Abstract
Objective: Odontoblasts are thought to be involved in innate immunity but their precise role in this process is not fully understood. Here, we assess effects of lipopolysaccharide (LPS) and lipoteichoic acid (LTA), produced by Gram-negative and Gram-positive bacteria, respectively, on matrix metalloproteinase-8 (MMP-8), interleukin-6 (IL-6) and cathelin-related antimicrobial peptide (CRAMP) expression in odontoblast-like MDPC-23 cells.
Material and methods: Gene activity and protein production was determined by quantitative real-time RT-PCR and ELISA, respectively. Cellular expression of CRAMP was determined by immunocytochemistry.
Results: Stimulation with LTA (5 and 25 µg/ml) but not LPS (1 and 5 µg/ml) for 24 h enhanced IL-6 mRNA expression. The LTA-induced up-regulation of IL-6 mRNA levels was associated with increased IL-6 protein levels. Stimulation with either LPS or LTA for 24 h lacked effect on both MMP-8 transcript and protein expression. Immunocytochemistry disclosed that MDPC-23 cells expressed immunoreactivity for CRAMP. MDPC-23 cells showed mRNA expression for CRAMP, but stimulation with either LPS or LTA did not modulate CRAMP transcript expression.
Conclusions: We show that MDPC-23 cells possess immune-like cell properties such as LTA-induced IL-6 production and expression of the antimicrobial peptide CRAMP, suggesting that odontoblasts may modulate innate immunity via these mechanisms.
Introduction
Dental caries is the most prevalent non-communicable disease in the world, affecting billions of people around the world and leading to a poorer quality of life [Citation1]. Untreated, the carious lesions will progress into dentine, thus affecting the dental pulp. Odontoblasts are lining the outer layer of the pulp cavity forming the interface between pulp tissue and dentine. They produce type I collagen-rich predentin, and are thought to be involved in the reparative process of dentine [Citation2].
Odontoblasts are exposed to oral bacteria and bacterial products when these have access to the dentine, suggesting that the odontoblasts also take part in the first line of defence against bacteria. Human odontoblast-like cells have been shown to express Toll-like receptors (TLRs), and, furthermore, they seem to respond to stimulation with the Gram-positive bacterial cell wall constituent lipoteichoic acid (LTA) by increased chemokine expression and reduced dentine formation [Citation3]. Moreover, mature human odontoblasts are reported to express virus-recognizing TLRs [Citation4]. Thus, odontoblasts seem to play a role in the innate immune system through these mechanisms but their precise role is not well understood. In inflammation, chemokines enhance recruitment of white blood cells to the inflamed area, whereas the pleiotropic pro-inflammatory cytokine interleukin-6 (IL-6) contributes to synthesis of acute phase proteins by hepatocytes, formation of neutrophils by the bone marrow and growth of B cells [Citation5]. Interestingly, IL-6 is thought to play an important role in the pathogenesis of periodontitis, and it is also supposed to be associated with vascular leakage and oedema formation in the inflamed pulp tissue [Citation6–8]. The human antimicrobial peptide LL-37 and its mouse ortholog CRAMP (cathelin-related antimicrobial peptide) exert antibacterial activity and protect against invading bacteria mainly through permeabilization of the bacterial cell wall and subsequent cell lysis [Citation9–12]. LL-37 shows high expression in neutrophils but also in epithelial cells such as keratinocytes [Citation13]. LL-37 has been detected in human saliva, and interestingly, neutrophils in human salivary glands show high expression of LL-37 [Citation14–18]. Odontoblasts may fulfil an important role in the first line of defence against bacterial invasion. Hence, it is of great importance to assess if odontoblasts can play a role in innate immunity by expression and production of pro-inflammatory IL-6 and the antimicrobial peptide CRAMP/LL-37.
Human odontoblasts have been shown to express the collagen cleaving endopeptidase matrix metalloproteinase-8 (MMP-8), also named human neutrophil collagenase [Citation19], but no results assessing the impact of bacterial endotoxins on odontoblast MMP-8 expression have been presented. It has also been demonstrated that subjects with manifest caries lesions have significantly elevated levels of MMP-8 in the saliva compared to caries-free subjects [Citation20]. Indeed, MMP-8 has been suggested to play a role in the pathogenesis of caries [Citation21]. Whether MMP-8 expression is an active process as a response to external stimulation by bacterial products, or if it is secreted into the predentin and then sequestered as the tissue is demineralized is currently not clear.
The spontaneously immortalized MDPC-23 odontoblast-like cell line obtained from mouse pulp tissue has been widely used as a model to investigate regulation of morphological and functional properties of odontoblasts [Citation22]. The MDPC-23 cells are epithelioid in shape, show expression of dentine-specific proteins and have high alkaline phosphatase activity [Citation22]. Thus, MDPC-23 cells display both structural and functional properties similar to those of primary human odontoblasts. Here, we assess effects of the bacterial endotoxin lipopolysaccharide (LPS) from Gram-negative bacteria and LTA from Gram-positive bacteria on MDPC-23 cell MMP-8, IL-6 and CRAMP expression. We demonstrate, on both mRNA and protein level, that LTA, but not LPS, enhances IL-6 production, suggesting that odontoblasts, via this mechanism, contribute to the inflammation-process observed in the pulp-tissue in response to stimulation with LTA from Gram-positive bacteria. Furthermore, we show that MDPC-23 cells express CRAMP, indicating that odontoblasts are equipped with a protective system against bacteria.
Materials and methods
Culture of odontoblast-like MDPC-23 cells
The MDPC-23 cells were kindly provided by Dr. Jacques E. Nör, University of Michigan, Ann Arbour. Cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM)/Ham’s F12 (1:1) (Biochrom GmbH, Berlin, Germany) with stable glutamine and supplemented with antibiotics (penicillin 50 U/ml and streptomycin 50 µg/ml, Biochrom GmbH) and 10% foetal bovine serum (Biochrom GmbH). The cells were cultured at 37 °C in a cell/tissue incubator (SANYO MCO-18AIC, Osaka, Japan) and trypsinized (0.25% trypsin/EDTA) and re-seeded when cells reached confluence. Cells were counted using the Luna™ Automated Cell Counter (Logos Biosystem, Annandale, VA, USA) and re-seeded at a density of 60,000 cells/ml. Cell morphology was assessed using an Olympus CKX41 microscope (Olympus Europa, Hamburg, Germany). Culture medium was exchanged every second day to new and fresh medium. Cells were used for experiments in passages 2 to 20, and they showed identical morphology and functional characteristics independent of passage number. Before experiments, the old culture medium was removed and instead fresh and pre-warmed medium was included. LPS (Escherichia coli 0111:B4) and LTA (Bacillus subtilis) were purchased from Sigma-Aldrich (St Louis, MO, USA) and dissolved in PBS. We used 1 and 5 µg/ml of LPS and 5 and 25 µg/ml of LTA throughout the experiments. These concentrations of Escherichia coli LPS and Bacillus subtilis LTA are in a range where they cause profound stimulation of cytokine and chemokine production in human periodontal ligament cells and human odontoblasts, respectively [Citation3,Citation23]. Controls received PBS as vehicle as appropriate.
Measurement of gene activity by quantitative real-time RT-PCR
Total RNA was extracted using a miRNeasy kit (Qiagen, Venlo, The Netherlands) according to manufacturer’s instructions. RNA concentration and quality was determined using the NanoDrop 2000 C spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Target mRNA expression was assessed using a Step One Plus real-time thermal cycler (Applied Biosystems, Waltham, MA, USA) and QuantiFast SYBR Green RT-PCR kit (Qiagen) and QuantiTect primer assays (Qiagen). Target gene expression was calculated using the delta CT method with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) included as reference gene [Citation24]. Each sample was analyzed in duplicate. Primers for MMP-8 (Mm_Mmp8_1_SG), IL-6 (Mm_Il6_1_SG), CRAMP (Mm_Camp_1_SG) and GAPDH (Mm_Gapdh_3_SG) were purchased from Qiagen.
Determination of protein production by enzyme-linked immunosorbent assay (ELISA)
Cells were scraped off in ice-cold PBS from the culture wells using cell scrapers (Sarstedt, Newton, NC, USA) and sonicated (2 × 10 s) on ice. The cell-homogenates were centrifuged (1700 g at 4 °C for 5 min) and the supernatants collected. MMP-8 and IL-6 protein production was determined in the cell supernatants using ELISA kits (MMP-8 and IL-6 kits purchased from LifeSpan BioSciences, Seattle, WA, USA and Nordic BioSite, Täby, Sweden, respectively). Determination of MMP-8 and IL-6 concentration was performed as recommended by the manufacturers. Each sample was analyzed in duplicate, and the concentrations of MMP-8 and IL-6 normalized to total protein concentration in each sample determined by a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, USA).
Assessment of CRAMP expression by immunocytochemistry
For immunocytochemistry, MDPC-23 cells were grown on glass coverslips. The cells were washed in PBS, fixed in 4% paraformaldehyde and then permeabilized for 10 min with 0.2% triton X-100. The non-specific binding sites were blocked by incubation with 2% bovine serum albumin before incubation for 2 h with a mouse monoclonal antibody raised against amino acids 6-175 of CRAMP of rat origin and used at a dilution of 1:50 (Santa Cruz Biotechnology, Dallas, TX, USA). After incubation with the primary CRAMP antibody, cells were washed with PBS and then incubated for 1 h with an anti-mouse IgG conjugated with Alexa Fluor 488 (Thermo Fisher Scientific). The coverslips were mounted on glass slides using mounting medium containing the nuclear marker DAPI (Fluoroshield, Sigma Aldrich) The CRAMP immunoreactive signal and DAPI staining were analyzed and depicted using an Olympus BX60 fluorescence microscope (Olympus) coupled to a DP72 digital camera (Olympus). For negative controls, the primary antibody was omitted.
Statistics
Summarised data are presented as means ± S.E.M. Each culture well represents one biological replicate (n = 1), and n-values are presented in the figure legends. Each type of experiment was repeated at least twice. Statistical significance was calculated by ANOVA followed by Tukey’s test for post hoc analysis for multiple comparisons as appropriate (GraphPad Prism5, GraphPad Software, San Diego, CA, USA). p-values <.05 were regarded statistically significant.
Results
LTA enhances pro-inflammatory IL-6 expression in odontoblast-like MDPC-23 cells
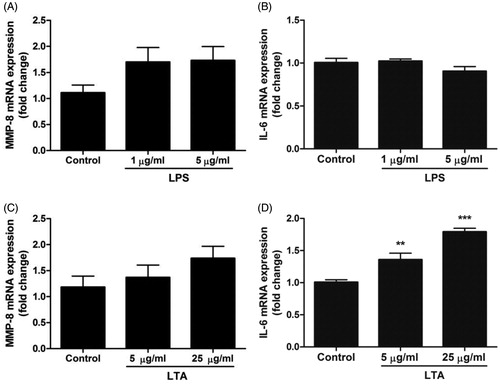
In order to characterize the structure of the odontoblast-like MDPC-23 cells used in this project, we examined their morphology using phase-contrast microscopy. depicts sub-confluent cells in passage 8. The cells grew in clusters and showed an epithelioid appearance with columnar cells as reported previously [Citation22,Citation25]. Cell morphology was not affected by treatment for 24 h with either LPS (1 and 5 µg/ml) or LTA (5 and 25 µg/ml) (data not shown). Next, we determined the effects of LPS on MMP-8 and IL-6 mRNA expression. Stimulation with LPS (1 and 5 µg/ml) for 24 h had no effect on either MMP-8 or IL-6 transcript expression (). Treatment with LTA (5 and 25 µg/ml) for 24 h showed a different transcript expression pattern compared to LPS. Stimulation with LTA (5 and 25 µg/ml) increased IL-6 mRNA by 35 and 80%, respectively (), but had no effect on MMP-8 transcript expression ().
Figure 1. Phase-contrast microscopy of odontoblast-like MDPC-23 cells. Phase-contrast microscopy image demonstrating sub-confluent MDPC-23 cells. The cells grow in clusters and show a columnar cell-shape. Phase-contrast microscopy was performed using an Olympus CKX41 microscope (Olympus). Bar represents 50 µm.
Figure 2. Treatment with LTA enhances IL-6 mRNA expression in MDPC-23 cells. (A-D) Cells were stimulated with either LPS (1 and 5 µg/ml) or LTA (5 and 25 µg/ml) for 24 h and mRNA expression for MMP-8 (A, C) and IL-6 (B, D) determined by quantitative real-time RT-PCR. Values are presented as means ± S.E.M. of 8–12 observations in each group. ** and *** represent p < .01 and p < .001, respectively, compared to control.
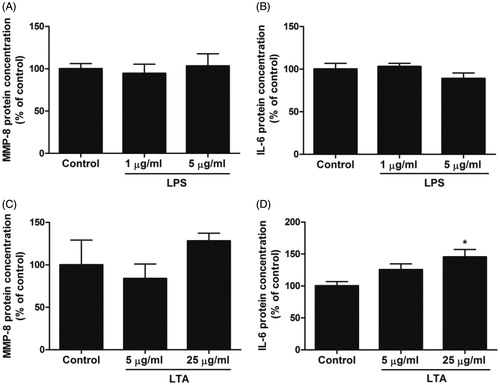
In the next experiments, we analyzed the effects of LPS and LTA on MMP-8 and IL-6 protein production using ELISA in order to confirm the mRNA data. Stimulation with LPS (1 and 5 µg/ml) for 24 h had no effect on either MMP-8 or IL-6 protein concentration in MDPC-23 cell supernatants (). Treatment with LTA (25 µg/ml) for 24 h increased IL-6 protein concentration by 45%, while a lower concentration of LTA (5 µg/ml) tended to enhance IL-6 protein production although this difference did not reach statistical significance (. Treatment with LTA (5 and 25 µg/ml) for 24 h had no effect on MMP-8 protein concentration (. Thus, we demonstrate, on both transcript and protein level, that LTA enhances IL-6 expression in MDPC-23 cells.
Figure 3. Stimulation with LTA increases MDPC-23 cell IL-6 protein production. (A-D) Cells were stimulated with either LPS (1 and 5 µg/ml) or LTA (5 and 25 µg/ml) for 24 h and MMP-8 (A, C) and IL-6 (B, D) protein concentration (ng/ml) was analyzed in cell supernatants by ELISA. The concentrations of MMP-8 and IL-6 protein was normalized to total protein concentration in each sample and expressed as % of control. Values are presented as means ± S.E.M. of 3–10 observations in each group. * represents p < .05 compared to control.
MDPC-23 cells express the antimicrobial peptide CRAMP
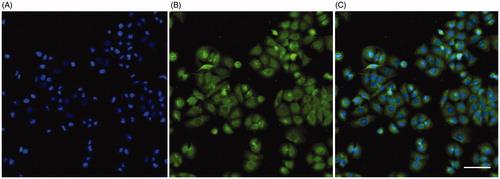
In order to further assess the role of MDPC-23 cells in innate immunity, we investigated their expression of the antimicrobial peptide CRAMP by immunocytochemistry. CRAMP immunoreactivity was observed in the cytoplasm of all cells (). The perinuclear region of the cytoplasm was especially rich in CRAMP immunoreactive signal. Cell nuclei are visualized by the nuclear marker DAPI, and the combined DAPI staining and fluorescence for CRAMP immunoreactivity is shown in the overlay (). The nuclei expressed no or very weak immunoreactivity for CRAMP. No CRAMP-immunoreactivity was observed after omission of the primary CRAMP antibody (data not shown).
Figure 4. MDPC-23 cells express cytoplasmic immunoreactivity for CRAMP. Cellular immunoreactivity for CRAMP was assessed by immunocytochemistry. (A) Staining with the nuclear marker DAPI (blue). (B) The same cells stained for CRAMP immunoreactivity (green). (C) Overlay of DAPI staining and CRAMP fluorescence. The DAPI signal and CRAMP fluorescence was analyzed using an Olympus BX60 fluorescence microscope (Olympus). The bar in panel C represents 50 µm and can be applied for all three panels.
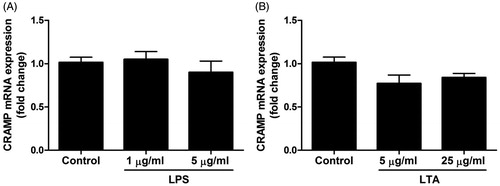
In the next experiments, we assessed effects of stimulation with LPS (1 and 5 µg/ml) and LTA (5 and 25 µg/ml) for 24 h on CRAMP mRNA expression (). MDPC-23 cells expressed mRNA for CRAMP, but neither LPS nor LTA modulated the CRAMP transcript expression (). We also investigated if a short-term (4 h) stimulation with a high concentration of LPS (5 µg/ml) and LTA (25 µg/ml), respectively, could affect the mRNA expression for CRAMP. Treatment with either 5 µg/ml LPS or 25 µg/ml LTA for 4 h had no effect on CRAMP mRNA expression (100 ± 6% in control cells vs. 114 ± 3% in LPS-stimulated cells and 100 ± 6% in control cells vs. 80 ± 10% in LTA-stimulated cells, n = 4 in each group). Thus, we demonstrate that stimulation with LPS and LTA for both 4 and 24 h has no effect on CRAMP transcript expression.
Figure 5. Treatment with LPS and LTA has no effect on CRAMP mRNA expression in MDPC-23 cells. (A, B) Cells were stimulated with 1 and 5 µg/ml LPS (A) or 5 and 25 µg/ml LTA (B) for 24 h and mRNA expression for CRAMP was determined by quantitative real-time RT-PCR. Values are presented as means ± S.E.M. of 8 observations in each group.
Discussion
In the present study, we demonstrate, both on mRNA and protein level, that LTA of Gram-positive bacteria, but not LPS from Gram-negative bacteria, triggers production of pro-inflammatory IL-6 in the odontoblast-like cell line MDPC-23. Furthermore, we show that MDPC-23 cells express the antimicrobial peptide CRAMP corresponding to human LL-37. Thus, our data suggest that MDPC-23 odontoblasts show immune-like cell properties and may modulate innate immunity through these different mechanisms. LTA, but not LPS, has previously been shown to stimulate chemokine expression in in vitro-differentiated human odontoblasts [Citation3] and furthermore promote expression of pro-angiogenic VEGF in MDPC-23 cells [Citation26], suggesting that Gram-positive bacteria via LTA secretion may stimulate recruitment of white blood cells to the inflamed area of the pulp tissue and moreover enhance formation of blood vessels. Interestingly, CD14 has been reported to be responsible for LPS-evoked signalling in macrophages raising the possibility that MDPC-23 cells lack CD14 expression, explaining why the MDPC-23 cells are not responsive to LPS. This explanation is however not likely, since Botero et al. have convincingly demonstrated that MDPC-23 cells express CD14 [Citation27]. The mechanism behind LTA-induced stimulation of chemokine expression in human odontoblasts probably involves up-regulation of its own TLR2 receptor as reported by Durand et al. [Citation3]. Hence, stimulation with LTA seems to enhance IL-6 cytokine production, as demonstrated in the present study, but also stimulate production of both chemokines and VEGF, indicating that LTA acts pro-inflammatory on odontoblasts through multiple mechanisms. It is widely recognized that MDPC-23 cells show similar structural and functional properties as human odontoblasts [Citation22], arguing that the data reported in the present study indeed reflect the human in vivo situation.
Besides enhancing chemokine production as shown by Durand et al. [Citation3], LTA may also modulate other aspects of odontoblast innate immune responses, e.g. by stimulating IL-6 formation as demonstrated in the present study. IL-6 is a pro-inflammatory cytokine affecting the innate immune system in many ways, e.g. by promoting synthesis of acute phase proteins by hepatocytes, enhancing neutrophil formation by bone marrow and stimulating growth of B cells [Citation5]. Previously, Farges et al. [Citation8] have reported that TLR2 agonists such as LTA and Pam2CSK4 enhance production of pro-inflammatory IL-6 and CXCL8 and anti-inflammatory IL-10 in human odontoblast-like cells, indicating that activation of odontoblast TLR2 induces both pro- and anti-inflammatory responses. Here, we demonstrate that LTA triggers IL-6 production, but neither LPS nor LTA regulates MMP-8 gene activity and protein expression in MDPC-23 cells. Thus, although odontoblasts express MMP-8, previously shown both on the mRNA and the protein levels [Citation19], the MMP-8 gene activity and protein production does not seem to be regulated by bacterial LPS and LTA. This may indicate that MMP-8 is produced by odontoblasts through a constitutive pathway or that other stimuli are required to up-regulate MMP-8 in odontoblasts. Interestingly, TGF-β1 has been demonstrated to down-regulate MMP-8 expression in human odontoblasts, suggesting that TGF-β1 signalling in fact represents a regulatory mechanism for MMP-8 in these cells [Citation19]. The results also lend support to the theory that MMP-8 is present in the intact dentine matrix and activated as a consequence of the caries process, rather than being produced as a direct response to bacterial challenge [Citation28]. In the present study, we used LTA from Bacillus subtilis and LPS from Escherichia coli. Although the structure of LTA as well as LPS may differ somewhat between different strains of bacteria they show similar structural and functional characteristics independent of their bacterial strain origin [Citation29,Citation30], implying that the present data are relevant for oral in vivo conditions.
Here, we show for the first time that odontoblast-like MDPC-23 cells express the important antimicrobial peptide CRAMP. Stimulation with either LPS or LTA had no effect on CRAMP gene activity, suggesting that CRAMP is constitutively produced by MDPC-23 cells. CRAMP and its human ortholog LL-37 are highly expressed by white blood cells and human epithelial cells such as skin keratinocytes [Citation9,Citation13]. The MDPC-23 cells show epithelioid appearance and columnar cell shape resembling keratinocytes and our data show that they express CRAMP/LL-37. Thus, in this perspective the MDPC-23 cells also share functional characteristics with keratinocytes of the skin. Interestingly, CRAMP expression has been reported in rat odontoblasts during the late bell stage of tooth development, whereas no expression of CRAMP was observed in mature teeth [Citation31]. Moreover, these authors show that odontoblast-like cells in contact with reparative dentine express CRAMP. Taken together, these findings indicate that CRAMP can be associated with dentine formation [Citation31]. Immunoreactivity for human antimicrobial beta-defensins has previously been demonstrated in the cytoplasm of odontoblasts [Citation32]. Hence, it seems that odontoblasts express both CRAMP/LL-37 and defensins, representing the two major groups of antimicrobial peptides in humans, suggesting that odontoblasts indeed play a role in innate immunity through their antimicrobial properties.
In conclusion, we demonstrate that MDPC-23 odontoblast-like cells show immune-like cell properties such as LTA-induced IL-6 production and expression of the antimicrobial peptide CRAMP, suggesting that odontoblasts can modulate the innate immune system via these mechanisms.
Acknowledgements
The authors wish to thank Katarzyna Kawka for excellent technical assistance. The study was conceived and designed by A.H-L. and BO.N.; K.O., F.F., S.D. and A.A. conducted the experiments. K.O., BO.N., D.E. and A.H-L. analyzed the data and wrote the paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Kassebaum NJ, Bernabé E, Dahiya M, et al. Global burden of untreated caries: a systematic review and metaregression. J Dent Res. 2015;94(5):650–658.
- Bleicher F. Odontoblast physiology. Exp Cell Res. 2014;325(2):65–71.
- Durand SH, Flacher V, Roméas A, et al. Lipoteichoic acid increases TLR and functional chemokine expression while reducing dentin formation in in vitro differentiated human odontoblasts. J Immunol. 2006;176(5):2880–2887.
- Pääkkönen V, Rusanen P, Hagström J, et al. Mature human odontoblasts express virus-recognizing toll-like receptors. Int Endod J. 2014;47(10):934–941.
- Saggini A, Chimenti S, Chiricozzi A. IL-6 as a druggable target in psoriasis: focus on pustular variants. J Immunol Res. 2014;2014:1.
- Okada H, Murakami S. Cytokine expression in periodontal health and disease. Crit Rev Oral Biol Med. 1998;9(3):248–266.
- Seymour GJ, Gemmell E. Cytokines in periodontal disease: where to from here?. Acta Odontol Scand. 2001;59(3):167–173.
- Farges JC, Carrouel F, Keller JF, et al. Cytokine production by human odontoblast-like cells upon Toll-like receptor-2 engagement. Immunobiology. 2011;216(4):513–517.
- Gallo RL, Kim KJ, Bernfield M, et al. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J Biol Chem. 1997;272(20):13088–13093.
- Burton MF, Steel PG. The chemistry and biology of LL-37. Nat Prod Rep. 2009;26(12):1572–1584.
- Cederlund A, Gudmundsson GH, Agerberth B. Antimicrobial peptides important in innate immunity. FEBS J. 2011;278(20):3942–3951.
- Hancock RE, Haney EF, Gill EE. The immunology of host defence peptides: beyond antimicrobial activity. Nat Rev Immunol. 2016;16(5):321–334.
- Zanetti M. The role of cathelicidins in the innate host defenses of mammals. Curr Issues Mol Biol. 2005;7(2):179–196.
- Murakami M, Ohtake T, Dorschner RA, et al. Cathelicidin antimicrobial peptides are expressed in salivary glands and saliva. J Dent Res. 2002;81(12):845–850.
- Tao R, Jurevic RJ, Coulton KK, et al. Salivary antimicrobial peptide expression and dental caries experience in children. Antimicrob Agents Chemother. 2005;49(9):3883–3888.
- Davidopoulou S, Diza E, Sakellari D, et al. Salivary concentration of free LL-37 in edentulism, chronic periodontitis and healthy periodontium. Arch Oral Biol. 2013;58(8):930–934.
- Colombo NH, Ribas LF, Pereira JA, et al. Antimicrobial peptides in saliva of children with severe early childhood caries. Arch Oral Biol. 2016;69:40–46.
- Svensson D, Aidoukovitch A, Anders E, et al. The host defense peptide LL-37 is detected in human parotid and submandibular/sublingual saliva and expressed in glandular neutrophils. Eur J Oral Sci. 2018;126(2):93–100.
- Palosaari H, Wahlgren J, Larmas M, et al. The expression of MMP-8 in human odontoblasts and dental pulp cells is down-regulated by TGF-beta1. J Dent Res. 2000;79(1):77–84.
- Hedenbjörk-Lager A, Bjørndal L, Gustafsson A, et al. Caries correlates strongly with salivary levels of matrix metalloproteinase-8. Caries Res. 2015;49(1):1–8.
- Tjäderhane L, Larjava H, Sorsa T, et al. The activation and function of host matrix metalloproteinases in dentin matrix breakdown in caries lesions. J Dent Res. 1998;77(8):1622–1629.
- Hanks CT, Fang D, Sun Z, et al. Dentin-specific proteins in MDPC-23 cell line. Eur J Oral Sci. 1998;106(S1):260–266.
- Jönsson D, Nebel D, Bratthall G, et al. LPS-induced MCP-1 and IL-6 production is not reversed by oestrogen in human periodontal ligament cells. Arch Oral Biol. 2008;53(9):896–902.
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45.
- Arana-Chavez VE, Massa LF. Odontoblasts: the cells forming and maintaining dentine. Int J Biochem Cell Biol. 2004;36(8):1367–1373.
- Telles PD, Hanks CT, Machado MA, et al. Lipoteichoic acid up-regulates VEGF expression in macrophages and pulp cells. J Dent Res. 2003;82(6):466–470.
- Botero TM, Shelburne CE, Holland GR, et al. TLR4 mediates LPS-induced VEGF expression in odontoblasts. J Endod. 2006;32(10):951–955.
- Tjäderhane L, Buzalaf MA, Carrilho M, et al. Matrix metalloproteinases and other matrix proteinases in relation to cariology: the era of ‘dentin degradomics’. Caries Res. 2015;49:193–208.
- Percy MG, Gründling A. Lipoteichoic acid synthesis and function in gram-positive bacteria. Annu Rev Microbiol. 2014;68(1):81–100.
- Yamaji Y, Kubota T, Sasaguri K, et al. Inflammatory cytokine gene expression in human periodontal ligament fibroblasts stimulated with bacterial lipopolysaccharides. Infect Immun. 1995;63(9):3576–3581.
- Horibe K, Hosoya A, Hiraga T, et al. Expression and localization of CRAMP in rat tooth germ and during reparative dentin formation. Clin Oral Invest. 2018;22(7):2559–2566.
- Dommisch H, Winter J, Acil Y, et al. Human beta-defensin (hBD-1, -2) expression in dental pulp. Oral Microbiol Immunol. 2005;20(3):163–166.
