Abstract
Objective: This is a rapid systematic review concerning taste alterations in 27,687 individuals infected with SARS-CoV-2, published in the worldwide literature.
Material and methods: Of the 485 articles recovered, 67 eligible studies (27,687 confirmed COVID-19 cases) were included in this analysis. We analysed the prevalence of the taste alterations in patients considering the country of origin of the studies.
Results: The results show strong important differences in the overall reported prevalence of taste alterations among the different countries (from 11% of Korea to 88.8% of Belgium).
Conclusions: These data highlight that there is a different geographical distribution of taste alterations in COVID-19 patients. Gustatory dysfunction seems to be an understudied symptom of COVID-19 and this may explain the inconsistencies of diagnostic criteria for COVID-19 case definition. Furthermore, this diagnostic underestimation can lead to an increased risk of contagion for the whole population and for the working classes most at risk, including the dental one.
Introduction
Starting from December 2019 in Wuhan, China, the new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread all over the world causing a pandemic of coronavirus disease 2019 (COVID-19). As at September 2020, SARS-CoV-2 has caused over one million deaths [Citation1]. The virus mainly targets the respiratory system causing cough, fever, and difficulty in breathing [Citation2]. Most cases result in mild symptoms, but some patients evolve to severe pneumonia and multi-organ failure. Among the most common symptoms (independent or in association with other manifestations), recent literature presents ageusia (loss of taste), with or without anosmia (loss of smell) [Citation3]. Loss of taste is now a distinguishing symptoms of COVID-19 with a high predictive value [Citation4]. The European Centre for Disease Prevention and Control (EDCD) was one of the first public health agencies that include sudden onset of ageusia, dysgeusia or anosmia as main clinical criteria for identifying probable COVID-19 cases [Citation5]. Anyway, until August 2020 these symptoms were not used by all clinical trials to identify COVID-19 cases and to prioritise diagnostic tests. Afterwards, in the USA, the Centres for Disease Control and Prevention (CDC) have modified the definition of COVID-19 case on 5 August, including taste disorders as a main clinical criterion for diagnosis [Citation6]. Soon after, the World Health Organisation (WHO) COVID-19 updated the case definition of COVID-19 and included onset of ageusia as suggestive of a probable COVID-19 case [Citation7].
The different geographical distribution of the prevalence of these symptoms may show differences in the method used to identify cases of national or local public health agencies, which may in turn affect the inclusion of taste assessment in COVID research. For this reason, we carried out a rapid systematic review with the aim of collecting comprehensive data on the taste alterations prevalence worldwide and, precisely, in 16 countries. Further, we investigated the predominance of this topic in the overall COVID-19 literature.
Methods
Study design
This study was performed in compliance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines and it used a rapid review method because of time constraints [Citation8] following the criteria for a restricted review. In this paper, were met the minimum requirements for completing a rapid systematic review [Citation9]. Accordingly, the search was performed by three investigators (N.C., M.E.B., L.L.M.), while N.C. and M.E.B carried out the verification of a sample of full texts for accuracy of title and abstract screening and data extraction. Key terms used were (COVID or COVID-19 or SARS-CoV-2) AND (hypogeusia or dysgeusia or gustatory or taste or ageusia). The search was conducted in PubMed/MEDLINE and COCHRANE Library using advanced search (all fields) tool. A second search was performed in order to correlate the number of papers aboutCOVID-19 in each country with the number of papers about taste alterations due to COVID-19 in each country. For this reason, we performed a new search on PubMed/MEDLINE using the keywords COVID-19, taste and name of each country.
Study selection and data extraction
The criteria of exclusion were: duplicate publications, irrelevant articles, articles not in English language, studies that not clearly confirmed infection status, studies that did not analyse gustatory outcomes in each patient, case reports and review/systematic reviews. Studies that used telephone surveys or Apps were only included in this paper if the patients had a confirmed COVID-19 diagnosis. Studies that reported cases from two or more geographical areas were included only if information from individual countries was available. The primary outcome was to establish the gustatory alterations (ageusia, hypogeusia, dysgeusia) prevalence in confirmed COVID-19 cases worldwide and in different geographical areas; the secondary aim was to assess a pattern of taste alterations in published cases. No limits were posted on the cohorts dimension to ensure a comprehensive search and to identify the maximum amount of useful articles.
Results
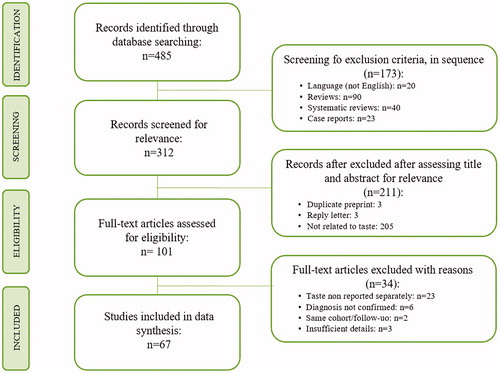
Our first search detected 485 studies and 101 full text articles, that meet the inclusion criteria, were evaluated. Of these 91 papers, 67 were included in the data synthesis (). Studies derived from 16 different countries and from multi-national cooperations. Most studies were from Europe (n = 45), followed by Middle East (n = 8), North America and South America (n = 9), East Asia (n = 6) and Africa (n = 1). Precisely: 1 study is from Belgium, 9 from France (of which 1 studying France, Germany and China), 4 from Germany, 22 from Italy, 1 from Poland, 7 from Spain, 2 from UK (of which 1 studying USA too), 1 from Brazil, 2 from Canada, 6 from USA, 2 from Israel, 1 from Qatar, 5 from Turkey, 3 from China, 1 from Hong Kong, 2 from Korea and 1 from Somalia.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart of study selection process.
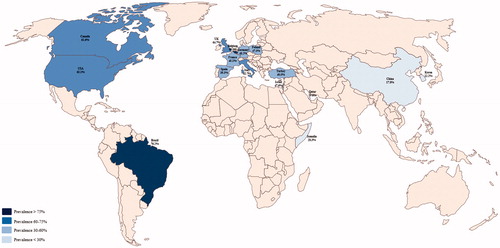
The prevalence of gustatory dysfunction in COVID-19 positive patients differed in different countries. Study from Belgium reported a prevalence of 88.8% [Citation10]; in France the prevalence reported ranges between 24% [Citation11] and 65% [Citation12] and in Germany between 14% [Citation13] and 69.4% [Citation14]; in Italy it varies from 25.4% to 96%; a polish study reported 47.5% [Citation15] of prevalence; in Spain and UK the prevalence respectively ranges from 6.2% [Citation16] to 70% [Citation17] and from 63.1% [Citation18] to 64.8% [Citation19]. Therefore, in Europe the prevalence reported is 48% (10033 out of 20859 patients with GD). The only one study from Brazil reported a prevalence of 76.2%, the two studies from Canada showed a prevalence range from 57.1% [Citation20] to 63.4% [Citation21] while studies from USA reported values from 10% [Citation22] to 71% [Citation23]. Thus, in America the GD is found in 1212 subjects on 1789 COVID-19 patients (67.8%). In Middle East, the reported average GD prevalence is 38.8% (386 out 994 patients); precisely: in Israel is between 33.3% [Citation24] and 52% [Citation25], in Qatar about 19.8% [Citation26] and in Turkey from 27.2% [Citation27] to 71.9% [Citation28]. In the East of the Asian continent is reported an average prevalence of 13.1% (522 out of 3985 patients COVID-19 positive): in China values range is from 5.6% [Citation29] to 38.4% [Citation30], Hong Kong Special Administrative Region of the People’s Republic of China (HKSAR) showed 43.4% [Citation31] of prevalence while in Korea GD is reported in 11–33.7% [Citation32,Citation33] out of COVID-19 patients. On the African continent, the literature reported a Somali study showing a prevalence of 28.3% [Citation34]. Therefore, worldwide 12.170 out of 27.687 confirmed COVID-19 cases (44%) reported subjective and/or objective gustatory dysfunction (GD) ().
Table 1. Studies included for the evaluation of gustatory dysfunction (GD).
In , study populations and average prevalence are graphically represented.
In we reported data concerning our second search regarding the ratio between articles concerning COVID-19 and those assessing taste alteration in COVID-19 confirmed cases, in the countries analysed in this manuscript.
Table 2. Ratio between papers about COVID-19 and papers about taste alteration in COVID-19 confirmed cases, in each country analysed in this manuscript.
Discussion
Taste alteration in COVID-19 positive patients could be an important factor in disease diagnosis [Citation76]. The results of this pilot search on international literature shows that there are different geographical patterns of Gustatory Dysfunction in patients with confirmed SARS-CoV-2 infection. In fact, taste alteration ranges from 13.1% in East Asia to 67.8% in American continent. However, we found remarkable differences in gustatory dysfunction also among countries of the same continent. For example, in Europe the average prevalence ranges from 39.3% of Spain to 88.8% of Belgium.
Several factors may be responsible for this considerable variability of prevalence in different countries. Among these, one of the most important is probably the different times in which patients were examined in the different studies and the consequent awareness of the presence of GD in COVID-19 patients.
The first study in international literature reporting a 5.6% prevalence of hypogeusia was a Chinese case series published on 10 April 2020 [Citation29]. Contrary, a recent meta-analysis evaluating taste and smell alterations highlighted that almost half of COVID-19 positive patients had these symptoms and, above all, that 15% of patients had olfactory and gustatory dysfunctions as their initial symptoms [Citation77]. For these reasons, most studies completed before April did not focussed on taste alterations and probably this can explain the low rate of GD prevalence in Chinese studies, the country first hit by the virus, and the high GD prevalence in countries where the pandemic has lately appeared, such as Brazil (76.2%).
Successively, the researchers started to examine GD in their patients. In fact, Aziz et al., in their evaluation, showed that about half of the COVID-19 patients (49.8%) had alteration of taste sensation [Citation78]. Our data are according to the current and relevant literature on the worldwide prevalence of COVID-related GD: out of 27.687 confirmed COVID-19 cases, 12.170 (44%) reported GD.
Another possible problem is that most studies are retrospective, cross-sectional, and observational, so, in our analysis, recollection bias may be present. The alteration of gustatory sense may not be reported when there are other severe symptoms, such as fever, dyspnoea, and productive cough. This absence of diagnostic data could explain the lack of association between taste alterations and COVID-19 in the first studies published in the period February–March 2020. Precisely for what has just been said, the real prevalence of taste alterations (in any form) may be more significant than actually reported [Citation78]. In fact, in the it is evident that several reports/studies on COVID-19 and related symptoms did not examine the GD. For example, only 56 studies from US considered GD versus 12230 studies on COVID-19 or in China 17 out of 6087 studies.
The difficulty in defining and measuring the grade of GD was another important variable. In fact, the altered taste has not been validated and the term ‘dysgeusia’ is not universally recognised. Furthermore, the diffusion of new knowledge about it and, therefore, the recognition of taste alterations as an early symptom of COVID-19 could have led to an increase in the reporting of this symptom by patients [Citation19]. Differently from other analyzes [Citation79] our study evaluated the different geographic position of the papers concerning the alterations of taste, rather than the ethnicity of the same. Although this type of study fails to identify the ethnic/genetic predispositions of the onset of this particular symptom and, more generally, of the infection, we think that our analysis can be considered suitable for studying the clinical manifestations of the disease in different countries. and, consequently, to help public health surveillance bodies in implementing the right measures to contain the infectious risk.
Another probable bias could be the heterogeneity of the examined studies, due to different sampling of examined studies in this review (direct data collection from hospitalised patients, data collection by telephonic survey, retrospective studies).
Furthermore, many studies, although valid, were excluded in this review because they analysed alterations in taste and smell as a single symptom and not as separate variables.
Furthermore, most of the studies in the literature are based on subjective (self-reported) impressions of patients and only a small number of studies used structured (objective) tests to assess GD. Subsequently, a comparison was made between subjective and structured (objective) gustatory functions and no significant differences were found [Citation80]. Hence, it can be said that self-reported alterations in taste can be considered a reliable parameter for studying the prevalence of this condition in COVID-19 patients.
Conclusion
Ageusia/hypogeusia/dysgeusia seem to be important and sometimes early symptoms inCOVID-19. For this reason, it is useful to detect the taste alterations by dentists, who can be an active part in identifying and diagnosing positive COVID-19 cases early in the near future.
Author contributions
N.C.: Contributed to design, data acquisition and interpretation and critically revised the manuscript
M.E.B.: Contributed to design, data acquisition and interpretation, drafted and critically revised the manuscript
E.L.M: Contributed to design, drafted and critically revised the manuscript
A.P.C.: Contributed to design, drafted and critically revised the manuscript
L.L.M.: Contributed to design, data acquisition and interpretation and critically revised the manuscript
All authors have given their final approval and they agree to be accountable for all aspects of the manuscript.
Acknowledgments
The authors have received no financial support in the writing of this paper, and they declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- WHO. WHO Coronavirus Disease (COVID-19) Dashboard 2020. Available from: https://covid19.who.int/
- Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179.
- Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. Apr 30
- Dawson P, Rabold EM, Laws RL, et al. Loss of taste and smell as distinguishing symptoms of Covid-19. Clin Infect Dis. 2020. DOI:10.1093/cid/ciaa799
- ECDC. Case definition for coronavirus disease 2019 (COVID-19) 2020. Available from: https://www.ecdc.europa.eu/en/covid-19/surveillance/case-definition.
- CDC. Coronavirus Disease 2019 (COVID-19) 2020 Interim Case Definition, Approved August 5, 2020. Available from: https://wwwn.cdc.gov/nndss/conditions/coronavirus-disease-2019-covid-19/case-definition/2020/08/05/
- WHO. WHO COVID-19 Case definition. [Published 2020 August 7].
- Khangura S, Konnyu K, Cushman R, et al. Evidence summaries: the evolution of a rapid review approach. Syst Rev. 2012;1:10.
- Pluddemann A, Aronson JK, Onakpoya I, et al. Redefining rapid reviews: a flexible framework for restricted systematic reviews. BMJ Evid Based Med. 2018;23(6):201–203.
- Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251–2261.
- Benezit F, Le Turnier P, Declerck C, et al. Utility of hyposmia and hypogeusia for the diagnosis of COVID-19. Lancet Infect Dis. 2020;20(9):1014–1015.
- Zayet S, Klopfenstein T, Mercier J, et al. Contribution of anosmia and dysgeusia for diagnostic of COVID-19 in outpatients. Infection. 2020. DOI:10.1007/s15010-020-01442-3
- Fistera D, Pabst D, Hartl A, et al. Separating the wheat from the chaff-COVID-19 in a German emergency department: a case-control study. Int J Emerg Med. 2020;13(1):44.
- Luers JC, Rokohl AC, Loreck N, et al. Olfactory and gustatory dysfunction in coronavirus disease 19 (COVID-19). Clin Infect Dis. 2020;71(16):2262–2264.
- Sierpinski R, Pinkas J, Jankowski M, et al. Sex differences in the frequency of gastrointestinal symptoms and olfactory or taste disorders in 1942 nonhospitalized patients with coronavirus disease 2019 (COVID-19). Pol Arch Intern Med. 2020;130(6):501–505.
- Romero-Sanchez CM, Diaz-Maroto I, Fernandez-Diaz E, et al. Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID registry. Neurology. 2020;95(8):e1060–e1070.
- Villarreal IM, Morato M, Martinez-RuizCoello M, et al. Olfactory and taste disorders in healthcare workers with COVID-19 infection. Eur Arch Otorhinolaryngol. 2020;1–5.
- Patel A, Charani E, Ariyanayagam D, et al. New-onset anosmia and ageusia in adult patients diagnosed with SARS-CoV-2 infection. Clin Microbiol Infect. 2020;26(9):1236–1241.
- Menni C, Valdes AM, Freidin MB, et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. 2020;26(7):1037–1040.
- Lee DJ, Lockwood J, Das P, et al. Self-reported anosmia and dysgeusia as key symptoms of coronavirus disease 2019. CJEM. 2020;22(5):595–598.
- Carignan A, Valiquette L, Grenier C, et al. Anosmia and dysgeusia associated with SARS-CoV-2 infection: an age-matched case-control study. CMAJ. 2020;192(26):E702–E707.
- Pinna P, Grewal P, Hall JP, et al. Neurological manifestations and COVID-19: experiences from a tertiary care center at the Frontline. J Neurol Sci. 2020;415:116969.
- Yan CH, Faraji F, Prajapati DP, et al. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. 2020;10(7):806–813.
- Levinson R, Elbaz M, Ben-Ami R, et al. Time course of anosmia and dysgeusia in patients with mild SARS-CoV-2 infection. Infect Dis (Lond). 2020;52(8):600–602.
- Biadsee A, Biadsee A, Kassem F, et al. Olfactory and oral manifestations of COVID-19: sex-related symptoms-a potential pathway to early diagnosis. Otolaryngol Head Neck Surg. 2020;163(4):722–728.
- Al-Ani RM, Acharya D. Prevalence of anosmia and ageusia in patients with COVID-19 at a primary health center, Doha, Qatar. Indian J Otolaryngol Head Neck Surg. 2020;1–7.
- Altin F, Cingi C, Uzun T, et al. Olfactory and gustatory abnormalities in COVID-19 cases. Eur Arch Otorhinolaryngol. 2020;277(10):2775–2781.
- Sayin I, Yazici ZM. Taste and smell impairment in SARS-CoV-2 recovers early and spontaneously: experimental data strongly linked to clinical data. ACS Chem Neurosci. 2020;11(14):2031–2033.
- Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690.
- Liang Y, Xu J, Chu M, et al. Neurosensory dysfunction: a diagnostic marker of early COVID-19. Int J Infect Dis. 2020;98:347–352.
- Cho RHW, To ZWH, Yeung ZWC, et al. COVID-19 viral load in the severity of and recovery from olfactory and gustatory dysfunction. Laryngoscope. 2020;130(11):2680–2685.
- Lee Y, Min P, Lee S, et al. Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J Korean Med Sci. 2020;35(18):e174.
- Kim GU, Kim MJ, Ra SH, et al. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19. Clin Microbiol Infect. 2020;26(7):948 e1–948 e3.
- Farah Yusuf Mohamud M, Garad Mohamed Y, Mohamed Ali A, et al. Loss of taste and smell are common clinical characteristics of patients with COVID-19 in Somalia: a retrospective double centre study. Infect Drug Resist. 2020;13:2631–2635.
- Lapostolle F, Schneider E, Vianu I, et al. Clinical features of 1487 COVID-19 patients with outpatient management in the Greater Paris: the COVID-call study. Intern Emerg Med. 2020;15(5):813–817.
- Zayet S, Kadiane-Oussou NJ, Lepiller Q, et al. Clinical features of COVID-19 and influenza: a comparative study on Nord Franche-Comte cluster. Microbes Infect. 2020;22(9):481–488.
- Tudrej B, Sebo P, Lourdaux J, et al. Self-reported loss of smell and taste in SARS-CoV-2 patients: primary care data to guide future early detection strategies. J Gen Intern Med. 2020;35(8):2502–2504.
- Poncet-Megemont L, Paris P, Tronchere A, et al. High prevalence of headaches during covid-19 infection: a retrospective cohort study. Headache. 2020. DOI:10.1111/head.13923
- Chary E, Carsuzaa F, Trijolet JP, et al. Prevalence and recovery from olfactory and gustatory dysfunctions in covid-19 infection: a prospective multicenter study. Am J Rhinol Allergy. 2020;34(5):686–693.
- Klopfenstein T, Zahra H, Kadiane-Oussou NJ, et al. New loss of smell and taste: uncommon symptoms in COVID-19 patients on Nord Franche-Comte cluster, France. Int J Infect Dis. 2020;100:117–122.
- Qiu C, Cui C, Hautefort C, et al. Olfactory and gustatory dysfunction as an early identifier of covid-19 in adults and children: an international multicenter study. Otolaryngol Head Neck Surg. 2020;163(4):714–721.
- Hintschich CA, Wenzel JJ, Hummel T, et al. Psychophysical tests reveal impaired olfaction but preserved gustation in COVID-19 patients. Int Forum Allergy Rhinol. 2020; Sep10(9):1105–1107.
- Paderno A, Schreiber A, Grammatica A, et al. Smell and taste alterations in COVID-19: a cross-sectional analysis of different cohorts. Int Forum Allergy Rhinol. 2020;10(8):955–962.
- Vaira LA, Deiana G, Fois AG, et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: single-center experience on 72 cases. Head Neck. 2020;42(6):1252–1258.
- Petrocelli M, Ruggiero F, Baietti AM, et al. Remote psychophysical evaluation of olfactory and gustatory functions in early-stage coronavirus disease 2019 patients: the Bologna experience of 300 cases. J Laryngol Otol. 2020;134(7):571–576.
- Giacomelli A, Pezzati L, Conti F, et al. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis. 2020;71(15):889–890.
- Vaira LA, Hopkins C, Petrocelli M, et al. Do olfactory and gustatory psychophysical scores have prognostic value in COVID-19 patients? A prospective study of 106 patients. J Otolaryngol Head Neck Surg. 2020;49(1):56.
- Mercante G, Ferreli F, De Virgilio A, et al. Prevalence of taste and smell dysfunction in coronavirus disease 2019. JAMA Otolaryngol Head Neck Surg. 2020;146(8):723.
- Liguori C, Pierantozzi M, Spanetta M, et al. Subjective neurological symptoms frequently occur in patients with SARS-CoV2 infection. Brain Behav Immun. 2020;88:11–16.
- Dell’Era V, Farri F, Garzaro G, et al. Smell and taste disorders during COVID-19 outbreak: Cross-sectional study on 355 patients. Head Neck. 2020; Jul42(7):1591–1596.
- Magnavita N, Tripepi G, Di Prinzio RR. Symptoms in health care workers during the COVID-19 epidemic. A cross-sectional survey. Int J Environ Res Public Health. 2020;17(14):5218.
- Meini S, Suardi LR, Busoni M, et al. Olfactory and gustatory dysfunctions in 100 patients hospitalized for COVID-19: sex differences and recovery time in real-life. Eur Arch Otorhinolaryngol. 2020;277(12):3519–3523.
- Vaira LA, Hopkins C, Salzano G, et al. Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck. 2020;42(7):1560–1569.
- Gelardi M, Trecca E, Cassano M, et al. Smell and taste dysfunction during the COVID-19 outbreak: a preliminary report. Acta Biomed. 2020;91(2):230–231.
- Vacchiano V, Riguzzi P, Volpi L, et al. Early neurological manifestations of hospitalized COVID-19 patients. Neurol Sci. 2020;41(8):2029–2031.
- De Maria A, Varese P, Dentone C, et al. High prevalence of olfactory and taste disorder during SARS-CoV-2 infection in outpatients. J Med Virol. 2020;92(11):2310–2311.
- Vaira LA, Hopkins C, Petrocelli M, et al. Smell and taste recovery in coronavirus disease 2019 patients: a 60-day objective and prospective study. J Laryngol Otol. 2020;134(8):703–709.
- La Torre G, Massetti AP, Antonelli G, et al. Anosmia and ageusia as predictive signs of COVID-19 in healthcare workers in Italy: a prospective case-control study. J Clin Med. 2020;9(9):2870.
- Paderno A, Mattavelli D, Rampinelli V, et al. Olfactory and gustatory outcomes in COVID-19: a prospective evaluation in nonhospitalized subjects. Otolaryngol Head Neck Surg. 2020;163(6):1144–1149.
- Cazzolla AP, Lovero R, Lo Muzio L, et al. Taste and smell disorders in COVID-19 patients: role of interleukin-6. ACS Chem Neurosci. 2020;11(17):2774–2781.
- Freni F, Meduri A, Gazia F, et al. Symptomatology in head and neck district in coronavirus disease (COVID-19): a possible neuroinvasive action of SARS-CoV-2. Am J Otolaryngol. 2020;41(5):102612.
- Vaira LA, Salzano G, Petrocelli M, et al. Validation of a self-administered olfactory and gustatory test for the remotely evaluation of COVID-19 patients in home quarantine. Head Neck. 2020;42(7):1570–1576.
- Barillari MR, Bastiani L, Lechien JR, et al. A structural equation model to examine the clinical features of mild-to-moderate COVID-19: a multicenter Italian study. J Med Virol. 2020. DOI:10.1002/jmv.26354
- Lovato A, Galletti C, Galletti B, et al. Clinical characteristics associated with persistent olfactory and taste alterations in COVID-19: a preliminary report on 121 patients. Am J Otolaryngol. 2020;41(5):102548.
- Martin-Sanz E, Riestra J, Yebra L, et al. Prospective study in 355 patients with suspected COVID-19 infection. Value of cough, subjective hyposmia, and hypogeusia. Laryngoscope. 2020;130(11):2674–2679.
- Abalo-Lojo JM, Pouso-Diz JM, Gonzalez F. Taste and smell dysfunction in COVID-19 patients. Ann Otol Rhinol Laryngol. 2020;129(10):1041–1042.
- Izquierdo-Dominguez A, Rojas-Lechuga MJ, Chiesa-Estomba C, et al. Smell and taste dysfunctions in COVID-19 are associated with younger age in ambulatory settings – a multicenter cross-sectional study. J Investig Allergol Clin Immunol. 2020;30(5):346–357.
- Rojas-Lechuga MJ, Izquierdo-Dominguez A, Chiesa-Estomba C, et al. Chemosensory dysfunction in COVID-19 out-patients. Eur Arch Otorhinolaryngol. 2020. DOI:10.1007/s00405-020-06266-3
- Beltran-Corbellini A, Chico-Garcia JL, Martinez-Poles J, et al. Acute-onset smell and taste disorders in the context of COVID-19: a pilot multicentre polymerase chain reaction based case-control study. Eur J Neurol. 2020;27(9):1738–1741.
- Brandao Neto D, Fornazieri MA, Dib C, et al. Chemosensory dysfunction in COVID-19: prevalences, recovery rates, and clinical associations on a large Brazilian sample. Otolaryngol Head Neck Surg. 2020. DOI:10.1177/0194599820954825
- Kempker RR, Kempker JA, Peters M, et al. Loss of smell and taste among healthcare personnel screened for coronavirus 2019. Clin Infect Dis. 2020. DOI:10.1093/cid/ciaa877
- Aggarwal S, Garcia-Telles N, Aggarwal G, et al. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): Early report from the United States. Diagnosis (Berl). 2020;7(2):91–96.
- Salepci E, Turk B, Ozcan SN, et al. Symptomatology of COVID-19 from the otorhinolaryngology perspective: a survey of 223 SARS-CoV-2 RNA-positive patients. Eur Arch Otorhinolaryngol. 2020;1–11.
- Sakalli E, Temirbekov D, Bayri E, et al. Ear nose throat-related symptoms with a focus on loss of smell and/or taste in COVID-19 patients. Am J Otolaryngol. 2020;41(6):102622.
- Calica Utku A, Budak G, Karabay O, et al. Main symptoms in patients presenting in the COVID-19 period. Scott Med J. 2020;65(4):127–132. 17:
- Cirillo N. COVID-19 outbreak: succinct advice for dentists and oral healthcare professionals. Clin Oral Investig. 2020;24(7):2529–2535.
- Chi H, Chiu NC, Peng CC, et al. One-seventh of patients with COVID-19 had olfactory and gustatory abnormalities as their initial symptoms: a systematic review and meta-analysis. Life (Basel). 2020;10(9):158.
- Aziz M, Perisetti A, Lee-Smith WM, et al. Taste changes (Dysgeusia) in COVID-19: a systematic review and meta-analysis. Gastroenterology. 2020;159(3):1132–1133.
- von Bartheld CS, Hagen MM, Butowt R. Prevalence of chemosensory dysfunction in COVID-19 patients: a systematic review and meta-analysis reveals significant ethnic differences. medRxiv. 2020 .DOI:10.1101/2020.06.15.20132134
- Agyeman AA, Chin KL, Landersdorfer CB, et al. Smell and taste dysfunction in patients with COVID-19: a systematic review and meta-analysis. Mayo Clin Proc. 2020;95(8):1621–1631.