Abstract
Objective: To study the effect of governmental strategies, to constitute and publish recommendations on antibiotic usage in dentistry, on the antibiotic consumption.
Material and methods: Descriptive statistics regarding antibiotic prescription between 2009 and 2017 was retrieved from two national registers, the Swedish Prescribed Drug Register and the Dental Health register, both administered by the National Board of Health and Welfare. Age standardization was applied to alleviate the comparison between gender, different regions and years.
Results: The number of dispatched prescriptions of antibiotics from dentists was reduced with 31% during the study period. 10% of the visits to a dentist generated an antibiotic prescription corresponding to 7% of the total number of antibiotic prescriptions. A decline in prescription was observed after publication of national recommendations for antibiotics prophylaxis in 2012 and antibiotic treatment in 2014. Unexplained geographical and gender differences in the rates of prescription were seen.
Conclusions: Data indicates a correlation between introduction of governmental strategies to reduce antibiotic usage and declining antibiotic prescription. Although a marked reduction in prescription was observed, the results indicate that there is further potential for improvement of antibiotic stewardship in odontology.
Introduction
Antibiotic resistance is a well acknowledged and increasing global problem with serious consequences for modern health and dental care. The correlation between antibiotic consumption and the development and propagation of resistance is well recognized [Citation1–4]. Besides antibiotic utilization factors such as hygiene measures in health care, the infrastructure of the health care system, environmental aspects, educational efforts, and governmental actions are of importance to counteract this development [Citation5–7]. Solid guidelines or recommendations can be considered a cornerstone in streamlining the clinicians’ decision process regarding antibiotic utilization. To obtain enough weight these should be evidence based. In the absence of solid evidence consensus recommendations formulated by experts, on behalf of authorities, can be an important intermittent action while awaiting more knowledge. In fact, it can be speculated that in the absence of solid evidence, recommendations are even more important, since the behaviour of the clinicians otherwise is bound to be divergent or even random. However, to ensure that these measures will be taken (provide such national guidelines, or recommendations), well-structured authorities with enough financial resources in the society for implementation and follow-up is required.
Sweden has a long tradition of strategic work against antibiotic resistance which is probably an important factor for the favourable rates of antibiotic resistance seen in comparison to many other countries [Citation8]. In 2011 the Swedish Medical Products Agency and Swedish Institute for Communicable Disease Control was given the assignment to assemble experts to formulate recommendations for antibiotic use in dentistry. This measure was a part of the governmental effort to increase patient safety with the aim of improving safety aspects in health care. This conveyed into two expert meetings one in 2012 with the topic of antibiotic prophylaxis in dentistry and one in 2014 concerning antibiotic treatment of dental infections. Representatives from different odontology and medical specialities, free from conflict of interest, participated in the expert meetings. As a result, national recommendations for antibiotic prophylaxis and for antibiotic treatment in dentistry were published in 2012 and 2014, respectively [Citation9,Citation10]. No financial support was allocated for implementation but through the dental section of the Swedish strategic programme against antibiotic resistance (Strama) lectures and workshops were given on a national scale upon request from different dental health care providers and course organizers. Strama started as a network of volunteers on both national and regional level. In 2007 a dental section within Strama National was formed, the Swedish strategic programme against antibiotic resistance in dentistry (StramaDent). Today Strama is funded by the Swedish government and incorporated in the Public Health Agency of Sweden [Citation11].
On an international scale it has been estimated that the dentistry accounts for 7–11% of all the antibiotics prescribed [Citation12]. In year 2017 dentists in Sweden accounted for 21 antibiotic prescriptions per 1000 inhabitants, which corresponded to approximately 7% of the total antibiotic usage in outpatient care [Citation8]. The antibiotic used in Swedish dentistry increased until 2007 after which a marked reduction has been observed. Noteworthy, in the available statistical reports, the large regional differences that still are found within the country, indicates that further improvement in antibiotic prudency might be achievable.
In order to optimize the resources allocated for the purpose of reducing over-prescription of antibiotics the publication of recommendations and their eventual implementations, needs to be evaluated. The aim of the current study was to study the effect of governmental strategies, to constitute and publish recommendations of antibiotic prophylaxis and treatment in dentistry, on the antibiotic consumption in Swedish dentistry.
Material and methods
Ethical considerations
Prior to onset the study an approval was obtained from the regional ethical committee (ref 2018/1616-31/2).
Registers and study population
Data for the study derived from two Swedish national registers, the Swedish Prescribed Drug Register and the Dental Health Register, both administered by the National Board of Health and Welfare in accordance to the law on health data register (1998:543). Part of the data is summarized in a report from The National Board of Health and Welfare composed by one of the authors (F.L.) and the other authors acting as external experts (B.L., M.H. and A.C.) [Citation13].
The Swedish Prescribed Drug Register has since 2005 a national coverage of all prescribed pharmaceutical compounds retrieved by patients from the pharmacies in Sweden [Citation14,Citation15]. Each dispatched antibiotic prescription is registered, meaning that non-attended prescriptions are not included in the statistics. Furthermore, medication distributed directly to the patients seeking health care is not registered. However, by Swedish regulation this accounts for a minimal amount of the total medication consumed. Data on all prescriptions from the period 2009 to 2017 regarding type of antibiotic compound, dosage, duration and occupation (medical doctor or dentist) were collected. The unit prescription/1000 inhabitants was used to described rate of to reflect the geographical distribution in relation to local population size. This way of displaying prescription is commonly used and is therefore suitable for comparison between different countries.
The Swedish Dental Health Register started in 2008 and registers all dental care subsided by public means, which accounts for more than 95% of all the dental care provided to the adult Swedish population [Citation16]. Children and adolescents dental care up to the age of 23 is not included in the register. However, at the time of study the register accounted for individuals above 21 years. Furthermore, dental care to patients with high requirements due to long-term disease or disability are not included in the data because of inadequate reporting. According to statistics from the counties of Sweden, this group of individuals accounts for approximately 2% of the total population [Citation17]. All the dental treatments in The Dental Health Register are coded with the primary aim to couple the diagnosis and type of treatment to the economical compensation system. This coding system was used to retrieve statistics on treatments with increased likelihood of being accompanied with antibiotic prescription. Consequently, visits regarding any type of dental treatment during the period 2009–2017 were selected for the analyses.
Statistical analyses
Descriptive statistics have been produced, using the software SAS Enterprise Guide 7.1 (SAS Institute, Cary, NC). Non-standardized proportions were compared using z score test. Statistical significance was set at a p-value level of .05 in all of the analyses.
Age standardization was applied to alleviate the comparison between gender, different regions and years, by eliminating the differences depending on inequalities in age distribution. Direct age standardization was calculated based on the total population in Sweden 2017. Five-year age distribution intervals were used. Prescription rates were compared by testing the log rate ratio.
Results
Antibiotic prescription during the period 2009–2017
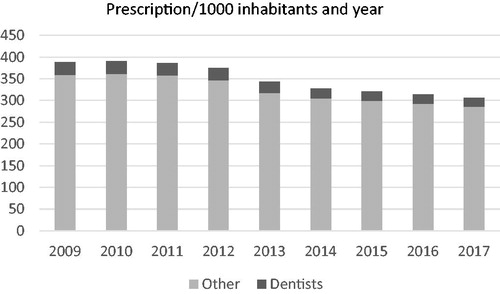
A total of 1,276,203 patients dispatched an antibiotic prescription prescribed by a dentist during the study period. The total number of antibiotic prescriptions per 1000 inhabitants and year was reduced with 21% during the study period (p < .0001). The number of dispatched prescriptions of antibiotics from dentists was reduced with 31% during the corresponding period (p < .0001). During the study period 10% of the visits to a dentist generated an antibiotic prescription corresponding to about 7% of the total number of antibiotic prescriptions per 1000 inhabitants. The largest reduction was seen between year 2012 and 2014 (). In the period from year 2000 to 2015 the prescription in dentistry peaked in 2007, after which a three-step decline was seen corresponding to initiation of StramaDent in 2007, followed by publication of national recommendations for antibiotics prophylaxis in 2012, and antibiotic treatment in 2014. Although less pronounced, a continuing decline was seen until 2017 in alignment with, but slightly more pronounced than, the total antibiotic prescription in the total outpatient care ().
Figure 1. Number of antibiotic prescriptions per 1000 inhabitants and year in dentistry and the timepoint for implementation of strategies to optimize the usage.
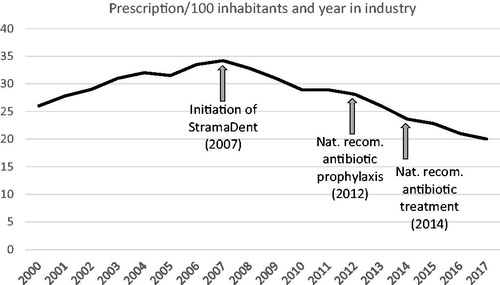
Figure 2. The total prescription of antibiotics in Swedish outpatient care and the fraction derived from dentistry.
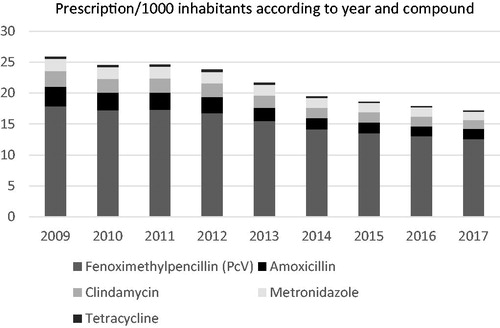
The main compounds prescribed in dentistry were in declining order: phenoxymethylpenicillin, amoxicillin, clindamycin, metronidazole and tetracycline (). Phenoxymethylpenicillin usage showed the lowest reduction (30%) during the study period and accounted for 73% of the antibiotics used in dentistry in 2017 (p < .0001). The largest change was seen for tetracycline where a decline of 53% occurred from 2009 to 2017 (p < .0001). The reduction in clindamycin, amoxicillin and metronidazole prescription was 42%, 49% and 31%, respectively (p < .0001). Between the year 2009 and 2012 the antibiotic utilization in dentistry was relatively stable. The major change in antibiotic usage in dentistry was seen during the years 2013 and 2014. Antibiotic consumption continued to fall during 2015–2017 but to a lesser degree.
Figure 3. Number of antibiotic prescriptions in dentistry per 1000 inhabitants according to year and type of compound.
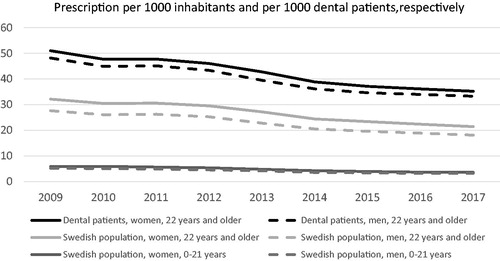
When relating the antibiotic prescription to the number of individuals attending dental care in comparison to the total population, in an age standardized manner, the number of individuals per 1000 dental patients and year was reduced from 50 in 2009 to 35 in 2017, while the corresponding figure for the total population was a reduction from 30 in 2009 to 20 in 2017 (p < .0001; ). This indicates that the likelihood of receiving an antibiotic prescription was strongly reduced in individuals at risk, that is, patients attending a dental office.
Influence of gender and age
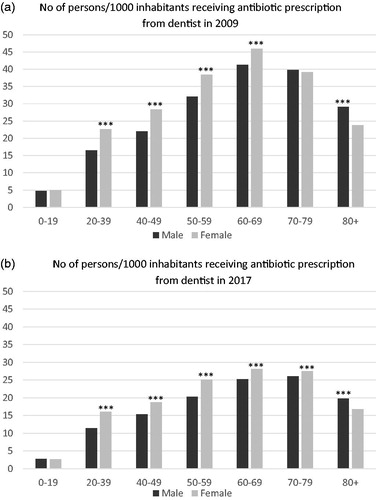
The total antibiotic prescription in the entire population is evenly distributed in the age groups 0–49 years with levels between 156 and 159 prescriptions per 1000 inhabitants and year. Thereafter an increase occurs for each age group. In dentistry, the influence of age on prescription shows maximum prescription in the age group 60–79 years in both 2009 and 2017, with less difference between the different age groups in 2017. In adults, women receive more antibiotics than men throughout the study period except for individuals 80 years or older, where the gender distribution is reversed. In individuals below 20 years of age the total prescription shows no gender differences ().
Geographical differences
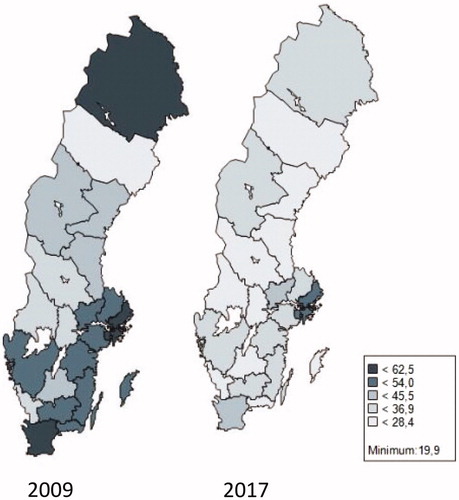
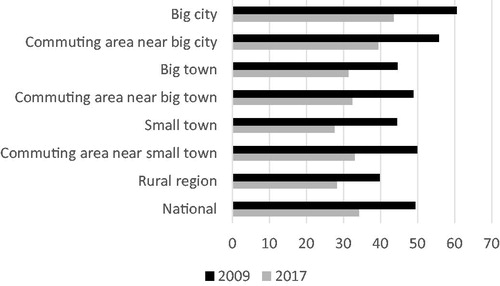
There is a wide geographical difference in rate of antibiotic prescription both in 2009 and 2017 (). Patients visiting a dental office in the Stockholm region had the highest risk of receiving an antibiotic prescription in 2009. Although the Stockholm prescription rate was reduced with 25% during the study period it still had highest figures in 2017 being 33% over the national average level. The prescription rate in Stockholm was more than twice the amount of the lowest region (Västerbotten). Also, the reduction in prescription during the study period was unevenly distributed. The highest reduction of almost 50% was observed in Gotland and the lowest of 10% in Västmanland. There was no association between the prescription levels in 2009 compared to the national average, and the size of reduction seen in 2017. This can be exemplified by the two regions Gävleborg and Västmanland, whom had approximately the same rate of prescription of antibiotics in 2009 of 45 versus 46 prescriptions per 1000 dental patients and year, while the corresponding figures for 2017 was 26 and 42 prescriptions per 1000 dental patients. The region with the lowest antibiotic usage in dentistry in 2009 showed a marked reduction of 24%, from 26 prescriptions per 1000 dental patients to 20. When the regions of Sweden are divided according to size of city, vicinity to a large city, smaller towns and rural areas the decline in prescription is marked in all the groups although the total prescription is substantially higher in urban areas and nearby commuting areas compared to rural regions ().
Discussion
The result of the study suggests that the constitution of national recommendations is an important mean to influence prescription of antibiotics in dentistry. However, it is not clear from de retrieved data whether the influence on antibiotic use is directly associated with the publication of the recommendations or if it is a consequence of the subsequent educational efforts that was undertaken. Previous research shows that effective actions to change behaviour are tutorial interactive sessions including components of feedback [Citation18]. Therefore, is it of importance to allocate financial means not only to produce guidelines but also for their implementation. The decline in prescription seen after 2007 may indicate an effect of mere educational efforts since it followed the formation of StramaDent, with aim of counteracting antibiotic resistance in dentistry. The guidelines strongly promote the use of narrow spectrum compounds as first choice of treatment. The result shows that the reduction was largest among broad spectrum compounds further supporting that the observed decline in prescription is in fact a consequence of the novel guidelines.
An interesting observation was that patients attending a dental office annually, or more frequent, were less likely to receive an antibiotic prescription compared to the total population. The correlation between oral and general health is multifactorial and probably bilateral [Citation19]. A possible explanation for this could therefore be that regular visits to a dentist are a confounding factor for generally health awareness and selfcare. Contradicting to this is the described observation that although women are more prone to seek dental care on regular basis [Citation20], the current study shows that females are prescribed more antibiotics than men in the ages between 21 and 80 years.
Ideally recommendations or guidelines should be based on scientific evidence. In the absence of solid evidence, the production of consensus-based recommendations can be even more important because it may have an even stronger impact on streamlining processes, assure equality and prevent overuse or misuse of antibiotics. However, recommendations based on consensus need frequent revision because even single studies may motivate alterations in suggested routines. Furthermore, achieving meaningful consensus requires assembly of expert groups and meetings enabling a stepwise iterative discussion procedure to converge opinions. Consensus recommendations can therefore be more costly both regarding production and maintenance. A current problem in Sweden is therefore that the allocation of financial means to provide recommendations and guidelines are mainly in temporary form. Further research is also needed to increase our knowledge regarding the most cost-effective means to implement new knowledge. Although the use of antibiotic in dentistry have reduced significantly both regarding total consumption but also regarding broad-spectrum antibiotics the data, especially regarding the large geographical differences in prescription, give strong indications that there is further potential for improvement. The current study highlights this issue but does not provide basis for identification of specific problem areas, which is necessary in order to pinpoint aberrant antibiotic utilization. Addressing this in future studies, in combination with efforts to educate and motivate antibiotic stewardship in odontology may be important actions to optimize the antibiotic use. In this context the components of antibiotic stewardship are to reduce over and under prescription or incorrect prescription regarding type of compound, dose or duration [Citation21]. Comparing educational efforts between the regions, such as Västmanland and Gävleborg, with initial equal prescription levels but markedly different after publication of guidelines, can be valuable guidance in this process. The concept of knowledge-based management, meaning steering health care practice through national evidence or expert-based guidelines seems to have important impact on dental care providers’ practice. However, knowledge-based management is dependent on the organization’s personnel, structural and economic capacities to spread information, follow-up and feed-back from top to bottom. This process may be a challenge in the small units often seen in dentistry.
In conclusion, although there seems to be a correlation between introduction of governmental strategies to reduce antibiotic usage in dentistry and declining antibiotic prescription, there is further need for improvement of antibiotic stewardship in odontology and to clarify effective measures to disseminate information.
Disclosure statement
The authors have no declaration of interest to declare.
Additional information
Funding
References
- Clatworthy AE, Pierson E, Hung DT. Targeting virulence: a new paradigm for antimicrobial therapy. Nat Chem Biol. 2007;3(9):541–548.
- Livermore DM. Minimising antibiotic resistance. Lancet Infect Dis. 2005;5(7):450–459.
- Foucault C, Brouqui P. How to fight antimicrobial resistance. FEMS Immunol Med Microbiol. 2007;49(2):173–183.
- Hsueh PR, Chen WH, Luh KT. Relationships between antimicrobial use and antimicrobial resistance in Gram-negative bacteria causing nosocomial infections from 1991–2003 at a university hospital in Taiwan. Int J Antimicrob Agents. 2005;26(6):463–472.
- WHO. Global strategy for containment of antimicrobial resistance. Geneva (Switzerland): World Health Organization; 2015. Available from: http://www.who.int/drugresistance/WHO_Global_Strategy.pdf
- Pittet D, Boyce JM. Revolutionising hand hygiene in health-care settings: guidelines revisited. Lancet Infect Dis. 2003;3(5):269–270.
- Baur D, Gladstone BP, Burkert F, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17(9):990–1001.
- Swedres-Svarm. Consumption of antibiotics and occurrence of resistance in Sweden. Public Health Agency of Sweden and National Veterinary Institute; 2018. Available from: https://www.folkhalsomyndigheten.se/contentassets/d118ac95c12d4c11b3e61d34ee6d2332/swedres-svarm-2016-16124.pdf
- Allander A, Aronsson B, Baecklund E, et al. Indikationer för antibiotikaprofylax i tandvården. Info Läkemedelsverket. 2012;5:22–35. Swedish.
- Skogh Andrén A, Aronsson B, Aust-Kettis A, et al. Rekommendationer för antibiotikabehandling i tandvården. (Publication inSwedish). Info Läkemedelsverket. 2014;25:19–30.
- Strama. Public Health Agency of Sweden. [cited 2020 Feb 17]. Available from: http://www.strama.se
- Johnson TM, Hawkes J. Awareness of antibiotic prescription and resistance in primary dental care. Prim Dent J. 2014;3(4):44–47.
- Antibiotikaförskrivning inom tandvården. Statistikrapport baserad på uppgifter från läkemedels- och tandhälsoregistret 2009–2017. Socialstyrelsen; 2019.
- Wettermark B, Hammar N, Fored CM, et al. The new Swedish Prescribed Drug Register–opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidem Drug Safe. 2007;16(7):726–735.
- Ludvigsson JF, Almqvist C, Bonamy A-KE, et al. Registers of the Swedish Total population and their use in medical research. Eur J Epidemiol. 2016;31(2):125–136.
- Ljung R, Lundgren F, Appelquist M, et al. The Swedish dental health register – validation study of remaining and intact teeth. BMC Oral Health. 2019;19(1):116.
- Johansson T. Uppföljning av landstingens särskilda tandvårdsstöd år 2017. Stockholm: Sveriges Kommuner och Landsting; PM 2018.
- Le Corvoisier P, Renard V, Roudot-Thoraval F, et al. Long-term effects of an educational seminar on antibiotic prescribing by GPs: a randomised controlled trial. Br J Gen Pract. 2013;63(612):e455–e464.
- Dörfer C, Benz C, Aida J, et al. The relationship of oral health with general health and NCDs: a brief review. Int Dent J. 2017;67:14–18.
- Talakey AA, Bernabé E. Long‐term regular dental attendance and tooth retention among British adults: A cross‐sectional analysis of national survey data. Int J Dent Hygiene. 2019;17(1):64–70.
- Sanchez GV, Fleming-Dutra KE, Roberts RM, et al. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1–12.