Abstract
Objective
Osteogenesis imperfecta (OI) is a rare, hereditary disease affecting collagen type-1 in connective tissue. Collagen type-1 is a substantial component of dentine, and it is speculated, whether affected dentine could cause altered mesiodistal tooth dimension possibly affecting restorative treatment regimen. Therefore, the aim of the present study was to measure mesiodistal tooth dimensions in individuals with OI and compare them with healthy controls.
Materials and methods
Fifty-seven individuals aged 20–77 years with OI type 1–4 were included and 70 control patients aged 11–34 years were drawn from an orthodontic database. Mesiodistal tooth dimensions of all tooth types, except third molars, were measured in mm (two decimals) on digital 3 D-models of the tooth-bearing arches.
Results
Multilevel mixed-effects linear regression analysis showed that mesiodistal tooth dimension on average was 0.17 mm (95% CI = (−0.33; −0.01)) reduced for the OI group compared to controls. The analysis revealed variation between tooth types; incisors and first premolars were most affected and molars minimally affected.
Conclusions
The mesiodistal tooth dimension in individuals diagnosed with OI is significantly smaller compared to healthy controls, which should be taken into consideration in the restorative treatment planning of individuals with OI, although the magnitude of the deviation is relatively small. The results on mesiodistal tooth dimensions of the present controls may be used as a standard for comparisons in future studies on tooth dimensions.
Introduction
Osteogenesis imperfecta (OI) is a rare hereditary disease caused by mutations in collagen type-1 encoding genes [Citation1]. The disease affects the connective tissue, leading to a heterogeneous spectrum of clinical symptoms. The main clinical symptom is fragile bones with an increased risk of fractures early in life. In Denmark, the prevalence of OI is estimated to 11 per 100,000 [Citation2]. The major causes of OI are autosomal dominant mutations in one of the two genes, COL1A1 and COL1A2 [Citation3–5] that encode the α1(I) and the α2(I) chains of collagen type-1, respectively. Collagen type-1 is an important component of bone and dentine, and mutations can lead to either quantitative or qualitative abnormalities; i.e. a reduced volume of structurally normal collagen or an abnormally structured collagen, respectively [Citation6].
In addition to OI, some individuals are diagnosed with dentinogenesis imperfecta (DI) as part of the same genetic disorder. A diagnosis of DI is established clinically by a characteristic greyish-blue to brown discolouration (opalescent) as well as pulp obliterations of the teeth [Citation7,Citation8]. The discolouration is due to the underlying affected dentine only, though, the enamel is fragile given this abnormality. Structurally, dentine is composed of hydroxyapatite crystals and an organic phase composed almost entirely of collagen type-1 and water. Depending on the impact of DI, the impaired collagen may affect the outer contours of the tooth and the dimension of the tooth crown. Furthermore, malocclusion in terms of mandibular overjet and open bite is a common trait in patients with OI [Citation9–12]. In a recent study, individuals with OI were shown to have more severe malocclusions than a control group, including a potential increased risk of crowding of maxillary incisors [Citation13]. Previous studies have demonstrated crowding in the dental arches to be positively correlated with mesiodistal dimension of teeth [Citation14–17]. Thus, it might be hypothesised that the mesiodistal dimension of teeth is increased in patients with OI, compared to healthy individuals. This is in contradiction to the hypothesised reduced tooth dimension due to the impaired collagen. Potentially, deviations in dimension might have restorative implications.
The aim of the present study was to measure and compare the mesiodistal dimension of all tooth types, except third molars, in a group of individuals with OI and a group of healthy individuals (controls). The null-hypothesis was no difference in mesiodistal tooth dimension between the two groups.
Materials and methods
Study design
The Danish individuals with OI (n = 57), aged 20–77 years (mean age: 44 years (SD = 16), 29 males (51%)) were initially recruited by Hald et al. as part of a cross-sectional study [Citation18]. Forty-seven individuals appeared with OI type 1, one with OI type 3 and nine individuals with OI type 4. The dental examination, carried out as part of this study by Hald et al. [Citation18], included a regular clinical examination, clinical photos, intraoral digital radiographs, a digital panoramic radiograph (orthopan) and an alginate impression of both tooth-bearing arches as previously described [Citation19]. The examinations took place at Department of Dentistry and Oral Health, HEALTH, Aarhus University, during the period January 2011 to February 2013. The present study addressed digital 3 D-models of the tooth-bearing arches, originating from plaster models of the alginate impressions.
The individuals making up the control group (n = 70), 35 males and 35 females aged between 11 and 34 years (mean age: 18 years (SD = 4)), were drawn from a database holding orthodontic data material, including digital 3 D-models. After extraction, post-orthodontic treatment data were saved anonymously using random numbers, according to the regulations of patient data protection. Inclusion criterion was presence of a fully erupted permanent dentition, except for third molars. Exclusion criteria comprised inherited syndromes (e.g. Down syndrome) or dysplasias (e.g. Cleidocranial dysplasia), chronic disease (except for allergies), missing teeth (except third molars), other dental abnormalities as, e.g. peg-shaped lateral incisors [Citation20], known anomalous tooth size, a history of supernumerary teeth, grave tooth wear, comprehensive enamel hypoplasia, or major deviations in craniofacial morphology, such as severe maxillary or mandibulary retrognathism. Thus, the individuals in the control group had neutral molar occlusion. Existing radiographs were used to screen for the inclusion.
On tooth level, for all individuals (both the OI and the control group), exclusion followed where the mesial and/or distal surface had undergone dental treatment (e.g. a filling or a crown) or had caries, since the mesiodistal dimension of these teeth could not be determined.
Mesiodistal tooth dimensions were measured in mm with two decimals using O3DMTM software (OrtoLab, Częstochowa, Poland) on digital 3 D-models of the tooth-bearing arches, originating from plaster models of alginate impressions. All measurements were carried out in triplicate by a single examiner (KJT). The examiner was blinded concerning the existing data when measuring the models for the second and third time, but not blinded regarding the two groups (OI versus control).
The initial study [Citation18], involving the recruitment and examination of individuals with OI, was conducted in accordance with the guidelines of the Helsinki Declaration II, and approved by the Central Denmark Region Committees on Biomedical Research Ethics (protocol number: M-20100108). All adult individuals gave written informed consent to participate in the study. Considering minors (<18 years), a parent or guardian gave the written informed consent.
Data analysis
The data comprised triplicate mesiodistal tooth dimension measurements (in mm) for up to 28 teeth for each participant. Probability plots and histograms showed that the data on tooth type level, 28 types when differentiating between lefts and rights, could be described by normal distributions. The data had a hierarchical structure with four levels: group (n = 2), individual (n = 127), tooth type (n = 14), and side (n = 2) with three replicate measurements at the bottom level. The statistical analysis was based on a multilevel mixed-effects linear regression model (STATA 13.1; StataCorp, College Station, TX, USA). The initial model for mesiodistal tooth dimension included four components of variance (individual, tooth type, side, and replicate) and fixed effects of group (OI/control), gender (female/male), age at examination, and dimension for the 14 different tooth types categorised as I1sup, I2sup, I1inf, I2inf, Csup, Cinf, P1sup, P2sup, P1inf, P2inf, M1sup, M2sup, M1inf and M2inf. Further analyses assessed the interaction between group and jaw, the interaction between group and gender and the interaction between group and tooth type. The initial multilevel model was repeated including only individuals diagnosed with OI not DI (OI-non-DI group); comparing this group with healthy controls. Furthermore, in order to estimate a possible difference in mesiodistal tooth dimension in individuals diagnosed with both OI and DI compared to individuals diagnosed with OI only, the initial multilevel model was also repeated assessing three groups (OI-non-DI/OI-DI/control). In the statistical model, the mean mesiodistal tooth dimension, including the 95% confidence intervals, was estimated for each of the 14 tooth types in a 20-year-old female and a 20-year-old male control individual.
Results
The Danish individuals with OI comprised 57 participants. Sixteen individuals who originally participated in the dental part of the study by Hald and colleagues [Citation18,Citation19] were excluded in the present study owing to missing digital 3 D-models. The reasons for absent models were various logistic challenges. The control group comprised 70 individuals. Among the 127 individuals, the data on mesiodistal tooth dimension included 8778 observations, comprising triplicate measurements (in mm) for up to 28 teeth for each participant from the two groups (). Multilevel mixed-effects linear regression analysis showed that the difference in mesiodistal tooth dimension on average (across all tooth types) was statistically significant and −0.17 mm (95% CI = (−0.33; −0.01)) when comparing the OI group with the control group. The effect of gender was also significant, and the analysis returned −0.28 mm (95% CI = (−0.38; −0.18)) in favour of the men (). A significant effect of age was not found, although a tendency towards a smaller mesiodistal tooth dimension with increasing age was shown (). The estimated variance components showed greatest variance between tooth types, followed by individuals, then sides, and finally the lowest variation between triplicates ().
Table 1. Data at tooth type level.
Table 2. Multilevel analysis of mesiodistal tooth dimension.
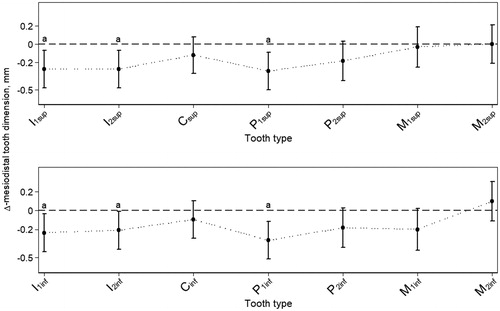
Multilevel mixed-effects linear regression analysis showed no significant interaction between group and gender (coefficient: 0.074, 95% CI = (−0.130; 0.278)). Also, multilevel mixed-effects linear regression analysis showed no significant interaction between group and jaw (coefficient: 0.005, 95% CI = (−0.070; 0.079)). The analysis disclosed significant variation of the effect of OI on mesiodistal tooth dimension at tooth type level (). Incisors and first premolars were significantly and most negatively affected, and the maxillary molars were hardly affected. The pattern of the effect of OI on mesiodistal tooth dimension between identical tooth types in the two jaws was almost similar, except for the molars showing more variation between jaws ().
Figure 1. Mesiodistal dimension across tooth types. Estimated mean effect of OI on mesiodistal tooth dimension (mm) at tooth type level, shown as the difference (Δ) between individuals with OI (n = 57) and controls (n = 70). The estimates hold both right and left side. Bars represent the 95% CIs. Letters (a) show tooth types where the effect of OI compared to controls is statistically significant. The dashed horizontal lines are for reference only (Δ = 0). The dotted lines connecting the means of the different tooth types illustrate the overall pattern of the effect of OI on mesiodistal tooth dimension in the two jaws. This pattern manifests almost alike in the two jaws. As multilevel mixed-effects linear regression analysis showed no significant interaction between group and gender, the data shown are pooled across gender.
Multilevel mixed-effects linear regression analysis, including individuals diagnosed with OI and not DI (OI-non-DI group, n = 46; excluding individuals diagnosed with DI, n = 11), returned a statistically non-significant difference in mesiodistal tooth dimension of −0.13 mm (95% CI = (−0.30; 0.04)) when comparing with the control group (n = 70).
Multilevel mixed-effects linear regression analysis, assessing the three groups OI-non-DI (n = 46), OI-DI (n = 11), and control (n = 70), returned a statistically significant difference in mesiodistal tooth dimension of 0.24 mm (95% CI = (0.04; 0.43)) when comparing the OI-non-DI group with the OI-DI group.
Considering all control data, mesiodistal tooth dimensions were estimated for 20-yr-old controls, categorised by gender and tooth type, showing smaller mesiodistal tooth dimension for all tooth types for women compared to men ().
Table 3. Mesiodistal tooth dimension in 20-year-old controls.
Six measurements (two teeth, in triplicate, from two different individuals) presented with high values. A sensitivity analysis was conducted in STATA (STATA 13.1, StataCorp) in which these measurements were omitted from the analysis. The results indicated no noticeable difference from the original analysis based on all individuals. Though, one tooth type (P2inf), which presented with a Δ-mesiodistal tooth dimension of non-significance (95% CI = (−0.392; 0.026); ), presented with borderline statistically significance after omission (95% CI = (−0.442; −0.003)). No further shifts concerning significance were reached. The analysis of the full data is reported in the present paper.
Discussion
The mesiodistal tooth dimension in individuals with osteogenesis imperfecta (OI) is reduced compared to healthy controls. The reduction is on average 0.17 mm, and apparently affects the different tooth types to a varying extent. The incisors and first premolars are affected the most, whereas the molars are barely affected. Analysis showed no significant interaction between group and gender nor between group and jaw, indicating an equal effect of OI on mesiodistal tooth dimension for men and women as well as for maxillary and mandibular teeth. The low triplicate measurement variance demonstrated a high level of reproducibility ().
An increased mesiodistal dimension of teeth to be associated with potential dental crowding in individuals with OI has to be discarded, as the study shows a reduced mesiodistal tooth dimension for the OI group, although of limited magnitude. Deviations in the dental arches and in dental occlusion in OI groups are supposedly mainly associated to abnormalities in craniofacial growth and development as previously suggested [Citation21].
The alternative hypothesis saying that OI is associated with reduced tooth dimension due to impaired collagen seems to be justified in our study. It is suggested that the degree of reduced tooth size is associated with the type of collagen abnormality, which is either qualitative or quantitative [Citation6]. The results indicate that an individual diagnosed with both OI and DI having qualitatively abnormal collagen might have harder affected dentine than an individual diagnosed with OI only, leading to narrower teeth.
The present results on mesiodistal tooth dimension in healthy 20-yr-old controls corroborate Townsend’s results from 1983 [Citation22]. The age of twenty was chosen to match Townsend’s control group comprising 265 children and young adults [Citation22]. Considering gender dimorphism, both studies show that women’s teeth in general are smaller than men’s teeth. This finding is in line with men exhibiting a higher frequency of hyperdontia and macrodontia than women, and women having a higher prevalence of microdontia and hypodontia than men [Citation20]. The present gender difference () is in the same order of magnitude as Townsend found [Citation22], with women’s teeth being in average 2% smaller in the mesiodistal dimension compared to men’s teeth. Townsend made measurements on plaster models using a dial calliper [Citation22] as compared to the present digital measurements on 3 D-models. A digital approach was chosen since a recent study has shown that measurements on digital models present with less variation than measurements on plaster models [Citation23].
Importantly, since a (non-significant) tendency towards a smaller mesiodistal tooth dimension with increasing age was shown (), and the mean age between the individuals with OI and controls differed considerably, the analyses adjusted for age. However, the overall estimated effect of age on mesiodistal tooth dimension was a negligible, statistically insignificant reduction (). This is in line with the slow continuous physiological attrition (approximal wear) taking place lifelong reducing the mesiodistal tooth dimension [Citation20].
Interestingly, the findings in the present study, demonstrating different effect of OI on the mesiodistal tooth dimensions for the different tooth types, do not follow ‘the normal reduction pattern’. Second premolars and maxillary lateral incisors are the tooth types most often prone to bypass development (be congenitally missing) leading to the diagnosis dental aplasia/hypodontia. Additionally, maxillary lateral incisors appear in reduced form (isolated microdontia) nominated peg-shaped laterals [Citation20]. Thus, the mesiodistal dimension is determined by other factors than lack of teeth or tooth reduction. A previous study, using a mouse model of chain deficiency OI, demonstrated that morphological abnormalities were more pronounced in incisors than in molars and depended on the dosage of the mutant allele [Citation24]. This finding might be associated with the impact of OI on variation in tooth dimension according to tooth type demonstrated in the present study on humans.
According to Kjaer [Citation25], the development of the jaws, including teeth, occur in a number of separate developmental fields. Each of the developmental fields has a common embryological origin, and each of them contains specified tooth types. In the present OI group, the size of molars, in contrast to other tooth types, is nearly unaffected. This observation might be described as a minimal or absent impact of OI on tooth dimension in the palatine developmental field as well as in the posterior part of the mandibular developmental field. The reason for the differentiated impact according to developmental fields remains unclear. However, selection bias could also be involved in the scant effect on the molars, masking the true effect of OI on molars. Considering selection bias, the teeth mostly affected by OI would already have undergone dental treatment or may even have been extracted and consequently not included in the study. Hence, we speculate that the molars in individuals with OI are worse affected than we were able to show.
Some individuals with OI included in the study were closely related (two or three persons from the same family; in total 11 families). We did not adjust for this in the analysis.
Keeping in mind that heterogeneity appears for OI, the representation of the different OI types might be of importance for the results, since individuals with a diagnosis of OI type 3 and OI type 4 are more often diagnosed with DI than individuals with OI type 1 [Citation19,Citation26]. Examining dot plots of the data on mesiodistal tooth dimension with emphasis on the different OI types, did not indicate any pattern (data not shown).
No attempt was made to blind the examiner regarding the two groups (OI versus control) owing to the characteristic tooth morphology of teeth in individuals with OI, and the obvious load of dental treatment in the OI group not found in the control group; both as an effect of the clinical symptoms of OI [Citation19] and the age difference between the groups.
Considering a possible impact of OI on mesiodistal tooth dimension in individuals diagnosed with OI and not DI (OI-non-DI group) compared to healthy controls, an analysis excluding individuals diagnosed with (both OI and) DI was conducted. The analysis returned a statistically non-significant difference in mesiodistal tooth dimension of −0.13 mm when comparing the OI-non-DI group with the control group. Although not statistically significant, the result implies by the skewed confidence interval that the mesiodistal tooth dimension is affected in individuals diagnosed with OI not diagnosed clinically with the tooth-related diagnosis DI compared to healthy controls.
We speculated whether the effect of OI on the mesiodistal tooth dimension in individuals diagnosed with both OI and DI, compared to individuals diagnosed with OI only, would show. The related analysis returned a statistically significant difference of 0.24 mm showing that the mesiodistal tooth dimension of individuals diagnosed with both OI and DI indeed is more affected than in individuals diagnosed with OI only; the estimate must, however, be interpreted keeping in mind the low number of individuals in the OI-DI group (n = 11).
In conclusion, the estimated difference in mesiodistal tooth dimension in individuals diagnosed with OI compared to healthy controls sums up to approximately 2.5 mm in each of the tooth-bearing arches. The impact of OI on mesiodistal tooth dimension is more pronounced in individuals diagnosed with both OI and DI compared to individuals diagnoses with OI alone. The results add to our knowledge about the clinical symptoms of OI, but are not expected to clinically affect individuals with OI substantially. However, despite the magnitude of the deviation being relatively small, the reduced mesiodistal dimension might be of relevance for the dentist when carrying out certain types of restorative dental treatment. The results on mesiodistal tooth dimensions of the present control group may be used as a standard for comparisons in future studies on tooth dimensions.
Acknowledgements
The authors would like to thank dental hygienist Rikke Munk Frandsen, Department of Oral and Maxillofacial Surgery, Aarhus University Hospital, and dentist Malene Hørnø Schmidt, Aarhus Municipal Dental Service, for their great effort in conducting dental clinical examinations. In addition, a special thanks to Professor Bente Lomholt Langdahl and consultant in endocrinology Torben Harsløf, Department of Endocrinology and Internal Medicine, for their contribution to establishing the study population comprising individuals with osteogenesis imperfecta.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet. 2004;363(9418):1377–1385.
- Andersen PE, Jr., Hauge M. Congenital generalised bone dysplasias: a clinical, radiological, and epidemiological survey. J Med Genet. 1989;26(1):37–44.
- Forlino A, Cabral WA, Barnes AM, et al. New perspectives on osteogenesis imperfecta. Nat Rev Endocrinol. 2011;7(9):540–557.
- Marini JC, Forlino A, Bachinger HP, et al. Osteogenesis imperfecta. Nature Rev Dis Primers. 2017;3:17052.
- Van Dijk FS, Sillence DO. Osteogenesis imperfecta: clinical diagnosis, nomenclature and severity assessment. Am J Med Genet A. 2014;164A(6):1470–1481.
- Lund AM, Muller J, Skovby F. Anthropometry of patients with osteogenesis imperfecta. Arch Dis Child. 1999;80(6):524–528.
- Barron MJ, McDonnell ST, Mackie I, et al. Hereditary dentine disorders: dentinogenesis imperfecta and dentine dysplasia. Orphanet J Rare Dis. 2008;3:31.
- Levin LS. The dentition in the osteogenesis imperfecta syndromes. Clin Orthop Relat Res. 1981;159:64–74.
- Stenvik A, Larheim TA, Storhaug K. Incisor and jaw relationship in 27 persons with osteogenesis imperfecta. Scand J Dent Res. 1985;93(1):56–60.
- Jensen BL, Lund AM. Osteogenesis imperfecta: clinical, cephalometric, and biochemical investigations of OI types I, III, and IV. J Craniofac Genet Dev Biol. 1997;17(3):121–132.
- Saeves R, Lande Wekre L, Ambjørnsen E, et al. Oral findings in adults with osteogenesis imperfecta. Spec Care Dentist. 2009;29(2):102–108.
- Bendixen KH, Gjørup H, Baad-Hansen L, et al. Temporomandibular disorders and psychosocial status in osteogenesis imperfecta - a cross-sectional study. BMC Oral Health. 2018;18(1):35.
- Rizkallah J, Schwartz S, Rauch F, et al. Evaluation of the severity of malocclusions in children affected by osteogenesis imperfecta with the peer assessment rating and discrepancy indexes. Am J Orthod Dentofacial Orthop. 2013;143(3):336–341.
- Bernabé E, Flores-Mir C. Dental morphology and crowding. A multivariate approach. Angle Orthod. 2006;76(1):20–25.
- Poosti M, Jalali T. Tooth size and arch dimension in uncrowded versus crowded Class I malocclusions. J Contemp Dent Pract. 2007;8(3):45–52.
- Puri N, Pradhan KL, Chandna A, et al. Biometric study of tooth size in normal, crowded, and spaced permanent dentitions. Am J Orthod Dentofacial Orthop. 2007;132(3):279.e7.
- Das PJ, Dkhar W, Pradhan A. An evaluation of dental crowding in relation to the mesiodistal crown widths and arch dimensions in southern Indian population. J Clin Diagn Res. 2017;11:TC10–TC13.
- Hald JD, Folkestad L, Harslof T, et al. Skeletal phenotypes in adult patients with osteogenesis imperfecta-correlations with COL1A1/COL1A2 genotype and collagen structure. Osteoporos Int. 2016;27(11):3331–3341.
- Thuesen KJ, Gjorup H, Hald JD, et al. The dental perspective on osteogenesis imperfecta in a Danish adult population. BMC Oral Health. 2018;18(1):175.
- Neville BW, Damm DD, Allen CM, et al. Abnormalities of the teeth. In: Neville BW, Damm DD, Allen CM, Bouquot JE, editors. Oral and maxillofacial pathology. Philadelphia (PA): W.B. Saunders Company; 1995. p. 44–95.
- Waltimo-Siren J, Kolkka M, Pynnonen S, et al. Craniofacial features in osteogenesis imperfecta: a cephalometric study. Am J Med Genet A. 2005;133A(2):142–150.
- Townsend GC. Tooth size in children and young adults with trisomy 21 (Down) syndrome. Arch Oral Biol. 1983;28(2):159–166.
- Dalstra M, Melsen B. From alginate impressions to digital virtual models: accuracy and reproducibility. J Orthod. 2009;36(1):36–41. discussion 14.
- Lopez Franco GE, Huang A, Pleshko Camacho N, et al. Dental phenotype of the col1a2(oim) mutation: DI is present in both homozygotes and heterozygotes. Bone. 2005;36(6):1039–1046.
- Kjaer I. Orthodontics and foetal pathology: a personal view on craniofacial patterning. Eur J Orthod. 2010;32:140–147.
- Andersson K, Dahllof G, Lindahl K, et al. Mutations in COL1A1 and COL1A2 and dental aberrations in children and adolescents with osteogenesis imperfecta - a retrospective cohort study. PLoS One. 2017;12(5):e0176466.
