Abstract
Objectives
The aim was to identify caries risk factors in 1-year-olds predicting dentine caries in 6-year-olds.
Materials and methods
Caries risk assessment was performed in 804 one-year-olds. Their parents answered a questionnaire, regarding family factors, general health, food habits and oral hygiene. Clinical examinations and caries risk assessments at 1, 3 and 6 years of age were performed. Simple and multiple regression analyses were used for identification of caries-associated factors.
Results
Caries risk was found in 5% of the 1-year-olds, and 12% of the 3-year-olds. Dentine caries was found in 3% of the 3-year-olds and in 16% of the 6-year-olds. Caries risk assessment was associated with caries at 6 years of age (OR = 5.1, p < .001). Multiple logistic regression analysis found the following variables associated with caries at 6 years of age: Caries in sibling (OR = 2.1, p = .012), Beverage other than water (OR = 2.1, p < .001), Night meal (OR = 1.9, p = .002), Presence of mutans streptococci (MS) (OR = 1.6, p = .033) and Male gender (OR = 1.5, p = .053). An overall caries risk assessment was more reliable than any single caries risk factor.
Conclusions
Caries risk assessment for 1-year-olds in a region with low caries prevalence has limited accuracy to predict dental caries at 6 years of age. Caries risk often changes over time and should be reassessed on a regularly basis. The presence of MS in 1-year-olds did not increase the prognostic accuracy at 6 years of age.
Keywords:
Introduction
Dental caries is still one of the most common preventable diseases during childhood with prevalence in some countries exceeding 90% in 3–5-year-olds [Citation1–3]. It is caused by several interacting factors [Citation4] and is clearly a public health problem in both developing and industrialized countries [Citation3,Citation5–8]. In Sweden, there has been a decreasing caries prevalence among children for decades [Citation9,Citation10], but this trend now seems to be broken. Recently published statistics from The Swedish National Board of Health and Welfare showed that the proportion of caries-free 6-year-olds has decreased from 79 to 73 percentage points from 2011 to 2017, and was still in 2018 (73%) at the same level as in 2005 [Citation11]. The proportion of caries-free Swedish 3-year-olds has not changed since 2005 and was still 95% in 2018 [Citation11]. Caries is known to have a negative effect on children’s quality of life due to pain, discomfort, infection, and the ability to chew and eat [Citation12–16], and obviously affects both oral and general health [Citation12,Citation17]. Early identification of individuals with a high caries risk is important in helping clinicians plan and perform caries preventive programmes, minimize negative dental experience and subsequent dental fear [Citation14,Citation15], increase oral health-related quality of life [Citation4,Citation18] and general health [Citation12,Citation17], and provides a long-term economic gain for both society and individuals [Citation19,Citation20].
Variables directly or indirectly related to risk for disease are either named risk indicators or risk factors. Some confusion exists regarding this nomenclature and there is no consensus at the present time [Citation21]. Some authors include environmental and behavioural as well as biological variables in the term risk factors [Citation22], while others have defined risk indicators as characteristics or exposures that co-exist with an increased possibility to develop a disease, while risk factors are characteristics or exposures playing an essential role in the development of a disease. Risk indicators are useful in identifying groups at risk [Citation23], while risk factors are more important when identifying individuals at risk. The use of microbiological screening to improve the prediction of caries risk has been used in research studies among pre-school children [Citation24–27], but the method is rarely used in clinical praxis. Studies have been carried out to find methods for aiding clinicians in identifying children with caries risk. Even if some efforts have been made to identify health factors contributing to the avoidance of caries [Citation28], most studies focus on pathogenic factors – how risk factors influence future caries development.
The use of caries risk assessment is not uniform and several standardized, computerized or individual approaches have been used to identify individuals at risk for dental caries [Citation28–34]. Some clinicians also tend to rely on the presence of dental caries as the most reliable indicator of future caries [Citation30,Citation33–36]. A project was started at a Public Dental Clinic in Norrköping, Sweden, to screen 1-year-olds regarding caries-related risk factors/indicators. In a previous study, we presented the results from the two-year follow-up of the initial caries risk assessment for 1-year-olds [Citation27]. We found it important to follow the children for another three years (until the age of 6 years), since the development of dental caries could be a relatively slow process [Citation37,Citation38], and because the influence of different caries risk indicators may differ during a longer time perspective [Citation38]. The aims were: (1) to identify separate risk factors/indicators in 1-year-olds predicting dentine caries at the age of 6 and (2) to analyse the sensitivity and specificity of a caries risk assessment performed by dental professionals in relation to caries prevalence (deft) at age 6.
The hypothesis was that caries risk assessment performed at 3 years of age is more reliable than if performed on 1-year-olds, when predicting dentine caries at age 6, as verified by larger AUC (area under the curve) in the ROC (receiver operating characteristics) curve.
Materials and methods
The Regional Ethics Board at Linköping University, Linköping, Sweden, approved the study (Dnr: M126-06, Dnr: 2011/465-31 and Dnr: 2018/344-32).
Study population
A total of 1013 one-year-old children, living in the catchment area of the Public Dental Clinic, were examined between 2002 and 2010. The social background of the population living in the region served by the Public Dental Clinic, where the study was performed, was considered to be representative for the region. The catchment area included both urban and rural areas with different kinds of residential areas with a mixture of villas, attached houses, condominium apartments and rental apartments. The overall caries prevalence at the clinic was 7% for the 3-year-olds and 26% for the 6-year-olds in 2002. Of the original cohort, 804 were included in the study at the age of 6. Reasons for exclusion were: moved from district (24), chose private dentist (182), no-show for examination at 3 years of age (2) and blocked dental record (1). Analysis of the dropout group (209) was performed to compare with the children included in the study.
A retrospective cohort study design was used. The children were followed from 1 to 6 years of age. All 1-year-olds from 2002 to 2010, living in the catchment area of the public dental clinic in Norrköping, Sweden, were invited by regular mail to participate. A total of 1013 children were examined at the age of one. The parents of the 1-year-olds received a questionnaire (Supplementary Appendix 1) on caries-associated factors. The parents were asked not to brush their children’s teeth the morning of the visit. Children who did not show up or whose parents had not completed the questionnaire were excluded from the study. At the visit, parents received information on oral health, including how to avoid dental caries.
The clinical examination comprised a visual inspection of the teeth, an assessment of visible dental plaque, and collection of a bacterial sample with a Quick-Stick® (Dentsolv AB, Saltsjö-Boo, Sweden). If a tooth – preferably an upper incisor – had erupted, a bacterial sample was taken from the buccal surface close to the marginal gingival sulcus. If no tooth had erupted, the bacterial sample was taken – in a similar way as from the teeth – from the tongue and cheek on one side of the mouth. Plaque on the Quick-Stick® was transferred to an incubation strip and cultivated according to the manufacturer’s instructions for the Dentocult® Strip Mutans test (Orion Diagnostica, Espoo, Finland). The sample was incubated in a heat chamber (model; Memmert GmbH, Hannover, Germany) at the clinic for 48 h at 37 °C before analysis. A dental hygienist/dental assistant assessed the number of adherent colonies according to a chart supplied by the manufacturer and assigned a score between 0 and 3 to indicate low to high levels of mutans streptococci (MS). An MS score of 0 included all MS bacterial samples with less than 104 CFU/ml [Citation26].
Only one dental professional, a dental hygienist or a dental assistant, was present at each visit and examined the child. Four experienced dental professionals were involved in the examinations during the study period. The four examiners were well trained in the examination procedures and bacterial sample evaluation. Assessment of caries risk followed the recommendations of the Östergötland County Council caries risk programme, including medical-, familial- or social factors and visual plaque (Supplementary Appendix 2). Caries risk was considered present if the child was expected to develop dental caries during the coming years. The overall caries risk assessment performed at the age of one year was evaluated based on clinical and anamnestic findings including responses from the questionnaire (Supplementary Appendix 1). The caries risk was assessed in two categories as ‘no risk’ or ‘risk’. If the child was assessed as having a caries risk a caries preventive programme, with further information to the parents, was implemented.
Examination at 3 years
At 3 years of age, the children were invited for a dental examination at the Public Dental Clinic. A dental hygienist or dentist recorded the findings from this dental examination in the clinic’s electronic patient file system. The clinical examination took place in the dental chair without an X-ray examination. The clinician diagnosed dentine caries (d3) using visual tactile inspection with a dental mirror and a dental explorer, according to the criteria by Koch [Citation39]. The caries experience was registered using the deft (decayed/extracted-due-to-caries/filled primary-teeth) index. Enamel caries was not registered. The caries risk assessment performed at 3-years-age was graded into either ‘no risk’ (0), ‘low risk’ (1) or ‘moderate to high risk’ (2–3) (Supplementary Appendix 2). Risk 2 (moderate risk) and 3 (high risk) were merged before statistical analysis. If the child was assessed as having a caries risk an individual caries preventive programme was implemented.
Teeth with dentine caries were treated with glass-ionomer- or composite fillings. Teeth with deep caries lesions affecting the pulp were extracted. Enamel caries was treated with fluoride varnish and caries-preventive information (depending on anamnestic findings) regarding food habits, tooth-brushing routines and use of fluoride tooth-paste. The children had regular dental check-ups from the age of 3 years, performed with a varying interval between 12 and 18 months – based on an individual assessment of caries risk. Children with high caries risk were offered additional visits at the dental clinic to check up oral hygiene and to evaluate if given recommendations regarding food habits and additional caries preventive recommendations had been followed, and to motivate parents/guardians and child to hold on to the given recommendations.
Examination at 6 years
At the age of 6, the same procedure was used as at the examination at 3 years of age – except that two dental bite-wing-X-rays were sometimes taken on individual indications, according to the dental care guidelines of the organization. Dentine caries in permanent teeth were excluded from the material to avoid confounding influences from mineralization disturbances such as MIH at this early age [Citation40].
Children who had been assessed at the Public Dental Clinic at the age of one, and who were examined in the Public Dental Service in Östergötland County at the ages of 3 and 6, were included in the study. Children, whose families had moved from the county, or who had chosen a private dental clinic, or for other reasons had not been examined in the Public Dental Service in Östergötland County during the year of their 3rd and 6th birthday, or whose dental records from the visit to the Public Dental Clinic at ages 3 and 6 were incomplete, were excluded from the study.
Statistics
For statistics, we used MedCalc for ROC-curve analysis (MedCalc Statistical Software version 19.2.1, MedCalc Software Ltd, Ostend, Belgium) and Statistica for all other analyses (Statistica v.12 StatSoft Inc., Tulsa, OK). Simple and multiple logistic regression analyses were used to identify caries risk factors at age one associated with dentine caries prevalence (deft > 0) at the age of 6. The significance level was set to 5%. The ROC analyses were performed using a non-parametric method according to DeLong et al. [Citation41].
Results
A total of 804 children (410 boys, 394 girls) (79% of the 1013 children examined at baseline) were assessed at the ages of 1, 3 and 6 years. A dental hygienist or dental assistant assessed 39 (4.9%) of the 1-year-olds to be at risk of developing dental caries during the coming years. MS was present in 250 (31%) of the 1-year-olds. At 3 years of age, a dentist or dental hygienist assessed 100 (12.4%) of the children to be at risk for developing caries. None of the one-year-olds were diagnosed with dental caries. The distribution of dentine caries (deft) at 3 and 6 years of age is shown in . At the age of 3, 25 (3.1%) of the children (18 boys, seven girls) were diagnosed with dentine caries lesions. At the age of 6, 127 (16%) of the children (75 boys, 52 girls) were diagnosed with dentine caries (deft > 0) in the primary dentition. The mean ± SD caries prevalence (deft) at 3 years was 0.10 ± 0.73. The mean ± SD caries prevalence (deft) at 6 years was 0.48 ± 1.50.
Table 1. Frequency distribution of dentine caries (deft) at the age of 3 and 6 years (N = 804).
Analysis of the 209 drop-outs showed that ‘Beverage other than water’ was more common among drop-outs than among the 804 participants (p = .005). No other variables from the simple logistic regression analysis () – male gender (p = .716), night meal (p = .351), presence of MS (p = .653), caries in sibling (p = .866), breast-feeding (p = .255), disease (p = .963), tooth brushing (p = .748) or caries risk assessment (CRA1) at the age of 1 year (7.2% for drop-outs and 4.9% for included) (p = .246) – differed significantly between the drop-outs and the participants according to Chi2-test with Yates’s correction.
Table 2. Results from simple (left) and multiple (right) logistic regression analysis of the associations between variables – from the questionnaire (Supplementary Appendix 1), MS scores and caries risk assessments at 1 year of age (CRA1) – and dental caries experience (deft > 0) at 6 years of age (N = 804).
Outcome of risk assessment at 1 year
The answers from the questionnaire at the 1-year examination are presented in . Seven (18%) of the 39 one-year-olds, who were considered at risk of developing caries, had developed dentine caries at age 3. Eighteen (46%) of the 39 one-year-olds, who were considered at risk of developing caries, had developed dentine caries until the age of 6. The 39 one-year-olds assessed to have caries risk included only 18 (14%) of the 127 children who developed caries until the age of 6. Of the 764 children who had been assessed ‘no risk’ at one-year-age were 109 (14%) diagnosed with dentine caries at the age of 6.
Results from simple and multiple regression analyses are presented in . In the multiple regression analysis, five variables at 1 year showed statistically significant association to dentine caries at the age of 6 years. From these five variables, we formed an index built upon the summary of the regression coefficients (log odds ratio including MR1_MS). MR1_MS = Gender×(0.398)+Night meal×(0.631)+Beverage other than water×(0.732)+Presence of MS×(0.459)+Caries in Sibling×(0.660), where Gender was 0 for girl and 1 for boy, Night meal, Beverage other than water, Presence of MS and Caries in sibling was 0 for no (including no sibling) and 1 for yes. Although MS was statistically significant in the multiple logistic regressions, the influence was clinically negligible (see ROC-curve, ). Hence, corresponding index excluding MS was (MR1): MR1 = Gender×(0.395)+Night meal×(0.642)+Beverage other than water×(0.810)+Caries in Sibling×(0.691). At the age of 3, none of the 25 children who had a chronic disease at the age of 1 year were diagnosed with dentine caries, while two (8%) had caries at 6 years of age. Separate variables used in the 1-year-questionnaire showed only weak correlation to dentine caries at 6 years of age with a lower OR than the overall caries risk assessment performed at 1 year of age (CRA1) (OR = 5.1, p < .001) (). A total of 28 (72%) of the 39 one-year-olds, who were assessed to have a caries risk, were not considered to have any caries risk when assessed at 3 years of age. Of the remaining 11 (28%) of the 39 with caries risk at both 1 and 3 years of age, seven (58%) were diagnosed with dentine caries at 3 years of age, and nine (75%) up to 6 years of age.
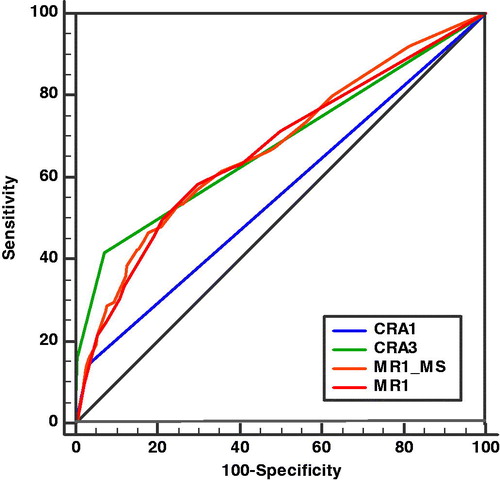
Figure 1. Receiver operating characteristic (ROC) curve describing the accuracy of four different methods used for caries risk assessment (CRA) at the ages of 1 (CRA1) and 3 years (CRA3) and from a multiple regression analysis of risk factors (Gender, Night meal, Beverage other than water, and Caries in Sibling) () at 1 year evaluated with (MR1_MS) and without (MR1) mutans Streptococci test (MS). Two different cut-off values – no caries risk and caries risk – were used for caries risk assessment performed at the age of 1 year (CRA1). Three different cut-off values – no caries risk (0), low caries risk (1) and moderate to high caries risk (2–3) – were used for the caries risk assessment performed at the age of 3 years (CRA3). CRA1 was performed by a dental hygienist or a dental assistant, while CRA3 was performed by a dentist or a dental hygienist according to a regional CRA protocol (Supplementary Appendix 2).
Of the 248 one-year-olds, who were assessed not to have any caries risk, and who did not present any caries risk variables at the examination at 1 year of age, 13 (5%) had developed dentine caries at 3 years of age and 27 (11%) had developed dentine caries at 6 years of age.
Caries risk assessment and dentine caries at 3 and 6 years
A total of 52 (52%) of the 100 children with an assessed caries risk at the age of 3 had developed dentine caries at the examination at 6 years of age.
Of the 25 (3%) children, who had been diagnosed with dentine caries at 3 years of age, 16 children (64%) had developed further dentine caries from 3 to 6 years of age.
At examination of the 6-year olds’ 18 children had more than two decayed teeth (deft > 2), even if they had been assessed as having no caries risk at 3 years of age. Five of these 18 had been assessed to be at risk as 1-year-olds. Two of the children with dentine caries at 3 years of age were, in any case, not assessed to have a caries risk at 3 years of age, according to the dental records.
In our study, 15% (117 children) changed risk group between the ages of 1 and 3. Forty-three (48%) of the 89 children, whose caries risk had increased (from ‘no risk’ to ‘risk’) between 1 and 3 years of age, had dentine caries at 6 years of age, and 11 (39%) of the 28 children, whose caries risk had decreased (from ‘risk’ to ‘no risk’) between 1 and 3 years of age, had dentine caries at 6 years of age. Sensitivity and specificity for risk assessment performed at 1 (CRA1) and 3 years of age (CRA3), are presented in . AUC for the four different methods () used for caries risk assessment showed the highest value for caries risk assessment at 3 years age (CRA3) with AUC 0.677 and slightly close was MR1_MS (0.672), for significant variables from multiple logistic regression analysis (). Almost the same value was seen for MR1 (0.665), for significant variables from multiple logistic regression analysis without bacterial test. The caries risk assessment at 1 year age (CRA1) had the lowest value (0.557). Pairwise comparison of ROC curves showed a significant difference between CRA1–CRA3 (p < .0001), CRA1–MR1_MS (p < .0001) and CRA1–MR1 (p = .0002), while the difference between CRA3–MR1_MS (p = .87) and MR1_MS–MR1 (p = .47) was not significant.
Discussion
In our study, we found that a caries risk assessment performed at 3 years of age had a higher accuracy than caries risk assessment performed at age 1 (). Among the children who developed caries before the age of 6 years, 14% had been identified with caries risk at 1 year and 52% at the caries risk assessment at 3 years of age, indicating that a regular re-assessment of caries risk is necessary to increase the sensitivity of the assessment, which is in agreement with the conclusions from several other studies [Citation32,Citation34].
Our finding that single caries risk factors () had a weaker predictive value (OR) than an overall caries risk assessment where several factors were weighted together (OR = 5.1) is in accordance with previous findings by Pienihäkkinen et al. [Citation42] and a review by Twetman [Citation34].
With the exception of the variable ‘Beverage other than water’, the drop-outs did not differ from the included group of children regarding any of the analysed variables from the 1-year assessment, why the excluded children were not expected to have caused any severe bias to the analyses.
The permanent teeth with diagnosed dentine caries at 6 years of age were not included in this study (four children), since caries in permanent teeth are not included in the definition of early childhood caries (ECC) [Citation43], and since caries in permanent teeth at this early age is often due to mineralization defects such as MIH [Citation44] that would confuse the evaluation of the caries risk assessments.
Caries prevalence varies both internationally and nationally, but also in different areas partly due to socio-economic factors [Citation45]. In Sweden, the proportions of dentine caries-free 3- and 6-year-olds varied between 91–98% and 59–82%, respectively, in 2017 [Citation11]. In the County/Region of Östergötland, the equivalent figures were 94% for 3-year-olds and 72% for 6-year-olds [Citation11]. The national and regional data correspond well to the prevalence found in the present study, where 3% of the 3-year-olds and 16% of the 6-year-olds were diagnosed with dentine caries in the primary dentition. Compared to other countries, the prevalence of caries was low at the clinic where our study was performed [Citation46].
Caries risk assessment
The results from another Swedish County/Region presented in 2009 by Holgerson et al. [Citation31], where 2-year-olds were assessed with Cariogram and evaluated regarding caries at 7 years of age, showed a higher sensitivity (46%) but lower specificity (88%) than what was found in our study (14% and 97%, respectively). Cariogram is, anyhow, primarily designed for adults [Citation47], why that method can possibly be developed further.
The conclusion by Twetman [Citation34] – that a combined weighting of several caries risk factors is better than using individual factors and that there is no reliable method, model, programme or technique for predicting future caries – is in agreement with the results from our study where separate caries risk factors had lower OR than an overall caries risk assessment including several factors performed on 1-year-olds by dental professionals. Therefore, we found it relevant that our study was designed locally according to factors expected to be caries-related risk factors/indicators among the pre-school children in the area. A large number of risk factors and -indicators could be used for the ECC risk assessment [Citation48]. Since excessive questionnaires are known to cause drop-outs [Citation49], we used a short questionnaire of caries-related variables expected to be relevant in the local clinical setting.
The caries risk assessments at 1 and 3 years of age were designed in a similar way as described by several authors, who have used a weighting of several risk factors but also the clinician's experience or ‘gut-feeling’ in the caries risk assessment [Citation33–35,Citation50,Citation51]. There might be a difference between caries risk assessment performed by dentists, dental hygienists and dental assistants. We did not evaluate this possible difference, but since the caries risk assessments were performed in the same way – using the same caries risk manual (Supplementary Appendix 2) – for all participants, we consider the risk for bias – due to the different staff categories involved in the study – as limited. The risk assessment performed on 1-year-olds identified only 18% of the 3-year-olds with dentine caries, but as much as 46% of the 6-year-olds with dentine caries, which could be due to the gradual long term process to develop a caries lesion [Citation30,Citation52]. The individual caries preventive measures implemented during the time of the study might also have influenced these results.
The AUC for the CRA3 (at 3 years of age) was larger than the AUC for the CRA1 (at age 1) (), why the hypothesis that ‘caries risk assessment performed at 3 years of age is more reliable than if it is performed at 1 year of age predicting caries at 6 years of age, as verified by a larger AUC in the ROC’, was accepted. We could conclude that significant caries risk factors from the multiple regression analysis (MR1_MS) () were not significantly better if MS was included. This showed that including the MS-bacterial test in the caries risk assessment at the age of 1 year did not increase the accuracy of the caries risk assessment when evaluated at the age of 6 years. The diagnosis of dental caries has been suggested as the single best predictor for new caries lesions [Citation33,Citation35,Citation53,Citation54], a statement that was not possible to verify with our study design, since the caries risk assessment performed at the age of 3 included the knowledge of possible dental caries and no separate risk factors were registered at 3 years of age.
Our finding that two children, who were diagnosed with dentine caries at the age of 3 without being assessed to have any caries risk, shows that the caries risk assessment was not optimized at the age of 3. A possible reason for bias is the possibility of an inadequate adherence to the guidelines regarding caries diagnostics or caries risk assessment, or that the registrations in the dental records were incorrect, but since the material includes a considerable number of patients, we consider the possible influence for bias due to this reason as limited. Grindefjord et al. [Citation24] showed – in her study of one-year-olds and follow-up after 2.5 years – that caries risk assessment, which included variables such as socio-demography, dietary habits and the incidence of MS had a sensitivity of 87% and a specificity of 83%, which was a higher value for sensitivity, but a lower value for specificity than we found (28% and 97%, respectively) after a 2-year-follow up of caries risk assessments performed at 1 year of age. A reason for these differences may be that the study by Grindefjord et al. [Citation24,Citation55] was conducted in an area with a 50% immigrant background and high caries prevalence at the age of 3 (30%) while the caries prevalence among the 3-year-olds in the catchment area of the clinic where our study was performed was considerably lower (3–7%). Another similar study made in Finland between 1989 and 1993 among 2- to 5-year-olds with higher caries prevalence showed a sensitivity of 32% and a specificity of 98% [Citation42], which is close to the results of our study of 1- to 6-year-olds (14% and 97%, respectively). The incidence of MS at low age has previously been found to have high validity for future caries incidence [Citation13,Citation24,Citation48], which we also found in our study regarding the presence of MS at 1 year of age and caries at 3 years of age with a sensitivity of 56% and a specificity of 70%.
In the study by Grindefjord et al. [Citation24], the presence of MS at 1 year showed a lower sensitivity (13%) and a higher specificity (97%) for caries at 3 years of age, as compared to our study (56% and 70%, respectively). However, Grindefjord et al. [Citation24] used a different bacteriological test, which could have contributed to the differences. In our study, the sensitivity and specificity (including bacterial test at 1 year) evaluated at 6 years (43% and 71%, respectively) was similar to what was found at 3 years age.
Caries
Due to the fear that the registration of enamel caries was often incomplete in the dental records, we decided to include only dentine caries in our study protocol in order to avoid bias from inadequate caries registration. Enamel caries has been found to be a risk factor for ECC, but dentine caries is, anyhow, found to be the strongest risk factor for ECC in high-income countries [Citation48].
The caries prevalence found among the 3- (3%) and 6-year-olds (16%) in our study was lower than in most other districts in the County Council (6% and 25%, respectively) and Sweden (2–6% and 15–31%, respectively) during the time of the study (2013) [Citation56], which must be considered when interpreting the results.
Holgerson et al. [Citation31] followed 2-year-old children for five years and Petersson et al. [Citation32] followed 10- and 11-year-olds for two years, and both found that about 50% of the children changed their risk group. In our study, only 15% (117 children) changed risk group between 1 and 3 years of age. The reason for these differences could be that our first risk assessment was performed already at 1 year of age, and the new risk assessment only 2 years later. Establishment of interproximal contacts during the first years of life – due to eruption and mesialization of posterior teeth – could also increase the susceptibility to dental caries with the subsequent change of risk group as a consequence. We did, anyhow, not evaluate the influence of interproximal contacts in our study. The low caries prevalence in our study has probably influenced this difference regarding changed risk groups in other studies. Since many children change their risk group and many of the children are not identified by the methods frequently used today, there is a reason for early and regular re-assessment of the caries risk among children, which is supported by several other authors [Citation32,Citation34]. The new caries risk assessment performed at 3 years of age had a better accuracy for dentine caries development until 6 years of age than the risk assessment made at 1 year of age.
Bias/limitations
The previously mentioned method for caries diagnosis [Citation39] was the method most closely resembling the generally used method as described in the regional dental care programme. The more than 60 dentists and dental hygienists participating in the examinations were not calibrated, due to the retrospective study design. This is a possible factor for bias, which should be considered when interpreting the results of the study. The regional clinical guidelines, that were used by the clinicians in the present study, anyhow, included clear, well-established and well-known internationally accepted criteria for diagnosis of dentine caries, and since dentine caries lesions often are easier to detect than enamel caries, and since the examiners were well trained according to the methods, we consider the risk for bias – due to methodological diversity regarding the diagnosis of dentine caries [Citation57] – as limited.
The dental personnel should always be aware of the risk that the parents’ answers in a questionnaire might have been modified to satisfy the dental personnel, thereby reducing the reliability of the assessment of both caries risk and adherence to a caries preventive programme. Since both standardized and individually designed caries preventive programmes existed and were known to the dental staff at the clinic, risk patients received individualized preventive care at the clinic during the time of the study. Therefore, the direct correlation between risk factor/variable and dentine caries could hardly be distinguished with the present study design. To exclude preventive care would, however, not have been an option due to ethical reasons. The amount of preventive dental care was not evaluated in this study, whereby the results should be regarded with caution, considering the possible limitations of the study design.
There are differences between residential areas in the county council, but also differences between districts in a city, regarding the occurrence of dental caries, markedly influenced by socio-economics. Socio-economics and residential area may change over time, and this could have influenced the results. The family could also have moved from the district during the study period. Other changes – such as the number of children in the family [Citation58], first-time parents and a general change in society’s view of dental care – might have influenced the commitment to their child’s oral health. These latter factors are to some extent known to influence the caries risk [Citation59], but were not evaluated in the study, which should be considered when applying the results from our study in a clinical setting. Hypomineralization in primary teeth has been found to increase the risk for dentine caries [Citation60], but was not registered in the present study. This should be considered when interpreting the results.
Since our study was extended during a long period of time, the perception of risk factors/indicators may have changed over the years due to e.g. changing attitudes towards oral hygiene habits, exposure time for risk factors/indicators, caries incidence and progression time, food habits, and exposure to fluoride, etc., whereby this might have influenced the results.
Conclusions
In a region with low caries prevalence, a caries risk assessment performed at 1 year of age has limited accuracy to predict dental caries at 6 years of age. Bacterial test (MS) at 1 year of age did not increase the accuracy of caries risk assessment to predict dental caries at 6 years of age. Reassessment of caries risk should be done on a regular basis, since several caries risk indicators/factors change over time. Further prospective longitudinal studies of caries development and caries risk assessment in different geographical regions and among subgroups with different socio-economic conditions will be valuable for dental clinicians and decision-makers.
APPENDIX_1and_2.docx
Download MS Word (17.4 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Gao XL, Hsu CY, Xu Y, et al. Building caries risk assessment models for children. J Dent Res. 2010;89(6):637–643.
- Manton DJ. Child dental caries – a global problem of inequality. Clin Med. 2018;1:3–4.
- [cited 2020 Apr 15]; 2020. Available from: https://www.who.int/oral_health/publications/en/orh_fact_sheet.pdf
- Colak H, Dülgergil CT, Dalli M, et al. Early childhood caries update: a review of causes, diagnoses, and treatments. J Nat Sci Biol Med. 2013;4(1):29–38.
- Wigen TI, Wang NJ. Maternal health and lifestyle, and caries experience in preschool children. A longitudinal study from pregnancy to age 5 yr. Eur J Oral Sci. 2011;119(6):463–468.
- Gaidhane AM, Patil M, Khatib N, et al. Prevalence and determinant of early childhood caries among the children attending the Anganwadis of Wardha district, India. Indian J Dent Res. 2013;24(2):199–205.
- Congiu G, Campus G, Lugliè PF. Early childhood caries (ECC) Prevalence and background factors: a review. Oral Health Prev Dent. 2014;12(1):71–76.
- Bissar A, Schiller P, Wolff A, et al. Factors contributing to severe early childhood caries in south-west Germany. Clin Oral Investig. 2014;18(5):1411–1418.
- Stecksén-Blicks C, Kieri C, Nyman JE, el al. Caries prevalence and background factors in Swedish 4-year-old children – a 40-year perspective. Int J Paediatr Dent. 2008;18(5):317–324.
- Koch G, Helkimo AN, Ullbro C. Caries prevalence and distribution in individuals aged 3–20 years in Jönköping, Sweden: trends over 40 years. Eur Arch Paediatr Dent. 2017;18(5):363–370.
- [cited 2020 Apr 15]; 2020. Available from: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/ovrigt/2020-2-6629.pdf
- Finucane D. Rationale for restoration of carious primary teeth: a review. Eur Arch Paediatr Dent. 2012;13(6):281–292.
- Leong PM, Gussy MG, Barrow SY, et al. A systematic review of risk factors during first year of life for early childhood caries. Int J Paediatr Dent. 2013;23(4):235–250.
- Welbury R. Summary of: influence of dental care on children's oral health and wellbeing. Br Dent J. 2013;214(11):568–569.
- Ng MW, Chase I. Early childhood caries: risk-based disease prevention and management. Dent Clin North Am. 2013;57(1):1–16.
- Shaghaghian S, Bahmani M, Amin M. Impact of oral hygiene on oral health-related quality of life of preschool children. Int J Dent Hyg. 2015;13(3):192–198.
- Crowe M, O’ Sullivan M, Cassetti O, et al. Weight status and dental problems in early childhood: classification tree analysis of a National Cohort. Dent J (Basel). 2017;5(3):25.
- Klingberg G. Dental fear and behavior management problems in children. A study of measurement, prevalence, concomitant factors, and clinical effects. Swed Dent J Suppl. 1995;103:1–78.
- Oscarson N, Källestål C, Fjelddahl A, et al. Cost-effectiveness of different caries preventive measures in a high-risk population of Swedish adolescents. Community Dent Oral Epidemiol. 2003;31(3):169–178.
- Jin LJ, Lamster IB, Greenspan JS, et al. Global burden of oral diseases: emerging concepts, management and interplay with systemic health. Oral Dis. 2016;22(7):609–619.
- Burt BA. Definitions of risk. J Dent Educ. 2001;65(10):1007–1008.
- Beck JD. Risk revisited. Community Dent Oral Epidemiol. 1998;26(4):220–225.
- Antunes LAA, Ornellas G, Fraga LS, et al. Oral health outcomes: the association of clinical socio-dental indicators to evaluate dental caries in preeschool children. Cien Saude Colet. 2018;23(2):491–500.
- Grindefjord M, Dahllöf G, Nilsson B, et al. Prediction of dental caries development in 1-year-old children. Caries Res. 1995;29(5):343–348.
- Pienihäkkinen K, Jokela J. Clinical outcomes of risk-based caries prevention in preschool-aged children. Community Dent Oral Epidemiol. 2002;30(2):143–150.
- Hultquist AI, Bågesund M. Dentin caries risk indicators in 1-year-olds. A two year follow-up study. Acta Odontol Scand. 2016;74(8):613–619.
- Ingemansson-Hultquist A, Lingström P, Bågesund M. Risk factors for early colonization of mutans streptococci – a multiple logistic regression analysis in Swedish 1-year-olds. BMC Oral Health. 2014;14(1):147.
- Wendt LK, Hallonsten AL, Koch G, et al. Analysis of caries-related factors in infants and toddlers living in Sweden. Acta Odontol Scand. 1996;54(2):131–137.
- Hänsel Petersson G, Twetman S, Bratthall D. Evaluation of a computer program for caries risk assessment in school children. Caries Res. 2002;36(5):327–340.
- SBU: Swedish Council on Technology Assessment in Health care. Karies – diagnostik, riskbedömning och icke-invasiv behandling. En systematisk litteraturöversikt [Caries – diagnosis, risk assessment and non-invasive treatment. A systematic review. Summary and conclusions]. Report no. 188. Swedish; 2007.
- Holgerson PL, Twetman S, Stecksèn-Blicks C. Validation of an age-modified caries risk assessment program (Cariogram) in preschool children. Acta Odontol Scand. 2009;67(2):106–112.
- Petersson GH, Isberg PE, Twetman S. Caries risk profiles in schoolchildren over 2 years assessed by Cariogram. Int J Paediatr Dent. 2010;20(5):341–346.
- Mejàre I, Axelsson S, Dahlén G, et al. Caries risk assessment. A systematic review. Acta Odontol Scand. 2014;72(2):81–91.
- Twetman S. Caries risk assessment in children: how accurate are we? Eur Arch Paediatr Dent. 2016;17(1):27–31.
- Twetman S, Fontana M, Featherstone J. Risk assessment – can we achieve consensus? Community Dent Oral Epidemiol. 2013;41(1):e64.
- Senneby A, Neilands J, Svensäter G, et al. Threshold values affect predictive accuracy of caries risk assessment. Acta Odontol Scand. 2019;6:1–13.
- Guedes RS, Piovesan C, Ardenghi TM, et al. Validation of visual caries activity assessment: a 2-yr cohort study. J Dent Res. 2014;93(7 Suppl.):101S–107S.
- Drancourt N, Roger-Leroi V, Martignon S, et al. Carious lesion activity assessment in clinical practice: a systematic review. Clin Oral Investig. 2019;23(4):1513–1524.
- Koch G. Effect of sodium fluoride in dentifrice and mouthwash on incidence of dental caries in school-children. Odontol Rev. 1967;18:37–43.
- Zhao D, Dong B, Yu D, et al. The prevalence of molars incisor hypomineralization: evident from 70 studies. Int J Paediatr Dent. 2018;28(2):170–179.
- DeLong E, DeLong D, Clarke-Pearson D. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845.
- Pienihäkkinen K, Jokela J, Alanen P. Assessment of caries risk in preschool children. Caries Res. 2004;38(2):156–162.
- Pitts NB, Baez RJ, Diaz-Guillory C. Early childhood caries: IAPD Bangkok Declaration. Int J Paediatr Dent. 2019;29:384–386.
- Llena C, Calabuig E. Risk factors associated with new caries lesions in permanent first molars in children: a 5-year historical cohort follow-up. Clin Oral Invest. 2018;22(3):1579–1586.
- Julihn A, Soares FC, Hjern A, et al. Socioeconomic determinants, maternal health, and caries in young children. JDR Clin Trans Res. 2018;3(4):395–404.
- Phantumvanit P, Makino Y, Ogawa H, et al. WHO global consultation on public health intervention against early childhood caries. Community Dent Oral Epidemiol. 2018;46(3):280–287.
- Jørgensen MR, Twetman S. A systematic review of risk assessment tools for early childhood caries: is there evidence? Eur Arch Paediatr Dent. 2020;21(2):179–184.
- Kirthiga M, Murugan M, Saikia A, et al. Risk factors for early childhood caries: a systematic review and meta-analysis of case control and cohort studies. Pediatr Dent. 2019;15:95–122.
- Phillips AW, Reddy S, Durning SJ. Improving response rates and evaluating nonresponse bias in surveys: AMEE Guide No. 102. Med Teach. 2016;38(3):217–228.
- Hallett KB. The application of caries risk assessment in minimum intervention dentistry. Aust Dent J. 2013;58:26–34.
- Lovell B, Cooksley T. Editorial – assessing, treating and prognosticating from the front door. Acute Med. 2018;17(4):174–175.
- Tickotsky N, Petel R, Araki R, et al. Caries progression rate in primary teeth: a retrospective study. J Clin Pediatr Dent. 2017;41(5):358–361.
- Disney JA, Graves RC, Stamm JW, et al. The University of North Carolina caries risk assessment study: further developments in caries risk prediction. Community Dent Oral Epidemiol. 1992;20(2):64–75.
- Isaksson H. On dental caries and dental erosion in Swedish young adults. Swed Dent J Suppl. 2013;232:1–60.
- Grindefjord M, Dahllöf G, Nilsson B, et al. Stepwise prediction of dental caries in children up to 3.5 years of age. Caries Res. 1996;30(4):256–266.
- [cited 2020 Apr 15]; 2020. Available from: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/statistik/2015-3-20.pdf
- Rechmann P, Jue B, Santo W, et al. Calibration of dentists for Caries Management by Risk Assessment Research in a Practice Based Research Network – CAMBRA PBRN. BMC Oral Health. 2018;18(1):2.
- Julihn A, Soares FC, Hammarfjord U, et al. Birth order is associated with caries development in young children: a register-based cohort study. BMC Public Health. 2020;20(1):218
- Fontana M, Jackson R, Eckert G. Identification of caries risk factors in toddlers. Int J Paediatr Dent. 2010;2010:1–657.
- Elfrink ME, Schuller AA, Veerkamp JS, et al. Factors increasing the caries risk of second primary molars in 5-year-old Dutch children. Int J Paediatr Dent. 2010;20(2):151–157.
