Abstract
Objective: The aim was to study prevalence of xerostomia, hyposalivation and quality of life among caries active younger adults.
Materials and methods: A questionnaire regarding oral and general health, xerostomia and quality of life was mailed to 134 caries active (CA) and 40 caries inactive (CI) patients, 25–50 years of age (mean age 39.9 ± 6.2 years) treated at a Swedish Public Dental Service clinic, regarding oral and general health, xerostomia and quality of life. Caries data and unstimulated whole salivary flow rates were obtained from dental records.
Results: The overall response rate was 69%. Dental records confirmed that CA patients had more decayed teeth over time than CI patients (p < .001). The CA group reported worse oral health (p < .001) and general health (p < .01), more xerostomia (p < .001) and lower salivary flow rate (p < .01) compared to CI patients. Xerostomia was inversely related to unstimulated whole salivary flow rates as well as to oral and general health (p < .01). There were no differences between groups in quality of life.
Conclusion: Younger caries active adult patients reported significantly more xerostomia and hyposalivation compared to caries inactive patients. Xerostomia and hyposalivation were inversely related to perceptions of oral and general health, but not to quality of life.
Introduction
Untreated dental caries in the permanent dentition is the most prevalent disease worldwide, affecting 35% of the global population [Citation1]. In spite of this, longitudinal caries studies among adults are rare, but available data indicate that individuals with a caries active disease continue to be disease active for many years [Citation2–5]. Knowledge regarding how development of new caries lesions over time is perceived by this group of individuals in relation to oral health and quality of life is incomplete. In older people, periodontal disease, dental caries and xerostomia are increasing in prevalence, and have been found to be the most common factors with a negative impact on oral health and quality of life [Citation6–10], the most important risk factor for xerostomia in these age groups is medication use [Citation6,Citation9,Citation11–13].
Xerostomia, a subjective feeling of dry mouth, is an increasing condition in the elderly [Citation11,Citation12] and affects oral health negatively [Citation13,Citation14]. According to the systematic review by Orellana et al. [Citation14], only a few studies have been conducted in younger groups. However, xerostomia is a common condition that can affect quality of life among people of all ages [Citation15], including younger adults [Citation16], and individuals with Sjögren’s syndrome [Citation17], HIV [Citation18], diabetes mellitus [Citation19] and Parkinson’s disease [Citation20]. One has to distinguish between ‘xerostomia’ and ‘hyposalivation’, an objective measure of low salivary flow rates [Citation11,Citation21–23]. These two conditions may not be equivalent in one and the same patient. It has for example been shown that only half of those with very low unstimulated flow rates experienced xerostomia [Citation11]. Evaluating xerostomia by questionnaires may be easier and more convenient than measuring salivary flow rates in the clinic, but the latter might be more relevant when relating the amount of saliva to caries [Citation24,Citation25] as hyposalivation increases the risk for caries [Citation25]. Thus, questionnaires and measurements of salivary flow may complement each other. To the best of our knowledge, there are no studies among younger adults that have examined both xerostomia and hyposalivation in relation to caries and how these conditions affect oral health and quality of life.
The aim of this study was to compare xerostomia and hyposalivation in caries active and caries inactive younger adults, and to examine the relationship to self-reported oral and general health and quality of life.
Material and methods
Setting and participants
The study was performed at the Public Dental Clinic in the municipality of Sala, Sweden. A total of 134 caries active (CA) and 40 caries inactive (CI) individuals were recruited during 2007 and have been described in three earlier studies [Citation3,Citation26,Citation27]. All participants were 25–50 years of age, the mean age was 39.5 ± 6.2 and 41.0 ± 6.3 for the CA and CI groups, respectively.
Caries active (CA) and caries inactive (CI) group
The following definitions of the two groups were used: ‘CA group’ included individuals who had developed manifest primary or secondary caries lesions in 2 or more teeth in the last 3 years. ‘CI group’ was individuals who had been free from manifest caries for 3 years or more. Caries prevalence among the two groups was recorded retrospectively [Citation3], with significant differences between the CA and CI groups for all caries-related variables (p < .001). The CA group had a greater number of decayed teeth (DT), decayed, missing and filled tooth surfaces (DMFS), and a longer caries active time during the follow-up period. ‘Caries active time’ was defined as the time between two examinations where the patients showed development of manifest caries.
Questionnaire
A questionnaire with detailed information about the study was mailed to all 174 (134 + 40) individuals, with two reminders sent 3 and 6 weeks later to those who did not respond to the first invitation. The participants also returned a signed consent form. The questionnaire was developed for this study and has been described previously [Citation3,Citation26,Citation27]. It included items related to general health, diet, oral hygiene habits, sociodemographic variables and perception of caries active time [Citation3]. Questions regarding caries prophylaxis [Citation26] carried out at the clinic and at home were also included, as well as patient-reported problems and negative experiences related to caries and dental treatment [Citation27].
Self-reported oral health was measured by a single global rating question, ‘How do you rate your oral health?’ This was rated on a five-point scale from score 1 (‘very bad’) to 5 (‘very good’). This question and a similar one to rate general health have been used annually since 2004 by the public health agency of Sweden on a random sample of 20,000 individuals [Citation28], and these two questions have also been analysed for oral health [Citation29,Citation30] or for both oral health and general health [Citation31,Citation32], in several other Swedish studies.
The short-form Oral Health Impact Profile (OHIP-14) [Citation33,Citation34] was used to measure oral health-related quality of life (OHRQoL). The questionnaire encompasses seven domains: functional limitation, physical pain, physical disability, psychological discomfort, psychological disability, social disability and handicap. It contains 14 questions (2 per domain). Each question seeks to determine the frequency of impact of various oral disorders during the last year on a five-point scale from score 0 (never) to 4 (very often). In addition, respondents could reply that the question was not applicable.
Xerostomia was determined using the single item: ‘How often has your mouth felt dry?’, with response options ‘Never’ (scoring 1), ‘Hardly ever’ (2), ‘Occasionally’ (3), ‘Often’ (4) and ‘Very often’ (5).
Dental records
Dental records were reviewed retrospectively to the patient age of 20 years or as far back as possible. Theoretically, this would provide a minimum follow-up period of 5 years among the youngest participants. Information regarding caries prevalence and received caries prophylaxis was registered.
Salivary flow measurement
All participants had their unstimulated whole salivary flow rate measured at the dental clinic at least once; if more than once, the mean value was used. Unstimulated whole saliva (UWS) was collected for 15 min. This was carried out between 7.00 and 9.30 a.m. The subjects were instructed not to eat, drink or use any form of tobacco 2 h before the collection and to relax for a couple of minutes before the test. At the saliva collection, the subject sat bent forward in an ordinary chair and was told to place his/her tongue on the lingual surfaces of the upper incisors and to hold the mouth open and remain still, letting the saliva drip into a pre-weighed disposable cup held to the lower lip. The UWS flow rate was determined by gravitation, using a scale with an accuracy of 0.01 g (Sartorius, BP310P, Sartorius AG, Goettingen, Germany), presuming that 1 g of saliva is equivalent to 1 ml. All saliva collections were supervised by specially trained assistants with extensive experience with the procedures. Prevalence of hyposalivation was determined by analysing the number of individuals with very low UWS flow rates, defined as <0.1 ml per minute or low rates <0.2 ml/min. [Citation35,Citation36].
Ethics
The regional ethical committee at Uppsala University, Sweden, did not review the application (Dnr: 2006/310), as the current project does not encompass any physical or other intervention on research participants as defined in paragraph 4 of the Ethical Review Law. The application included permission to access and use dental records. All participants in the study returned a signed consent form that also included consent for their dental records to be accessed and used.
Statistical methods
Bivariate correlations between oral and general health, xerostomia and hyposalivation were analysed using Spearman’s rho. Differences between caries active and caries inactive patients were compared by t-tests for continuous variables and by chi-square test for categorical variables. All tests were two-sided and p-values less than .05 were considered significant. We intended to test the hypothesis that the caries active and inactive groups varied in dental treatment and patient experiences of oral and general health, oral health-related quality of life, and xerostomia via a series of two-tailed t-tests. In order to detect a medium effect size (Cohen’s d = 0.65), expected in patient-reported outcomes research of this type [Citation37], with a power of .80 and a p-value of .05, a sample size of 77 for the caries active group and 25 for the caries inactive group was required (total n = 102). Regarding patient dental records, calculation of sample size was based on data from a pilot sample, where the caries active group had received a mean number of 1.5 basic prophylaxis activities per year while the caries inactive group received 0.4 activities. To detect an expected difference of 1.1 mean number of basic prophylaxis activities per year with a power of 80% and a significance level of 5%, assuming a standard deviation of 1.5, it would require a sample size of 60 persons including 30% drop outs.
OHIP-14 scores were computed in two ways: (1) an overall OHIP-14 score was calculated by summing responses over all 14 items; (2) the severity of oral disorders was computed as the sum of individuals who reported experiencing negative impacts ‘Very often’ or ‘Fairly often’ [Citation16,Citation38]. Differences between groups in scores of oral and general health, OHIP– 14 scores, were analysed with t-tests. Non-parametric tests were also performed and the conclusions were identical.
One-way analysis of variance (ANOVA) with post hoc tests using Bonferroni was used to compare unstimulated whole saliva flow rates by participants’ ratings of xerostomia. All analyses were conducted using IBM SPSS version 24.0, Chicago, IL.
Results
A correlation matrix for all study variables is presented in . There were significant correlations (p < .01) between all four factors. Patient ratings of their general health (rho = 0.50) and unstimulated whole saliva flow (rho = 0.38) were positively correlated with perceived oral health, while xerostomia ratings were inversely correlated (−0.47, p < .01).
Table 1. Correlation matrix with means, standard deviations, and bivariate associations between oral health (1), general health (2), xerostomia (3) and unstimulated whole saliva (UWS) flow (4).
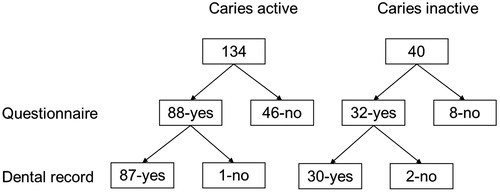
The overall response rate to the postal questionnaire was 69% (120/174). For the 120 patients who responded, complete dental records could be obtained for 87 out of the 88 in the CA group and 30 out of the 32 in the CI group, see flow chart .
Figure 1. Flow chart showing eligible individuals. Number receiving questionnaire, number of returned questionnaires and number of retrieved dental records.
Background characteristics of the caries active (CA) and caries inactive (CI) patients are compared in . The two groups did not differ significantly by gender, (p = .93), age (p = .27) or follow-up time (p = .22). At the end of the follow up period the CA patients had significantly higher cumulative disease activity measured as DMFS (p < .001). There was also a significantly greater increase in DMFS during the follow up period (p < .001). More details concerning caries prevalence among the two groups have been described previously [Citation3].
Table 2. Background characteristics of the caries active (CA) and caries inactive (CI) patients (for details, see references [Citation3,Citation26]).
The CA individuals had received more frequent information about caries and recommendations of caries prophylaxis than the CI individuals, and had also made more extra caries prophylaxis efforts at home. However, 60% of the CA individuals had not experienced that they had become free from caries (i.e. not needing fillings). This was confirmed by data from the dental records [Citation26].
Oral and general health
A comparison of all study outcomes between the CA and CI groups is summarised in .
Table 3. Oral and general health, OHIP 14 sum (OHIP: Oral Health Impact Profile), xerostomia and unstimulated whole saliva (UWS) flow rates of the caries active (CA) and caries inactive (CI) patients.
Self-reported oral health differed significantly between groups, with significantly lower mean scores among the CA patients (3.5 ± 0.7) compared to the CI group (4.2 ± 0.7, p < .001). Similarly, the CA group rated their general health significantly worse than the CI group (3.9 ± 0.8 and 4.4 ± 0.6, respectively, p = .002).
Ohip
There was no statistically significant difference between groups in mean total sum OHIP 14 scores (). However, the groups differed significantly in the functional limitation and physical pain domains, with the CA group reporting greater limitations and physical pain than the CI group (). The distribution of the OHIP score was skewed, with scores of 0 reported by 20% (n = 17) in the CA group versus 34% (n = 11) in the CI group (p = .14).
Table 4. Comparison of OHIP-14 scores, per dimensions and total sum for caries active (CA) vs. caries inactive (CI) patients.
Xerostomia
Among those experiencing xerostomia sometimes, fairly often or very often, 43 out of 44 were from the CA group. The CA group reported higher mean scores (2.6 ± 1.2) versus the CI group (1.3 ± 0.54, p < .001, ).
When dichotomising answers among those who experienced xerostomia very often or often, it occurred in 24% of the CA group, while none of the patients in the group CI group reported this (p = .002).
When comparing OHIP-14 among those 44 who experienced xerostomia (Sometimes, fairly often or very often) vs. those 94 who did not (seldom or never), there was a significant difference for the mean total sum of OHIP–14 between the groups, 4.88 (5.66) for the CA group vs. 7.59 (6.86) for the CI group, (p = .02), respectively.
Hyposalivation
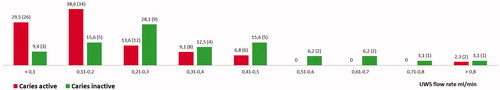
The distribution of unstimulated whole saliva (UWS) flow rates are presented in . There were significant differences between the groups in the prevalence of hyposalivation with regards to very low unstimulated whole saliva (UWS) flow rates (<0.1 ml/min). For the CA group, the prevalence was 29.5% compared to 9.4% for the CI group (p < .001). With a level of low unstimulated whole saliva (UWS) flow rates <0.2 ml/min, the corresponding values were 68.1% and 25%, respectively (p < .001). There was also a significant difference for mean flow rates of unstimulated whole saliva (UWS) for the two groups, 0.20 ± 0.21 and 0.35 ± 0.23 for CA and the CI groups, respectively (p = .002).
Figure 2. Distribution of the percentage of individuals (number of individuals) with different flow rates (ml/min) in the caries active (CA) group and in the caries inactive (CI) group. Limits: Very low flow rates = <0.1 ml/min; Low flow rates = 0.11–0.2 ml/min; Normal flow rates = >0.2 ml/min.
The relationship between frequency of perceived xerostomia and mean UWS flow rates is shown in . Those who never or seldom perceived xerostomia had ‘normal’ mean flowrates, while those perceiving xerostomia more often all had mean flow rates below the ‘low’ limit. There were statistically significant differences in flow rates between those who never experienced xerostomia and those who experienced it sometimes (p < .05) or often (p < .05).
Table 5. Responses to the question, ‘How often have you experienced xerostomia’ correlated to unstimulated whole saliva (UWS) flow rates for all participants.
Discussion
This study examined patient-reported oral and general health, xerostomia, hyposalivation and quality of life and compared differences among caries active (CA) and caries inactive (CI) younger adults. Self-rated oral and general health were rated significantly lower among CA patients, compared to the CI group. A significantly larger proportion of CA individuals reported xerostomia as well as had hyposalivation compared to the CI group. The two groups did not differ significantly on overall oral health impact scores. However, the CA group rated the functional limitation and physical pain domains significantly higher than the CI group, indicating a higher degree of negative impact on their oral health.
There are indications that the association between xerostomia and general health needs further investigation [Citation39]. Different magnitudes of hyposalivation have been related to diagnosed disease and high BMI in younger adults but to different medications in older adults [Citation11]. In this sample we have previously reported a significant increased risk for xerostomia and sleep disturbances among the CA group [Citation3]. Sleep disturbances related to xerostomia have been mentioned in different medical condition [Citation40–43]. Sleep disturbances in relation to caries activity must be considered unique and the relationship to general health needs more research.
The OHIP-14 total score did not differ between CA and CI in this study among younger adults. It seems like caries activity over time in younger adults alone does not increase OHIP-14. Compared to other younger individuals, from the Dunedin longitudinal cohort study reported by Thomson et al. [Citation16], the mean OHIP-14 sum scores were lower for both the CA and the CI groups. However, when focussing on the half of the caries active group experiencing xerostomia, compared with all others, the difference in OHIP-14 was significant. This difference for the mean total sum of OHIP–14 related to xerostomia seems to be in line with other studies of New Zealanders of different ages [Citation15,Citation16].
In population-based studies, ∼10–25% of 40 year-olds have reported that their mouth frequently or usually feels dry. In a majority of these studies xerostomia has been more common among women than among men [Citation11,Citation15,Citation44]. In the present study, the prevalences were similar to the upper end, 24% in the CA group had xerostomia frequently or very often, while it did not occur in the CI group. Both groups contained more women, approximately 70% [Citation3]. The clear difference in perception of xerostomia between the two groups was a very obvious finding in this sample, independent of different limits for xerostomia. This calls for more studies among young adults who are caries active.
To the best of our knowledge there is just one population-based prevalence study of hyposalivation among different age groups. In that study, 11–13% reported having hyposalivation, defined as a very low unstimulated whole saliva flow rate, in the age group 40–49 years [Citation11]. In the present study, the CI group had a slightly lower prevalence while in the CA group, the prevalence was almost three times higher. This difference might be one reason for a higher caries activity over time among the caries active group. This finding must be considered the most important of this study and needs further attention.
At least one review of caries risk in relation to hyposalivation found an increased risk, even if studies are difficult to compare as there are no gold standard methods for measuring caries activity or salivary flow rates [Citation24]. Different flow rates of unstimulated whole saliva have been investigated in relation to caries, identifying limits of increased risk [Citation25]. As mentioned above several different methods have been used to determine hyposalivation, for example, the caries risk software Cariogram uses stimulated whole saliva to diagnose hyposalivation [Citation45], as well as other caries management system for caries prevention [Citation46,Citation47].
The prevalence of hyposalivation diagnosed by using limits for unstimulated or stimulated whole saliva are completely different [Citation11]. The limits for hyposalivation by unstimulated flow rates will incorporate more individuals compared with the limits for stimulated saliva [Citation11]. This relationship has been described in different ways over the years [Citation11,Citation22,Citation48,Citation49], but is probably considered and known by a few. The use of the stimulated whole saliva limits might be one reason for the presumption that ‘extreme reduction in salivary flow is very rare’ [Citation46] while actual hyposalivation might be common in regards to unstimulated flow rates [Citation11]. It could be presumed that the use of unstimulated whole saliva in the current study clarified the difference in the proportions of individuals with hyposalivation in the CA and the CI groups.
It is not known if the CA group’s more negative perceptions of oral and general health were due to lower salivary flow rates, the perception of xerostomia, or the actual experience of more caries-related dental treatments. If a relationship between hyposalivation and caries could be identified in larger longitudinal population-based studies, it could motivate more extensive caries prophylaxis for individuals with repeated caries activity, as there are so far no known treatments that can permanently increase salivary flow [Citation50,Citation51]. Extra fluoride might yet be the only way to offer this group a cavity free future [Citation52]. More caries prophylaxis will hopefully stop caries progression and treatment needs, but further research on the role of hyposalivation and xerostomia is needed.
The main strength of this study is that it is the first to examine both xerostomia and hyposalivation among young adults in relation to caries as well as oral and general health.
This study, along with previously reported data from this sample [Citation3,Citation26,Citation27] has focussed on questions that might be important to individuals that are caries active repeatedly over time [Citation53]. Limitation of this study include the small sample size and especially the small control group (the caries inactive group). Even if the salivary flow measurements followed a strict protocol to eliminate systematic errors, there are aspects concerning hyposalivation and xerostomia that are not yet fully understood and investigated, and therefore also difficult to control. For example, changes of salivary flow rate over time are not known, as studies of repeated measurement of saliva in general populations do not yet exist. We have presumed that salivary flow rates could be considered as fairly consistent over time, until proven otherwise, but decline in older ages independent of medication use [Citation54]. Due to the limitations the results must be interpreted with caution, as further studies in larger samples are needed.
To conclude, caries active younger adults reported significantly more xerostomia which was related to hyposalivation defined by unstimulated whole saliva flow rates. These conditions were negatively related to perceptions of both oral and general health. There is a need for learning more about the prevalence and the pathogenesis of xerostomia and hyposalivation in younger adults that are caries active.
Acknowledgements
This is the last out of four articles planed and outlined together with the late Professor Folke Lagerlöf, whom the authors want to acknowledge, honour and keep in warm memory. The authors also want to express their gratitude to Tony Wiklund at the Centre for Clinical Research, Västerås, for valuable and helpful software support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Availability of data and materials
Data are available on request to the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Marcenes W, Kassebaum NJ, Bernabe E, et al. Global burden of oral conditions in 1990-2010: a systematic analysis. J Dent Res. 2013;92:592–597.
- Broadbent JM, Thomson WM, Poulton R. Trajectory patterns of dental caries experience in the permanent dentition to the fourth decade of life. J Dent Res. 2008;87:69–72.
- Flink H, Tegelberg Å, Arnetz J, et al. Correlation between perceived experience of caries disease and recorded caries activity among adult patients at a Swedish Public Dental Clinic: a longitudinal study. Acta Odontol Scand. 2013;71:1486–1492.
- Söderström U, Johansson I, Sunnegårdh-Grönberg K. A retrospective analysis of caries treatment and development in relation to assessed caries risk in an adult population in Sweden. BMC Oral Health. 2014;14:126.
- Broadbent JM, Foster Page LA, Thomson WM, et al. Permanent dentition caries through the first half of life. Br Dent J. 2013;215:E12.
- Murray Thomson W. Epidemiology of oral health conditions in older people. Gerodontology. 2014;31 :9–16.
- Masood M, Newton T, Bakri NN, et al. The relationship between oral health and oral health related quality of life among elderly people in United Kingdom. J Dent. 2017;56:78–83.
- Åkesson ML, Wärnberg Gerdin E, Söderström U, et al. Health-related quality of life and prospective caries development. BMC Oral Health. 2016;16:15. :
- Barbe AG. Medication-induced xerostomia and hyposalivation in the elderly: culprits, complications, and management. Drugs Aging. 2018;35:877–885.
- Johansson AK, Johansson A, Unell L, et al. Self-reported dry mouth in 50- to 80-year-old Swedes: longitudinal and cross-sectional population studies. J Oral Rehabil. 2019. DOI:10.1111/joor.12878
- Flink H, Bergdahl M, Tegelberg Å, et al. Prevalence of hyposalivation in relation to general health, body mass index and remaining teeth in different age groups of adults. Community Dent Oral Epidemiol. 2008;36:523–531.
- Thomson WM. Issues in the epidemiological investigation of dry mouth. Gerodontology. 2005;22:65–76.
- Ship JA, Pillemer SR, Baum BJ. Xerostomia and the geriatric patient. J Am Geriatr Soc. 2002;50:535–543.
- Orellana MF, Lagravere MO, Boychuk DG, et al. Prevalence of xerostomia in population-based samples: a systematic review. J Public Health Dent. 2006;66:152–158.
- Benn AM, Broadbent JM, Thomson WM. Occurrence and impact of xerostomia among dentate adult New Zealanders: findings from a national survey. Aust Dent J. 2015;60:362–367.
- Thomson WM, Lawrence HP, Broadbent JM, et al. The impact of xerostomia on oral-health-related quality of life among younger adults. Health Qual Life Outcomes. 2006;4:86. :
- Stewart CM, Berg KM, Cha S, et al. Salivary dysfunction and quality of life in Sjogren syndrome: a critical oral-systemic connection. J Am Dent Assoc. 2008;139:291–299; quiz 358–299.
- Jeganathan S, Carey H, Purnomo J. Impact of xerostomia on oral health and quality of life among adults infected with HIV-1. Spec Care Dentist. 2012;32:130–135.
- Busato IM, Ignacio SA, Brancher JA, et al. Impact of clinical status and salivary conditions on xerostomia and oral health-related quality of life of adolescents with type 1 diabetes mellitus. Community Dent Oral Epidemiol. 2012;40:62–69.
- Barbe AG, Bock N, Derman SH, et al. Self-assessment of oral health, dental health care and oral health-related quality of life among Parkinson’s disease patients. Gerodontology. 2017;34:135–143.
- Sreebny LM, Valdini A. Xerostomia. Part I: relationship to other oral symptoms and salivary gland hypofunction. Oral Surg Oral Med Oral Pathol. 1988;66:451–458.
- Wang SL, Zhao ZT, Li J, et al. Investigation of the clinical value of total saliva flow rates. Arch Oral Biol. 1998;43:39–43.
- Flink H, Tegelberg Å, Lagerlöf F. Influence of the time of measurement of unstimulated human whole saliva on the diagnosis of hyposalivation. Arch Oral Biol. 2005;50:553–559.
- Bardow A, Nyvad B, Nauntofte B. Relationships between medication intake, complaints of dry mouth, salivary flow rate and composition, and the rate of tooth demineralization in situ. Arch Oral Biol. 2001;46:413–423.
- Leone CW, Oppenheim FG. Physical and chemical aspects of saliva as indicators of risk for dental caries in humans. Proceedings of the NIH Consensus Development Conference on Diagnosis and management of dental caries throughout life; 2001 Mar 26–28. Bethesda, MD: National Institutes of Health; 2001.
- Flink H, Tegelberg Å, Arnetz J, et al. Patient-reported outcomes of caries prophylaxis among Swedish caries active adults in a long-term perspective. Swed Dent J. 2016;40:101–110.
- Flink H, Tegelberg A, Arnetz JE, et al. Patient-reported negative experiences related to caries and its treatment among Swedish adult patients. BMC Oral Health. 2017;17:95.
- The Public Health Agency of Sweden. Health on equal terms - National public health survey. Available from: https://snd.gu.se/en/catalogue/study/ext0118
- Wamala S, Merlo J, Boström G. Inequity in access to dental care services explains current socioeconomic disparities in oral health: the Swedish National Surveys of Public Health 2004-2005. J Epidemiol Community Health. 2006;60:1027–1033.
- Molarius A, Engström S, Flink H, et al. Socioeconomic differences in self-rated oral health and dental care utilisation after the dental care reform in 2008 in Sweden. BMC Oral Health. 2014;14:134.
- Engström S, Holmlund A. Self-estimated oral and general health are related and associated with clinically investigated dental health. Swed Dent J. 2011;35:169–175.
- Ekbäck G, Persson C, Linden-Boström M. What factors can be protective for both self-rated oral health and general health?. Swed Dent J. 2015;39:99–107.
- Slade GD. Derivation and validation of a short-form oral health impact profile. Commun Dent Oral Epidemiol. 1997;25:284–290.
- Larsson P, John MT, Hakeberg M, et al. General population norms of the Swedish short forms of oral health impact profile. J Oral Rehabil. 2014;41:275–281.
- Ericsson Y, Hardwick L. Individual diagnosis, prognosis and counselling for caries prevention. Caries Res. 1978;12:94–102.
- Pedersen AM, Nauntofte B. Primary Sjögren’s syndrome: oral aspects on pathogenesis, diagnostic criteria, clinical features and approaches for therapy. Expert Opin Pharmacother. 2001;2:1415–1436.
- Revicki D, Hays RD, Cella D, et al. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61:102–109.
- Dahl KE, Wang NJ, Skau I, et al. Oral health-related quality of life and associated factors in Norwegian adults. Acta Odontol Scand. 2011;69:208–214.
- da Silva L, Kupek E, Peres KG. General health influences episodes of xerostomia: a prospective population-based study. Community Dent Oral Epidemiol. 2017;45:153–159.
- Lavigne GJ, Goulet JP, Zuconni M, et al. Sleep disorders and the dental patient: an overview. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:257–272.
- Lopez-Jornet P, Lucero Berdugo M, Fernandez-Pujante A, et al. Sleep quality in patients with xerostomia: a prospective and randomized case-control study. Acta Odontol Scand. 2016;74:224–228.
- Oksenberg A, Froom P, Melamed S. Dry mouth upon awakening in obstructive sleep apnea. J Sleep Res. 2006;15:317–320.
- Tishler M, Barak Y, Paran D, et al. Sleep disturbances, fibromyalgia and primary Sjogren’s syndrome. Clin Exp Rheumatol. 1997;15:71–74.
- Nederfors T, Isaksson R, Mörnstad H, et al. Prevalence of perceived symptoms of dry mouth in an adult Swedish population–relation to age, sex and pharmacotherapy. Commun Dent Oral Epidemiol. 1997;25:211–216.
- Hänsel Petersson G, Åkerman S, Isberg PE, et al. Comparison of risk assessment based on clinical judgement and Cariogram in addition to patient perceived treatment need. BMC Oral Health. 2017;17:13.
- Evans RW, Pakdaman A, Dennison PJ, et al. The Caries Management System: an evidence-based preventive strategy for dental practitioners. Application for adults. Aust Dental J. 2008;53:83–92.
- Rechmann P, Jue B, Santo W, et al. Calibration of dentists for Caries Management by Risk Assessment Research in a Practice Based Research Network - CAMBRA PBRN. BMC Oral Health. 2018;18:2.
- Becks H, Wainwright W. Human Saliva. IX. The effect of activation of salivary flow. J Dent Res. 1939;18:447–456.
- Sreebny LM, Valdini A, Yu A. Xerostomia. Part II: relationship to nonoral symptoms, drugs, and diseases. Oral Surg Oral Med Oral Pathol. 1989;68:419–427.
- Flink H. Studies on the prevalence of reduced salivary flow rate in relation to general health and dental caries, and effect of iron supplementation. Swed Dent J Suppl. 2007;(192):3–50.
- Salum FG, Medella-Junior FAC, Figueiredo MAZ, et al. Salivary hypofunction: an update on therapeutic strategies. Gerodontology. 2018;35:305–316.
- Pitts NB, Grant J, Hinrichs-Krapels S, et al. Towards a Cavity Free Future: how do we accelerate a policy shift towards increased resource allocation for caries prevention and control? London: The Policy Institute at King’s; 2017.
- Chiang HM, Tranaeus S, Sunnegårdh-Grönberg K. Caries as experienced by adult caries active patients: a qualitative study. Acta Odontol Scand. 2019;77:15–21.
- Affoo RH, Foley N, Garrick R, et al. Meta-analysis of salivary flow rates in young and older adults. J Am Geriatr Soc. 2015;63:2142–2151.
