antibiotics is believed to be excessive duration [Citation57].
The WHO suggests that unnecessary use of antibiotics for infections is one of the main etiological factors behind antibiotic resistance [Citation58]. Even a single dose of antibiotic prophylaxis has been shown to induce a selection of resistant strains in the oral microflora [Citation9], illustrating that any dosage and its consequences should be carefully considered. Antibiotic-resistant bacteria have become a major public health crisis and a threat to both global stability and national security [Citation58]. At the same time, it is not possible to develop and manufacture new antibiotics to satisfy the increasing demand, resulting in an irreversible phenomenon that is difficult to manage [Citation59]. The present unnecessary use of common antibiotics may result in both expensive future antibiotics and an increase in bacterial resistance. Therefore, the need for recommendations to limit and optimise the utilisation of antibiotics are needed.
Limitations
The restriction in English language might potentially have resulted in missed relevant studies, which is a limitation in this review.
There were no signs indicating publication bias in the present review, yet there is a possibility that small negative studies might not have been published.
Conclusion
In conclusion, the scientific evidence regarding the use of antibiotic prophylaxis for reducing the risk of infection at bone augmentation procedures and subsequent dental implant placement is very limited. Infection rate has been shown to be low using a single dose of prophylactic antibiotics. However, the infection rate in comparison to nonusage of prophylactic antibiotics, the compound, and the duration of prophylactic treatment is still not known. Therefore, there is an urgent need for further primary RCTs.
Acknowledgments
The authors would like to acknowledge Carl Gornitzki and Gun Brit Knutssön at the Karolinska Institutet library for their skilful assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Davies SC, Fowler T, Watson J. Annual report of the Chief Medical Officer: infection and the rise of antimicrobial resistance. Lancet. 2013;381:1606–1609.
- Alanis AJ. Resistance to antibiotics: are we in the post-antibiotic era? Arch Med Res. 2005;36:697–705.
- Tomson G, Vlad I. The need to look at antibiotic resistance from a health system perspective. Ups J Med Sci. 2014;119:117–124.
- Hughes VM, Datta N. Conjugative plasmids in bacteria of the 'pre-antibiotic' era. Nature. 1983;302:725–726.
- Bronzwaer SL, Cars O, Buchholz U, et al. A European study on the relationship between antimicrobial use and antimicrobial resistance. Emerg Infect Dis. 2002;8:278–282.
- Livermore DM. Minimising antibiotic resistance. Lancet Infect Dis. 2005;5:450–459.
- Foucault C, Brouqui P. How to fight antimicrobial resistance. FEMS Immunol Med Microbiol. 2007;49:173–183.
- Guillemot D, Carbon C, Balkau B, et al. Low dosage and long treatment duration of beta-lactam: risk factors for carriage of penicillin-resistant Streptococcus pneumoniae. JAMA. 1998;279:365–370.
- Khalil D, Hultin M, Rashid M, et al. Oral microflora and selection of resistance after a single dose of amoxicillin. Clin Microbiol Infect. 2016;22:949.e1–949.e4.
- Hicks LA, Bartoces MG, Roberts RM, et al. US Outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60:1308–1316.
- Holyfield G, Karki A. Review of prescribing by dentists in Wales. NHS Wales. 2009. Available from: www.1000livesplus.wales.nhs.uk/opendoc/179908
- Norm-Norm-Vet. Usage of antimicrobial agents and occurrence of antimicrobial resistance in Norway. 2015 [cited 2018 Mar 15]. Available from https://unn.no/Documents/Kompetansetjenester,%20-sentre%20og%20fagr%C3%A5d/NORM%20-%20Norsk%20overv%C3%A5kingssystem%20for%20antibiotikaresistens%20hos%20mikrober/Rapporter/NORM_NORM-VET-2015.pdf.
- Pipalova R, Vlcek J, Slezak R. The trends in antibiotic use by general dental practitioners in the Czech Republic (2006–2012). Int Dent J. 2014;64:138–143.
- Swedres-Swarm. Consumption of antibiotics and occurrence of resistance in Sweden. Public Health Agency of Sweden and National Veterinary Institute; 2016 [cited 2018 Mar 15]. Available from https://www.folkhalsomyndigheten.se/contentassets/d118ac95c12d4c11b3e61d34ee6d2332/swedres-svarm-2016-16124.pdf.
- Albrektsson T, Dahl E, Enbom L, et al. Osseointegrated oral implants. A Swedish multicenter study of 8139 consecutively inserted Nobelpharma implants. J Periodontol. 1988;59:287–296.
- Lekholm U, Gunne J, Henry P, et al. Survival of the Brånemark implant in partially edentulous jaws: a 10-year prospective multicenter study. Int J Oral Maxillofac Implants. 1999;14:639–645.
- Eklund JA, Lindquist LW, Carlsson GE, et al. Implant treatment in the edentulous mandible: a prospective study on Branemark system implants over more than 20 years. Int J Prosthodont. 2003;16:602–608.
- Pjetursson BE, Tan K, Lang NP, et al. A systematic review of the survival and complication rates of fixed partial dentures (FPDs) after an observation period of at least 5 years. Clin Oral Implants Res. 2004;15:667–676.
- Jemt T, Johansson J. Implant treatment in the edentulous maxillae: a 15-year follow-up study on 76 consecutive patients provided with fixed prostheses. Clin Implant Dent Relat Res. 2006;8:61–69.
- Lekholm U, Grondahl K, Jemt T. Outcome of oral implant treatment in partially edentulous jaws followed 20 years in clinical function. Clin Implant Dent Relat Res. 2006;8:178–186.
- Roos-Jansåker AM, Lindahl C, Renvert H, et al. Nine- to fourteen-year follow-up of implant treatment. Part I: implant loss and associations to various factors. J Clin Periodontol. 2006;33:283–289.
- Roos-Jansaker AM, Lindahl C, Renvert H, et al. Nine- to fourteen-year follow-up of implant treatment. Part II: presence of peri-implant lesions. J Clin Periodontol. 2006;33:290–295.
- Astrand P, Ahlqvist J, Gunne J, et al. Implant treatment of patients with edentulous jaws: a 20-year follow-up. Clin Implant Dent Relat Res. 2008;10:207–217.
- Kim DM, Badovinac RL, Lorenz RL, et al. A 10-year prospective clinical and radiographic study of one-stage dental implants. Clin Oral Implants Res. 2008;19:254–258.
- Milinkovic I, Cordaro L. Are there specific indications for the different alveolar bone augmentation procedures for implant placement? A systematic review. Int J Oral Maxillofac Surg. 2014;43:606–625.
- Storgård Jensen S, Terheyden H. Bone augmentation procedures in localized defects in the alveolar ridge: clinical results with different bone grafts and bone-substitute materials. Int J Oral Maxillofac Implants. 2009;24:218–236.
- Chiapasco M, Casentini P, Zaniboni M. Bone augmentation procedures in implant dentistry. Int J Maxillofac Implants. 2009;24:237–259.
- SBU. Antibiotic prophylaxis for surgical procedures. In Swedish: Antibiotikaprofylax vid kirurgiska ingrepp. En systematisk litteraturöversikt. Stockholm: Statens beredning för medicinsk utvärdering (SBU); 2010. SBU-rapport nr 200. (ISBN 978-91-85413-36-2).
- Straus S, Moher D. Registering systematic reviews. CMAJ. 2010;182:13–14.
- Booth A, Clarke M, Ghersi D, et al. An international registry of systematic-review protocols. Lancet. 2011;377:108–109.
- Moher D, Liberati A, Tetzlaff J, PRISMA Group, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;339:b2535.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100.
- Shea BJ, Bouter LM, Peterson J, et al. External validation of a measurement tool to assess systematic reviews (AMSTAR). PLoS One. 2007;2:e1350.
- Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10.
- Shea BJ, Hamel C, Wells GA, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62:1013–1020.
- Whitlock EP, Lin JS, Chou R, et al. Using existing systematic reviews in complex systematic reviews. Ann Intern Med. 2008;148:776–782.
- Mejàre I, Klingberg G, Mowafi F, et al. A systematic map of systematic reviews in pediatric dentistry: what do we really know? PLoS One. 2015;10:e0117537.
- Guyatt G, Oxman A, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence – study limitations (risk of bias). J Clin Epidemiol. 2011;4:407–415.
- Balshem H, Helfanda M, Schunemann H, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;4:401–406.
- Chen ST, Wilson TG, Jr., Hammerle CH. Immediate or early placement of implants following tooth extraction: review of biologic basis, clinical procedures, and outcomes. Int J Oral Maxillofac Implants. 2004;19:12–25.
- Chrcanovic BR, Albrektsson T, Wennerberg A. Prophylactic antibiotic regimen and dental implant failure: a meta-analysis. J Oral Rehabil. 2014;41:941–956.
- Esposito M, Grusovin MG, Felice P, et al. The efficacy of horizontal and vertical bone augmentation procedures for dental implants – a Cochrane systematic review. Eur J Oral Implantol. 2009;2:167–184.
- Lang NP, Pun L, Lau KY, et al. A systematic review on survival and success rates of implants placed immediately into fresh extraction sockets after at least 1 year. Clin Oral Implant Res. 2012;23:39–66.
- Sharaf B, Dodson TB. Does the use of prophylactic antibiotics decrease implant failure? Oral Maxillofac Surg Clin North Am. 2011;23:547–550.
- Waasdorp JA, Evian CI, Mandracchia M. Immediate placement of implants into infected sites: a systematic review of the literature. J Periodontol. 2010;81:801–808.
- Brennan MT, Sasser HC, Fox PC, et al. Statistical analysis used in a prospective placebo-controlled double-blind trial of antibiotic prophylaxis in intraoral bonegrafting procedures: a pilot study. Oral Surg Med Oral Pathol. 2003;96:669–672.
- Chuvilkin VI, Chuvilkina EI, Tsarev VN, et al. Preventive antibacterial treatment in oral bone augmentation procedures. Stomatologiia (Mosk). 2013;92:84–87.
- Cohen BM. Antibiotics and intraoral bone grafts. J Oral Surg (Chic). 1955;13:34–43.
- Eickholz P, Röllke L, Schacher B, et al. Enamel matrix derivative in propyleneglycol alginate for treatment of Infrabony defects with or without systemic doxycycline: 12- and 24-month results. J Periodontol. 2014;85:669–675.
- Kulshrestha S, Khan S, Meena R, et al. A graphene/zinc oxide nanocomposite film protects dental implant surfaces against cariogenic Streptococcus mutans. Biofouling. 2014;30:1281–1294.
- Ngeow WC. Antibiotics for surgical prophylaxis. Aust Prescr. 2005;28:113–115.
- Lindeboom JA, Tuk JG, Kroon FH, et al. A randomized prospective controlled trial of antibiotic prophylaxis in intraoral bone grafting procedures: single-dose clindamycin versus 24-hour clindamycin prophylaxis. Mund Kiefer Gesichts Chir. 2005;9:384–388.
- Lindeboom JA, Frenken JW, Tuk JG, et al. A randomized prospective controlled trial of antibiotic prophylaxis in intraoral bone-grafting procedures: preoperative single-dose penicillin versus preoperative single-dose clindamycin. Int J Oral Maxillofac Surg. 2006;35:433–436.
- Lindeboom JA, Van den Akker HP. A prospective placebo-controlled double-blind trial of antibiotic prophylaxis in intraoral bone grafting procedures: a pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:669–672.
- Lee JW, Lee JY, Kim SM, et al. Prophylactic antibiotics in intra-oral bone grafting procedures: a prospective, randomized, double-blind clinical trial. J Kor Assoc Oral Maxillofac Surg. 2012;38:90–95.
- Schulz KF, Altman DG, Moher D. Consort 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:1.
- Khalil D, Hultin M, Andersson Fred L, et al. Antibiotic prescription patterns among Swedish dentists working with dental implant surgery: adherence to recommendations. Clin Oral Implant Res. 2015;26:1064–1069.
- World Health Organization (WHO). Global strategy for containment of antimicrobial resistance – 2015. Geneva, Switzerland: World Health Organization (WHO) [cited 2017 Mar 15]. Available at: http://www.who.int/drugresistance/who_global_strategy_english.pdf.
- Bencharit S. Bacterial resistance to antibiotics and implanted medical device. Adv Pharmacoepidemiol Drug Saf. 2012;1:5.