Abstract
Objectives
The aims of the present study were to evaluate the relative incidence of alveolar osteitis (AO) after mandibular third molar surgery, post-operative findings and local expression of bone markers and cytokines.
Study design
In 445 patients, unilateral surgical third molars extractions were undertaken (584 teeth). Bone markers and cytokines were explored at the AO side and on the un-operated contralateral side and compared with the levels in samples from a control group of 18 persons without AO.
Results
The relative incidence of AO was 4.6%. Patients (n = 27) with AO were invited to participate in the study and 21 (77.8%) did so. Patients with AO had 1–4 extra visits for treatment of AO, the mean follow-up time was 2.6 days for all patients. There were significantly higher levels of bone markers and cytokines in the AO site compared with the un-operated contralateral site, except for Epidermal growth factor (EGF). No significant difference in expression of bone markers and cytokines between the AO and control groups was found. Lower maximum inter-incisor opening (MIO) was correlated with increased Macrophage inflammatory protein 1 alpha. A negative correlation between patients’ complaint of trismus and MIO was seen.
Conclusions
The relative incidence of AO was low in our patient group treated with surgical removal of third molars. AO was more frequently seen in female patients. Treatment of AO required up to four extra visits. The study provides some information on the role of cytokines in AO; but further studies are required.
Introduction
Removal of impacted mandibular third molars is one of the most common procedures performed by oral and maxillofacial surgeons. Complications after third molar surgery are commonly of minor character and self-limiting. Serious complications related to third molar surgery, such as neurosensory disturbance, haemorrhage, jaw fracture, and life-threatening infections are rare.
Abnormal postoperative inflammation of the dental alveolus, known as ‘alveolar osteitis’ (AO), is a relatively common complication after third molar surgery. AO may occur after all types of dental extraction but is more often associated with third molar surgery. The prevalence of AO after third molar surgery ranges from 1 to 37.5% [Citation1–3].
The aetiology of AO is not fully understood. Focal fibrinolytic activity, with or without the influence of bacteria, leading to loss of the blood clot is an accepted theory explaining the development of AO. Previous studies have identified several surgical and patient related factors that may increase the risk of developing AO, including flap design, surgical trauma, the surgeon’s experience, gender, use of oral contraceptives, presence of local infection and a history of AO after previous dental extractions [Citation4–6].
The immune system’s response to removal of teeth is similar to any other tissue trauma. The immune system initiates an inflammatory response in the alveolus, eventually leading to bone and soft tissue healing. Proteins secreted from cells in the involved tissues, i.e. cytokines, are important signalling factors in both inflammatory response and healing. Both pro- and anti-inflammatory cytokines play a role in the normal immune response after tissue trauma [Citation7].
In a previous study of cytokine expression in relation to third molar surgery, the focus was on variations of IL-6 after different medication regimens [Citation8]. IL-6 is both a pro-inflammatory and an anti-inflammatory cytokine. It is secreted by T-cells and macrophages to stimulate the immune response and it is also an early marker of tissue damage. IL-6 is considered to have a dual role in bone remodelling, both in bone formation and in bone resorption [Citation9]. Other studies have focussed on cytokines in the oral and maxillofacial region; gingival crevicular fluid [Citation10], cleft lip and palate [Citation11] and the temporomandibular joint [Citation12].
To the best of our knowledge, local expression of different cytokines involved in alveolar osteitis has not previously been investigated.
Aims
The aims of the present study were to evaluate the relative incidence of AO after third molar surgery, the clinical course in patients diagnosed with AO, and to explore the local expression of some bone markers and cytokines in extraction sockets with AO.
Material and methods
Study design
The study was designed as a prospective controlled study. The study population comprised all patients having third molar surgery, during the period 1 January to 31 December 2014. Patients diagnosed with AO within one week after third molar surgery were selected for further examination. A control group, which had undergone third molar surgery without developing AO, was consecutively recruited during 2015 and 2016. The regional ethical committee for medical research (REK) approved the study (2013/2382 REK sør-øst). Patients were given written information about the study when presenting at the Department of Oral Surgery and Oral Medicine (DOS) with symptoms of AO within the first week after surgery. Those willing to participate in the study then signed informed consent forms. Inclusion criteria were age ≥18 years, symptoms and clinical findings according to the presence of AO and no previous treatment for AO. Diagnostic criteria for AO were defined as strong postoperative pain radiating from the surgical site intensified 2–4 days postoperatively, and possibly foetor. Subjective symptoms should correspond with clinical findings of an empty alveolus lacking a blood clot and/or exposed bone [Citation13,Citation14]. Exclusion criteria were age <18 years, postoperative symptoms not defined as AO, failure to present for further follow-up, and initiated treatment for AO.
A group of 18 consecutive persons who had undergone third molar surgery, were chosen as a control group for evaluation of the cytokines. In this group, the third molar had been removed, and at one-week follow-up, there were no signs of alveolitis. They were given written information about the study and signed informed consent forms. No economic compensation was offered.
Study variables
Several demographic variables were recorded for each patient, including age, gender, use of contraceptives, smoking habits, and occupation. Tooth number (FDI), indication for surgery, amount of local analgesia, intraoperative use of steroids, intraoperative use of antibiotics, duration of the surgical procedure, and the surgeon’s perception of the complexity of the surgery were obtained from the patients’ charts. Participants were asked about work or school related absence due to surgery, number of days using analgesics, use of antiseptic mouth rinse, postoperative antibiotics and specific complications. Clinical findings and AO treatment were also recorded. For the control group, age, gender, indication for third molar surgery, and relevant medical information and medication were recorded. All demographic and clinical data were recorded in a questionnaire accessed through InReach, a research module in University Health Network (InReach, University Health Network, www.uhnsl.com). This has previously been described by Øyri et al. [Citation5].
Indications for surgical removal of third molars were based on the recommendation of the Norwegian Centre for Evaluation of Medical Methods. The definition ‘therapeutic indication’ comprises third molars with evident clinical or radiological signs of local disease, i.e. pericoronitis, caries, resorption, pulpitis, marginal or apical periodontitis. Prophylactic indication for third molar surgery were only applicable in patients ≤30 years with partially erupted teeth (soft tissue impactions) with no visible clinical or radiological findings or previous episode(s) of pain associated with eruption and no sign(s) of pericoronitis within the preceding 12 months. In addition, prophylactic indication included teeth removed prior to orthognathic surgery. Asymptomatic, fully bony impacted teeth, with no associated pathological findings, were not removed.
Surgical procedure and medication
The surgical procedure and intraoperative medication have previously been described by Øyri et al. [Citation5]. Preoperative mouth rinsing with 0.2% chlorhexidine (Corsodyl 2 mg/ml, GlaxoSmithKline AS, Oslo, Norway) was routinely prescribed immediately before surgery. Surgery was exclusively performed under local analgesia (Xylocain Dental Adrenaline, Dentsply Ltd., Surrey, England). All patients underwent complete extractions. After removal of the third molar, a 3 × 1 cm oxytetracycline impregnated gauze drain (Terramycin-Polymyxin B, Pfizer, Pfizer Inc. New York, NY, USA) was routinely placed in the socket, and non-resorbable sutures (Supramid 3-0, B. Braun Melsungen AG, Melsungen, Germany) were used. Systemic antibiotics (SAB) or steroids were not routinely administered intraoperatively nor prescribed postoperatively. All patients were scheduled for removal of sutures and drain one week postoperatively but were encouraged to contact DOS at any time if necessary. Indications for surgery, surgical treatment and medications in both the patient group with AO and the control group without AO are presented in .
Table 1. Characteristics of patients with alveolar osteitis (AO).
Data collection methods
Patients presenting with symptoms of AO within the first week after third molar surgery were examined, along with a control group comprising persons who had undergone third molar surgery the week prior without symptoms of AO. Signs of local infection qualified for exclusion. Patients fulfilling the criteria for AO, who were willing to participate in the study, were then interviewed and asked to participate in the study. Three sterile endodontic paper points (ISO-Cells 50, Hygenic® Ster-I-Cell Paper Points, Coltene/Whaledent AG, Switzerland) were placed in the alveolus for 10 s. Gingival crevicular fluid (GCF) obtained from the most posterior point at the gingival sulcus on the contralateral side served as the control, and the sampling procedure was repeated. The paper points were placed in Eppendorf tubes (Axygen, Tewksbury, MA, USA) according to the test and the control sites and coded with a unique identifier. Samples were immediately frozen at −20° C after collection and later transferred to a high-performance laboratory freezer and stored at −80° C until analysis. Sampling was performed by four surgeons (two consultants and two residents), who had been calibrated in the sampling procedure. The surgeons were calibrated by training on performing the sampling procedure in 1–2 persons (clinical staff) that volunteered for this to be done. Patients with AO were scheduled for new follow-up appointments at intervals of 2–3 days. At the second appointment, the previously described sampling was repeated. For the control group, sampling was performed from the extraction alveolus at the one-week follow-up visit.
Laboratory analysis
All laboratory analyses were performed blinded with regard to the sample site, visit and participant information. The laboratory analyses were performed in duplo, and one person (laboratory engineer) prepared and analysed all the molecular data. Tris-buffered saline (100 µl) was added to each tube to elute the proteins from the paper point samples from AO sites and GCF respectively. The tubes were vortexed for 30 s and centrifuged and the paper points removed. Multianalyte profiling of the level of eluted bone markers (ACTH, DKK-1, IL-6, Insulin, Leptin, TNFa, OPG, OC, OPN, SOST, IL-1b, PTH, FGF-23) was performed on the Milliplex Human Bone Magnetic Bead Panel (HBN-51K, Merck Millipore, Merck KGaA, Darmstadt, Germany), and cytokines (EGF, IL-6, IL-8, MCP-1, MIP-1a VEGF) on the Human Cytokine Chemokine Magnetic Bead Panel (HCYTOMAG-60K, Merck Millipore, Merck KGaA, Darmstadt, Germany).
The following bone markers and cytokines were considered relevant and therefore selected for analysis: Dickkopf WNT signalling pathway inhibitor 1 (DKK-1), Osteoprotegerin (OPG), Osteocalcin (OC), Osteopontin (OPN), Sclerostin (SOST), TNF-α, IL-1ß, Interleukin 4 (IL-4), Interleukin 6 (IL-6), Interleukin 8 (IL-8), Epidermal growth factor (EGF), Monocyte chemoattractant protein 1 (MCP-1), Macrophage inflammatory protein 1 alpha (MIP-1α) and Vascular endothelial growth factor (VEGF). All analyses were performed according to the manufacturers’ protocols. Analyses showed that the following bone markers and cytokines had detectable values: EGF, IL-6, IL-8, MCP-1, MIP-1 and VEGF.
Data analysis
Clinical data were exported from InReach to the principal investigator’s database within UHN, consolidated and exported directly to SPSS (version 25.0, IBM Corp., Armonk, NY, USA). Chi-square and independent t-tests were used to analyse the data. Pearson’s correlation analysis was applied to estimate the correlation among data variables. The significance level was set to 5%. SPSS (version 25.0, IBM Corp., Armonk, NY) was used for data analysis.
Results
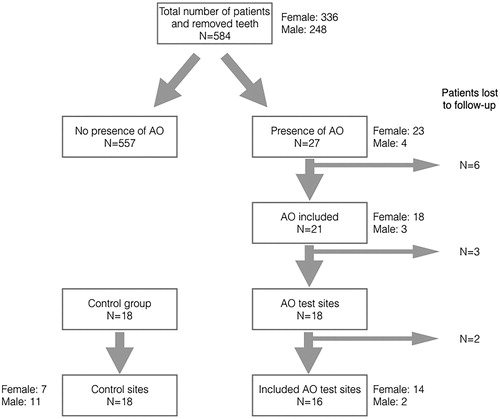
During the study period 584 third molars were removed in 445 patients, out of which 27 patients were diagnosed with alveolar osteitis. The relative incidence of alveolar osteitis was 4.6%. Residents in oral surgery performed most of the operations (88.9%, n = 24). Supervised dental students (final year of undergraduate training) performed two (7.4%) operations. One (3.7%) patient was treated by a staff surgeon. Mean surgical time was 22.9 min (range 8–45 min). Twenty-one patients (18 females, 3 males) consented to participate in the study and were followed prospectively until subjective symptoms resolved. The AO female:male ratio was 6:1. Patient demographics are presented in . A flow chart of patient inclusion is presented in .
Figure 1. Flowchart of distribution and inclusion of patients/alveoli (n) with alveolar osteitis (AO). Twenty-one of 27 patients/alveoli with AO were included, giving an inclusion of 78%. Eleven patients/alveoli were lost due to drop-out and technical errors: one did not want to participate, three were missing clinical recordings, two were not available for follow-up, three had missing test sites and two had tests with undetectable values.
Subjective symptoms and clinical findings
Among the 21 patients consenting to participate in the study, 86% were diagnosed with AO at recall one week after surgery. Three patients (14%) contacted DOS prior to their scheduled appointment due to intense pain: one patient at day four and two patients at day five. Pain was the chief complaint of all 21 patients. Swelling was considered neglectable by most patients (76%). One third of the patients complained of halitosis. Patient characteristics are presented in .
Table 2. Diagnostic and therapeutic characteristics of patients with alveolar osteitis (AO) and the control group without AO.
Clinical examination revealed that the drain was in place in 81% (n = 17) of the patients. In the remaining 19%, the drains had either been lost or accidentally removed by the patients. Nearly half of the patients (48%) had exposed bone in the alveolus. No severe infections were seen, but one patient had signs of surgical site infection.
Follow-up and treatment
Nearly all patients (95%) were followed until subjective symptoms had declined. Over half of the patients (57%) were discharged after two follow-up appointments. Mean follow-up time was 2.6 days for all patients (range 2–4), for female and male patients 2.7 and 2 days, respectively. One patient was not able to attend further follow-up and was discharged after diagnosis and initial treatment.
After drain removal, if the drain was still present, test sampling was performed according to the previously mentioned protocol. The alveolus was then irrigated with copious amounts of sterile saline (NaCl, 0.9%) and lightly packed with sterile gauze impregnated with oxytetracycline ointment. Two patients had one drop of eugenol added to the gauze for supplementary analgesic effect. Codeine (30 mg) and paracetamol (400 mg) tablets were prescribed for four patients (Paralgin Forte, Karo Pharma AS, Norway). Systemic antibiotics (penicillin V) were prescribed for one patient with surgical site infection.
Patients were discharged if pain and discomfort were acceptable and in correspondence with clinical findings of healing. After dismissal, patients were instructed to rinse the surgical site with sterile saline twice daily for seven days, using a blunt syringe. More than 50% were dismissed after the second appointment. Six patients were seen a third time and three patients were seen four times.
Pain
Pain was recorded on an 11- point scale (NRS-11) at patients’ postoperative appointments.
Mean pain score at first postoperative visit was 6.1 (SD 1.6) and after the initial treatment on the second 2.5 (SD ± 2.1) visit after. This reported reduction in pain was found to be statistically significant (p < .001).
Patients who underwent concomitant removal of an ipsilateral maxillary third molar (n = 4), reported higher pain scores initially than patients having mandibular third molar surgery only. This finding was statistically significant (p = .023).
No statistically significant differences were found between the first recorded pain score and the following variables; work status, smoking, indication, surgical complexity and intraoperative steroids (p > .05).
Analgesics
The mean number of days using analgesics was 8.6 (range 5–14, SD ± 2.9) for all patients. Male patients (n = 3) used analgesics for 10.3 (SD ± 3.8) days and female patients (n = 18) for 8.3 days (SD ± 2.8). The difference was not statistically significant (p = .281).
Absence from work/school
The mean number of days absent from work/school after surgery was 3.3 (range 0–14; SD ±3,7). Male patients (n = 3) reported being absent for 4.7 days (SD ± 5.5) and female patients (n = 18) for 3.1 days (SD ± 3.4). The difference was not statistically significant (p = .760).
Trismus
Five patients (24%) complained of trismus. Maximum inter-incisor opening (MIO) was recorded in 19 patients with a mean score of 29.8 mm. Patients complaining of trismus had lower MIO (24.2 mm) than those with no subjective trismus (35.1 mm). This finding was statistically significant (p = .021). Correlation analysis (Pearson Correlation) showed a negative correlation between patients’ complaint of trismus and MIO. No correlation between subjective trismus and pain scores (NRS-11) was found.
Bone markers and cytokines
Valid samples were obtained from the alveoli and from the most posterior point at the gingival sulcus on the contralateral side in 16 patients diagnosed with AO and from the alveoli of a healthy control group. The control group comprised 18 persons with a mean age of 27.8 years (SD 6.00) without signs or symptoms of AO one week after third molar surgery. The persons were treated by the same surgical protocol as previously described. The majority were males (N = 11 (61.1%)). There were significantly more female patients in the AO group compared with the control group (p = .002). Use of contraceptives was more commonly reported amongst females in the control group (p = .021). One person reported smoking (5.6%). Characteristics of patients with alveolar osteitis (AO) and persons in the control group without AO are given in .
There were significantly higher levels of all bone markers and cytokine levels at the AO site (alveoli), except for EGF, when compared with the un-operated contralateral site (gingival sulci). We could not identify any significant difference in cytokine concentration in the AO group at the two different time-points (postoperative control one week after surgery and subsequent clinical follow-up after 2–3 days). Furthermore, there were no significant differences in cytokine concentration in the alveoli in the AO group compared with the control group. Cytokine levels are presented in . There were no significant correlations between cytokine levels and clinical characteristics, except for MIP-1α and VEGF. When cytokine levels were correlated with MIO, a moderately significant correlation was seen for MIP-1α (r: 0.547, p = .035). VGEF showed a similar correlation to ROM (r: 0.515, p = .050).
Table 3. Local expression of cytokines in patients with alveolar osteitis (AO) compared with the patients’ un-operated contralateral side; and comparison between patients with AO and a control group without AO at the first postoperative control.
Discussion
The aims of the present study were to evaluate the relative incidence of AO after third molar surgery, the clinical course in patients diagnosed with AO, and to explore the local expression of some bone markers and cytokines in extraction sockets with AO.
The incidence of AO in the present investigation was low and comparable with previous studies using the same operative method including the use of a tetracycline drain [Citation5,Citation6]. The incidence of AO is also comparable with the results of other studies of third molar surgery [Citation15,Citation16]. Significantly more women than men developed AO. The population in our previous study regarding third molar removal had a female to male ratio of approximately 3:2 [Citation6], but the female to male ratio concerning alveolar osteitis was as high as 6:1 in the present study. A higher prevalence of AO is supported by other studies [Citation1,Citation4,Citation6]. Although female gender traditionally has been proposed to be a risk factor for developing AO, this is debated in a recent review [Citation17]. No statistical significance was observed for pain, analgesics and absence from work/school between the male and female patients with AO, but this may be related to the low number of men with AO in the present study.
The age of the patients has been discussed as an important factor in the development of AO [Citation16,Citation18–21]. The mean age of our patient population was 29.1 years and is somewhat lower than in other studies, possibly because our study also includes prophylactic removal of third molars [Citation22]. Thus, our low reported incidence of AO may reflect that the patient population included many younger patients.
Methods to reduce postoperative complications after third molar surgery have been thoroughly discussed [Citation23–26], and the systemic use of antibiotics seems to be quite a common approach in conjunction with third molar surgery. The use of local antibiotics as in our study, an oxytetracycline impregnated gauze drain, seemed to have a comparable positive effect in the reduction of AO as systemic antibiotics. In a study of third molar surgery patients, trismus as a complication was reported to be only 8%, whereas 72% reported pain [Citation16]. In our AO group, all patients reported high levels of pain, and trismus was seen in 24% of our patients, but there was no significant correlation between the two parameters.
In the present study we found that patients (with AO) reported a mean number of days (mean ± SD) requiring analgesics to be 8.6 ± 2.9, and absence from work/school to be 3.3 ± 3.7. These numbers are much higher compared with the findings (analgesics 3.8 ± 2.4 and absence 0.6 ± 1.2 days respectfully) in a previous study from our department on morbidity after third molar surgery [Citation5]. AO is a painful condition and patients are therefore more likely to use analgesics and stay at home for a longer period of time.
Our patients with AO needed a mean additional follow-up of 2.7 days (females) and 2 days (males), but the difference was not significant. Of the 21 patients with AO, most patients needed only one additional appointment, whereas six patients needed two, and three patients needed three additional appointments. For these patients, both pain and limitations of activity may have influenced their tab as emphasized in recent research [Citation16,Citation26].
The mechanism of AO is not clear, but inflammation may play an important role [Citation4,Citation5]. Cytokines most likely play an important role in the inflammation process, but what cytokines to focus on and how to most effectively sample them are not clear. Singh et al. [Citation8] found that three different medications (diclofenac, ketorolac and tramadol) all downregulated IL-6 after third molar surgery, but not specifically in relation to AO.
Our samples were obtained by inserting paper points in the alveolus and sampling the gingival crevicular fluid on the contralateral side in patients with AO and from the post-extraction alveolus in the control group. Bone markers and cytokines undergo a rapid degradation, and the collection method is therefore of great importance. The paper points, after placement in Eppendorf tubes, were immediately frozen at −20 °C after collection and later transferred to a high-performance laboratory freezer and stored at −80° C until analysis. Other ways of collecting cytokines may be developed in the future. The levels of cytokine expression in some studies of oral and maxillofacial tissues [Citation10–12] are not conclusive and cannot be directly compared with our study.
We found significant differences in the cytokine levels in five out of six examined cytokines when comparing the alveoli with the contralateral site. This has to the best of our knowledge not been described before. When correlating clinical parameters and cytokine levels, the only significant finding in our study was that MIP-1α was significantly higher in patients with reduced MIO. MIP-1α has mainly an inflammatory effect thus may play an important role in the development of trismus related to third molar surgery. Correlation analysis (Pearson Correlation) showed a negative correlation between patients’ complaint of trismus and MIO. In our opinion MIO can be regarded as a good measure for trismus.
In the present study, surgery was performed by professionals with different level of training; i.e. a few undergraduate dental students under supervision, oral surgery residents and staff surgeons in contrast to our previous study where only one surgeon (HØ) performed the surgical procedures [Citation6]. Christensen et al. [Citation27] found that when appropriately selected, patients operated on by dental students did not have more post-operative pain and complications, compared with patients operated by oral surgeons. Thus, it may be that the experience of the surgeon is of less importance in the development of AO [Citation17].
Conclusion
The relative incidence of AO after third molar surgery was low (4.6%). Patients diagnosed with AO required up to three additional follow-ups after the one-week follow-up, but more than half of the patients in the AO group needed only one additional appointment. In the present study, expression of cytokines was statistically significantly increased on the AO site compared with the non-AO site. Reduced levels of macrophage inflammatory proteins were correlated with reduced range of motion after third molar surgery. Further studies on the role of cytokines in the development of AO and refinement of the sampling methods are required.
Acknowledgements
The authors thank Dr. Paul Riordan (Write2Publish) for language corrections, Dr. Shoresh Afnan for participating in cytokine sampling and professor Per-Erik Isberg for assistance in the statistical analyses.
Disclosure statement
The authors report no conflict of interest.
References
- Fridrich KL, Olson RA. Alveolar osteitis following surgical removal of mandibular third molars. Anesth Prog. 1990;37:32–41.
- Vezeau PJ. Dental extraction wound management: medicating postextraction sockets. J Oral Maxillofac Surg. 2000;58:531–537.
- Caso A, Hung LK, Beirne OR. Prevention of alveolar osteitis with chlorhexidine: a meta-analytic review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:155–159.
- Veale B. Alveolar osteitis: a critical review of the aetiology and management. Oral Surgery. 2015;8:68–77.
- Øyri H, Bjørnland T, Barkvoll P, et al. Mandibular third molar surgery in 396 patients at a Norwegian university clinic: morbidity recorded after 1 week utilizing an e-infrastructure for clinical research. Acta Odontol Scand. 2016;74:148–154.
- Øyri H, Jonsdottir OH, Liaaen Jensen J, et al. The use of a tetracycline drain reduces alveolar osteitis: a randomized prospective trial of third molar surgery under local anaesthetics and without the use of systemic antibiotics. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;128:205–212.
- Hsing CH, Jhi-Joung Wang JJ. Clinical implication of perioperative inflammatory cytokine alteration. Acta Anaesthesiol Taiwan. 2015;53:23–28.
- Singh P, Rastogi S, Bansal M, et al. A prospective study to assess the levels of interleukin-6 following administration of diclofenac, ketorolac and tramadol after surgical removal of lower third molars. J Maxillofac Oral Surg. 2015;14:219–225.
- Blanchard F, Duplomb L, Baud'huin M, et al. The dual role of IL-6-type cytokines on bone remodeling and bone tumors. Cytokine Growth Factor Rev. 2009;20:19–28.
- Swathi B, Charitha M, Mandava D, et al. Evaluation of levels of proinflammatory chemokines MIP-1α and MIP-1β in gingival crevicular fluid of primary, mixed and permanent dentition. J Int Soc Prev Community Dent. 2019;9:205–209.
- Smane L, Pilmane M, Akota I. Local expression of inflammatory cytokines in a facial tissue of children with a cleft lip and palate. PoA. 2012;21:264–275.
- Olsen-Bergem H, Bjørnland T, Reseland JE. Temporomandibular joint pain is negatively correlated to TNF alpha and osteoprotegrin content in synovial fluid in patients with juvenile idiopathic arthritis. Endocrinol Metab Synd. 2014;03:145.
- Akota I, Alvsaker B, Bjørnland T. The effect of locally applied gauze drain impregnated with chlortetracycline ointment in mandibular third-molar surgery. Acta Odontol Scand. 1998;56:25–29.
- Daly B, Sharif MO, Newton T, et al. Local interventions for the management of alveolar osteitis (dry socket). Cochrane Database Syst Rev. 2012;12:CD006968.
- Mustafa NS, Kashmoola MA, Qader OAJA, et al. A retrospective study on the prevalence of dry socket in patients who attended a polyclinic for extraction. J Int Dent Med Res. 2018;11:527–531.
- Singh S. Comprehensive assessment of etiology, complications and quality of life as related to impacted third molar surgery: a questionnaire based original study. J Adv Med Dent Scie Res. 2019;7:74–78.
- Rakhshan V. Common risk factors of dry socket (alveolitis osteitis) following dental extraction: a brief narrative review. J Stomatol Oral Maxillofac Surg. 2018;119:407–411.
- Bruce RA, Frederickson GC, Small GS. Age of patients and morbidity associated with mandibular third molar surgery. J Am Dent Assoc. 1980;101:240–245.
- Fisher SE, Frame JW, Rout PG, et al. Factors affecting the onset and severity of pain following the surgical removal of unilateral impacted mandibular third molar teeth. Br Dent J. 1988;164:351–354.
- Chiapasco M, De Cicco L, Marrone G. Side effects and complications associated with third molar surgery. Oral Surg Oral Med Oral Pathol. 1993;76:412–420.
- Chaparro-Avendado AV, Perez-Garcia S, Valmaseda-Castellon E. Morbidity of third molar extraction in patients between 12 and 18 years of age. Med Oral Patol Oral Cir Bucal. 2005;10:422–431.
- Berge TI, Espeland LV, Klock K, et al. Profylaktisk fjerning av retinerte visdomstenner (Norwegian). SMM 10/2003. November 2003. Available from: https://www.fhi.no/globalassets/dokumenterfiler/rapporter/2009-og-eldre/smm-rapporter/smm-rapport_03-10_profylaktisk_fjerning_av_visdomstenner.pdf
- Coulthard P, Bailey E, Espsito M, et al. Surgical techniques for the removal of mandibular wisdom teeth. Cohrane Database Syst Rev. 2014;7:CD004345.
- Rodrígues Sánchez F, Rodrígues Andrés C, Artegoitia Calvo I. Does chlorhexidine prevent alveolar osteítis after third molar extractions? Systematic review and meta-analysis. J Oral Maxillofac Surg. 2017;75:901–914.
- Taberner-Vallverdú M, Sánchez-Garcés MÁ, Gay-Escoda C. Efficacy of different methods used for dry socket prevention and risk factor analysis: a systematic review. Med Oral Patol Oral Cir Bucal. 2017;22:e750–e758.
- Duarte-Rodrigues L, Miranda EFP, Souza TO, et al. Third molar removal and its impact on quality of life: systematic review and meta-analysis. Qual Life Res. 2018;27:2477–2489.
- Christensen J, Hauge Matzen L, Wenzel A, et al. Should removal of lower third molars be included in the pre-graduate curriculum for dental students? An evaluation of post-operative complications after student opérations. Acta Odontol Scand. 2012;70:42–48.
