Abstract
Objective
To investigate whether self-reported smoking and serum cotinine levels associate with periodontal pocket development and to determine the accuracy of self-reported smoking using serum cotinine.
Materials and methods
This 4-year prospective cohort study included data from 294 dentate adults, aged ≥30 years, who participated in both the Health 2000 Survey and the Follow-up Study of Finnish Adults’ Oral Health. Subjectively reported smoking status (daily smokers n = 62, occasional smokers n = 12, quitters n = 49, and never-smokers n = 171), serum cotinine levels, demographic factors, education level, dental behaviours and medical history were collected at baseline. The outcome measure was the number of teeth with periodontal pocketing ≥4 mm over 4 years.
Results
Self-reported daily smokers had 1.82 (95% CI: 1.32–2.50) higher incidence of deepened periodontal pockets than never-smokers. A positive association was observed between serum cotinine (≥42.0 μg/L) and the development of periodontal pockets. The misclassification rate of self-reported smoking was 6%.
Conclusions
Both self-reported daily smoking and higher serum cotinine were associated with periodontal pocket development. Self-reported smoking was fairly accurate in this study. However, higher cotinine levels among a few self-reported never-smokers indicated misreporting or passive smoking. Thus, self-reports alone are not enough to assess the smoking-attributable disease burden.
Introduction
The deleterious effect of smoking on the periodontium has been investigated in previous epidemiological studies [Citation1–3]. These studies have shown dose-response effect between daily cigarette consumption and the progression of periodontal disease [Citation3,Citation4]. It has been shown that smoking reduces beneficial bacteria and increases periodontopathogenic bacteria in the subgingival microbiome [Citation5]. The alteration in the virulence of bacteria and host immune response and facilitation of pathogen-enriched subgingival microbiome leads to a more severe periodontal destruction and to higher prevalence of periodontitis among smokers than non-smokers [Citation6].
The most common method for the assessment of tobacco use are self-reports that have been used in a majority of the previous studies focussing on the association between smoking and periodontal diseases [Citation7–10]. In self-report measures, the problem is that some people do not disclose their smoking status due to societal and peer pressure while others find it difficult to remember the number of cigarettes smoked. This causes social desirability and recall biases, thus endangering the validity of the study. Also, assessments using a questionnaire can lead to over- or underestimation of the amount of smoke inhaled per cigarette [Citation11].
The amount of tobacco products inhaled and absorbed varies among different individuals. It depends on the nicotine intake per cigarette and how the cigarettes are smoked. Thus, measuring tobacco exposure according to self-reports is difficult and therefore often imprecise [Citation12,Citation13].
Recent exposure to tobacco smoke can be assessed in a reliable and quantitative way by using serum cotinine levels. It is the primary metabolite of nicotine and has been used as a biomarker for tobacco exposure. It is the preferred biomarker over nicotine concentrations for monitoring smoking behaviour due to its longer half-life (∼20 h) compared to nicotine (∼2 h). The serum cotinine concentration relates directly to tobacco exposure and its measurement provides an estimation of passive and active exposure to tobacco [Citation14–17]. The biochemical verification of self-reported smoking should be considered to assess the disease outcomes related to smoking and cessation rates [Citation16].
Until today, only very few studies have validated self-reported smoking status against cotinine levels and studied the association between cotinine and periodontal health in a longitudinal setting [Citation18,Citation19]. In this paper, we aimed to study using a prospective cohort whether self-reported smoking and serum cotinine levels associate with the development of periodontal pockets. We also determined the accuracy of self-reported smoking status by employing serum cotinine measurements.
Materials and methods
Study design and subjects
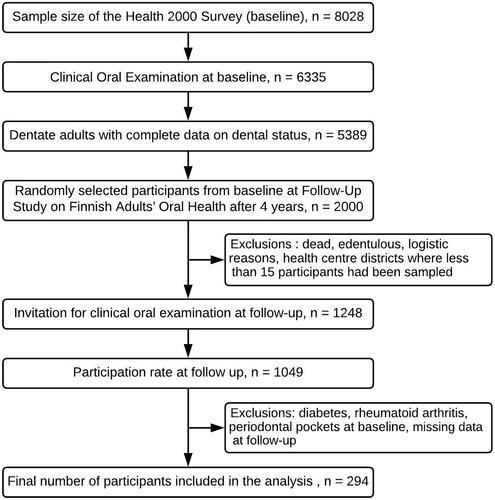
In 2000–2001, the National Public Health Institute (KTL, currently the Finnish Institute for Health and Welfare, THL) conducted the national Health 2000 Survey. A stratified two-stage cluster sampling was implemented consisting of a nationally representative sample of the Finnish population that includes 8028 participants aged 30 years or above [Citation20]. The clinical oral examinations were attended by 6335 participants [Citation21]. During 2004–2005, a follow-up study on Finnish Adults’ Oral Health [Citation22] was conducted with 2,000 randomly selected participants for clinical oral health examination in the Health 2000 Survey. Exclusion criteria were edentulousness, death since the first survey, logistic reasons and sampling limit of <15 subjects per health centre district. After exclusions, the data consisted of 1,248 participants who were re-invited for oral health examination. Of these, 1,049 participants turned up for the follow-up examination.
The number of participants who had no periodontal pockets ≥4 mm at baseline was 365; of these, 334 showed up for the follow-up examination. Based on this, complete data on the variables selected for analysis was available for 294 participants ().
Exclusion criteria
Participants who were diabetic, had rheumatoid arthritis, had teeth with periodontal pockets at baseline and those with missing data (either Health 2000 Survey or Follow-up study on Finnish Adults’ Oral Health) were excluded from the study.
Ethical approval
For the baseline and follow-up study, informed consent from the study participants and the approval of the Ethical Committee for Epidemiology and Public Health of the Hospital District of Helsinki and Uusimaa in Finland was obtained (T2000/version 0.7/21.9.1999/KTL6167 and FSAOH/479/E3/26.11.2003). Both the studies were conducted in accordance with the Helsinki Declaration of 1975, as revised in 2013.
Exposure
Self-reported smoking status
Smoking status was assessed at baseline through home interviews. The following questions recommended by the World Health Organization (WHO) were asked: ‘have you ever smoked?’, ‘have you ever smoked regularly (i.e. daily for at least 1 year)?’, ‘have you smoked at least 100 times?’ and ‘when did you last smoke?’ The subjects were categorized into the following groups: daily smokers, occasional smokers, quitters and never-smokers. Daily smokers were those who reported smoking at least 100 times in their lifetime with regularity for at least 1 year and most recently on the day of the survey or the previous day [Citation23,Citation24]. Occasional smokers were those who smoked, but not every day. Quitters were those who did not smoke in the 30 days before the survey. Never-smokers were those who did not smoke more than 100 cigarettes in their lifetime or did not smoke at all. Depending on the reported amount of daily smoking by the participants, four categories were formed: (0 cigarettes per day, 1–9 cigarettes per day, 10–19 cigarettes per day and 20+ cigarettes per day).
Serum cotinine levels
Cotinine concentrations were determined from serum samples collected at baseline using liquid-phase radioimmunoassay methodology (Nicotine Metabolite DOUBLE ANTIBODY kit, Diagnostic Products Corporation, Los Angeles, CA, USA) [Citation25].
Clinical oral examination
In the follow-up study, the clinical oral examination was the same as at the baseline. During the clinical examinations, WHO guidelines [Citation26,Citation27] and the methods adopted in standard clinical practice were followed. A dental chair, saliva suction, a syringe, a headlamp as a light source, a mouth mirror and the WHO periodontal probe were part of the standard dental unit and the examination was carried out by 5 dentists [Citation28]. Follow-up oral examinations were performed by one of the dentists who participated in the baseline oral examination.
To determine the periodontal status, the depth of periodontal pockets was assessed using the WHO periodontal probe (Plandent Oyj, no.19577). The depth of periodontal pockets was measured for all teeth except for the third molars and residual roots. The examination started from the ‘most posterior tooth in the upper right quadrant to the most posterior tooth in the lower left quadrant’. The measurement around each tooth was done at four points – ‘distal angle, midpoint on the buccal side, midpoint on the lingual side, and mesial angle’. In the baseline Health 2000 Survey, the kappa values for inter-examiner reliability and intra-examiner reliability for the periodontal pockets were 0.41 [Citation21] and 0.83 [Citation28], respectively. The outcome was the number of teeth with a periodontal pocket of ≥ 4 mm in the follow-up examination. The intra-examiner reliability of the field examiner, who performed all the follow-up measurements and who was one of the baseline examiners, was the following: the mean kappa value for the repeated periodontal pocket measurement of four index teeth (16, 21, 35, 46) was 0.89 and the intraclass correlation coefficient (ICC) for the number of teeth with deepened (≥ 4 mm) periodontal pockets was 0.97.
Covariates
Covariates included sociodemographic factors, dental behaviour inquired during the interview and oral hygiene determined in clinical examination. Education was categorized into three groups: basic, secondary, and higher education. For toothbrushing frequency, the subjects were asked ‘How often do you brush your teeth?’ and the response options were: (a) ‘more often than twice a day’, (b) ‘twice a day’, (c) ‘once a day’, (d) ‘less often than once a day’ and (e) ‘never’. The options were dichotomized into ‘twice a day or more’ versus ‘once a day or less’. For dental attendance pattern, the subjects had three response options (‘never’, ‘only for emergencies’, ‘regularly for check-ups’). This variable was dichotomized into ‘only for emergencies/never’ versus ‘regularly for check-ups’.
Oral hygiene was assessed by measuring the presence of dental plaque using a modified version of the scale developed by Silness and Löe [Citation29]. Dental plaque was visually assessed on three index teeth using the categories: ‘no plaque’, ‘marginal plaque only’ and ‘plaque also elsewhere’. The highest score indicated the subject’s oral hygiene level.
Statistics
Correlations between self-reported smoking and serum cotinine levels were calculated using Spearman’s correlation test (rs) due to skewed distribution. Negative binomial regression was used to estimate incidence rate ratios (IRRs). In regression models, the number of teeth at baseline was used as the offset variable.
Receiver operating characteristic (ROC) curve analysis and the corresponding area under the curve (AUC) was used to calculate the optimal cut-off point for serum cotinine to discriminate between smokers and non-smokers. Youden index (J), another diagnostic measure to determine the optimal cut-off point, was also calculated. The optimal cut-off point of 42.0 μg/L was used to separate smokers (daily, occasional) from non-smokers.
Covariates were added into regression models in a sequential way: age (continuous) and gender (Model 1B); age, gender and education (Model 1 C); age, gender, education, oral hygiene (plaque) and dental behaviours (toothbrushing frequency, dental attendance pattern) (Model 1 D). The same sequence of adjustments was done when modelling the association of the amount of daily smoking and serum cotinine levels with the number of teeth with deepened periodontal pockets (Models 2 A–2D, 3 A–3D).
Results
Socio-demographic and smoking-related characteristics of the study participants
The participants who developed periodontal pockets after 4 years were more likely to be males, less educated, daily smokers and had serum cotinine levels ≥ 42.0 µg/L than those with no periodontal pocket after 4 years (). The participants who smoked daily, who smoked 20+ cigarettes/day and those who had serum cotinine levels ≥ 42 µg/L were more often males, less educated, had more dental plaque and a less frequent dental attendance pattern than never-smokers or those who had serum cotinine levels <42 µg/L, respectively ().
Table 1. Baseline characteristics of the study participants by development of periodontal pocket after 4 years (n = 294)
Table 2. Baseline characteristics of the study participants according to self-reported smoking status (n = 294), amount of daily smoking (n = 238) and serum cotinine levels ((n = 294)
Incidence of periodontal pocketing during 4-year follow-up in relation to smoking status and serum cotinine levels
The unadjusted IRR for daily smokers was 1.82 (95% CI: 1.32–2.50) when compared with the never-smokers (reference category). After adjustment of all confounding factors, the IRR for daily smokers was 1.59 (95% CI: 1.12–2.23) in comparison to never-smokers (). Practically no elevated risk for periodontal pocket development was observed among occasional smokers and quitters.
Table 3. Negative binomial regression models for the association between baseline self-reported smoking status and the development of teeth with periodontal pockets ≥4 mm during the 4-year follow-up*
The amount of smoking was found to be associated with the development of deepened periodontal pockets in an exposure-dependent manner. The IRRs for the highest category (20+ cig/day) varied from 2.03 to 2.41. In a fully adjusted model, 1–9 cigarettes/day was not associated with periodontal pocket development ().
Table 4. Negative binomial regression models for the association between baseline amount of daily smoking and the development of teeth with periodontal pockets ≥4 mm during the 4-year follow-up*
Serum cotinine levels ≥42.0 µg/L were found to be associated with the number of teeth with deepened periodontal pockets. The association in all models was statistically significant with a p-value level of .005; the IRRs for those who had serum cotinine levels ≥ 42.0 µg/L varied between 1.56–1.74 compared to those with cotinine levels below 42.0 µg/L ().
Table 5. Negative binomial regression models for the association between baseline serum cotinine levels and the development of teeth with periodontal pockets ≥ 4 mm during the 4-year follow-up*
Accuracy of self-reported smoking status
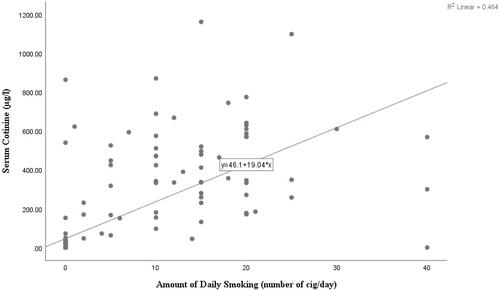
A strong positive correlation was found between the number of cigarettes smoked and serum cotinine levels among daily smokers (rs = 0.72, CI: 0.65–0.78) ().
Figure 2. Correlation between number of cigarettes smoked and serum cotinine levels among daily smokers.
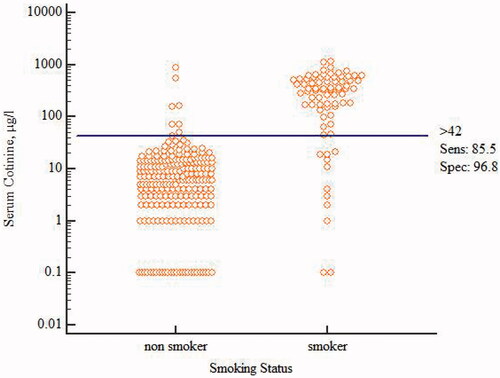
The ROC curve and the corresponding higher AUC has shown the predictive power of serum cotinine to discriminate smokers (daily, occasional) from non-smokers (AUC = 0.92, CI: 0.88–0.95). The optimal serum cotinine cut-off point was 42.0 µg/L with sensitivity of 86% and specificity of 97% (). To ensure the reliability of the cut-off point, Youden’s Index was also calculated (J = 0.82, 95% CI: 0.72–0.89).
Figure 3. Optimal cut-off point for serum cotinine levels (µg/L) to distinguish between smokers (daily, occasional) and non-smokers at baseline.
Using the cut-off point of 42 µg/L, there were 65 smokers who had cotinine levels ≥42 μg/L and 211 non-smokers who had cotinine levels <42μg/L. There were 11 smokers who had cotinine levels < 42 µg/L and 7 non-smokers who had cotinine levels ≥42 µg/L. The misclassification rate of self-reported smoking status was 6% (Supplementary Table S1). The minimum level for daily smokers was 2 µg/L, while the maximum for never-smokers was 864 µg/L. The mean values for quitters and never-smokers were approximately in the same range (Supplementary Table S2).
Discussion
In this paper, we aimed to study whether self-reported smoking and serum cotinine levels are related to the development of periodontal pockets and to validate self-reported smoking status by using serum cotinine levels. This study reveals that smoking is related to deterioration of periodontal health irrespective of whether the measurement of smoking is based on self-reports or serum cotinine level. These findings are consistent with those found in previous longitudinal studies regarding both self-reported smoking [Citation9,Citation30,Citation31] and serum cotinine levels [Citation32]. The validation of the self-reported smoking is fairly consistent with the associated serum cotinine levels in this study.
Regarding exposure-response, results show that the higher the cigarette consumption, the higher the incidence of periodontal disease among the daily smokers. These findings are in agreement with a 10-year [Citation30] and a 4-year long prospective study [Citation33]. On the other hand, it is demonstrated that former smokers have practically no increased risk for the deterioration of periodontal health when compared to never-smokers. This finding is in accordance with the previous studies reporting that smoking cessation improves periodontal health [Citation3,Citation4,Citation9,Citation30,Citation31,Citation34].
To date, the most common approach for the assessment of smoking status is to collect data about smoking habits by using a questionnaire or by interview. However, self-reported smoking data may not represent correct smoking status due to difficulties in recalling or societal or peer pressure. The change in social norms has made tobacco use less acceptable, which may increase the extent of social desirability bias over time [Citation35]. To overcome these possible sources of bias related to self-reports, this study concurrently uses serum cotinine levels to measure exposure to tobacco products. The results of this study are in agreement with a previous study that reported a positive association between cotinine levels and the development of periodontal disease [Citation36–38].
There are also other aspects that cannot be accurately assessed using standard questionnaires or interviews: exposure to passive smoke may not be accurately assessed [Citation39–41]. The odds of developing periodontitis are associated with environmental exposure to tobacco smoke. A study revealed environmental exposure to tobacco smoke increases the odds of developing periodontitis by two- to threefold [Citation42].
In this study, the misclassification rate was 6% when using the serum cotinine level cut-off point of 42.0 μg/L. In certain cases, passive smoking can produce serum cotinine levels greater than 10 ng/mL in non-smokers, meaning that a higher cut-off value may be needed to exclude most non-smokers [Citation43]. In this data set, there were 11 smokers who had cotinine levels <42 μg/L and 7 non-smokers who had cotinine levels ≥42 μg/L. The self-reported never-smoker had serum cotinine levels as high as 864 μg/L. This may be due to occasional smoking, passive smoking, laboratory error in the data or an error in filling in the questionnaire. Lastly, one reason for these exceptionally high values could be related to individual variation in biology, i.e. transformation of nicotine to cotinine, as well as to variation in the clearance of serum cotinine [Citation44].
Overlapping of serum cotinine levels between occasional smokers and non-smokers was also observed, whereas measurements based on cotinine level are shown to be accurate and able to distinguish daily smokers from non-smokers. This observation is consistent with the findings of an earlier study where self-reported smoking appeared to be a valid measurement of tobacco exposure [Citation36,Citation45].
An earlier study in Finland used the cut-off point of 100 μg/L to differentiate smokers from non-smokers, with high misclassification among non-smokers who exhibited serum cotinine levels ≥100 μg/L [Citation46]. In this study, the cut-off point of 100 μg/L led to sensitivity of 78% and specificity of 98%. The overlap among serum cotinine values in smokers (daily, occasional) and non-smokers was also observed at the cotinine cut-off value of 100 μg/L.
Biochemical measures that verify smoking status may help to determine bias in self-reporting information and thereby also reduce potential interpretation errors [Citation47]. In addition, this may provide better information about the commonness of smoking as well as exposure to tobacco smoke (passive smoking). Regarding the commonness of smoking, this study is in agreement with the previous studies that suggest that self-reported smoking history may underestimate the proportion of those who have been exposed to smoking [Citation47–49]. In this study, the proportion of daily smokers was 21.8%, whereas according to cotinine level, the proportion of those who had been exposed to tobacco smoke was 24.5%.
The validity of this study is increased by excluding the participants who had a known risk for periodontal disease such as diabetes and rheumatoid arthritis. The validity is also increased by excluding those participants from the study who had periodontal pockets at baseline, because it is known that previous disease history is one of the important disease indicators of periodontal diseases.
This study has some limitations. The presence of a periodontal pocket ≥4 mm in the follow-up examination was confined to four site/tooth assessments, which might underestimate the actual extent of periodontal disease. In most cases, the effect of the underestimation is likely to be negligible due to the fact that the participants had on average a fairly healthy periodontium. It is also important to note that the outcome variable ‘number of teeth with deepened periodontal pockets’ is based solely on probing depth, not on bleeding or attachment loss. The presence of deepened periodontal pockets is most likely related to periodontal pocketing, although in some cases it can be the result of other periodontal diseases, such as gingivitis or gingival overgrowth. Also, in general, incidence cannot be studied among those who already have the disease.
Exposure to smoking was assessed only at baseline; therefore, smoking habits do not represent changes in individuals’ smoking behaviour over time. This may have caused some misclassification. The magnitude of this misclassification is most likely small because smoking as a habit is in most cases quite permanent, although there might be some changes in smoking intensity. Furthermore, due to the sample of follow-up and attrition of the study population, the results of this study cannot be generalized to the whole Finnish population.
This study was conducted in a prospective longitudinal setting which allows assessing the effect of tobacco smoke on periodontal disease development, measured by means of periodontal pocket formation. The results show that both self-reported daily smoking and serum cotinine levels were associated with the deterioration of periodontal health. Self-reported smoking status was fairly accurate in this study. However, a few self-reported never-smokers had higher cotinine levels, indicating the importance of biochemical measurements to assess misclassified smoking status, actual dose of tobacco exposure and associated risks. Future studies should consider bio-verification for more accurate evaluation of active and passive smokers. The results of this study lend support to the conception that smoking has an adverse effect on the periodontium. Implementation of strict tobacco control laws, policies and anti-smoking campaigns are necessary to protect people from the harmful effects of tobacco.
Supplementary_Table_S2.docx
Download MS Word (17 KB)Supplementary_Table_S1.docx
Download MS Word (22.3 KB)Acknowledgements
This article is partly based on the master’s thesis titled ‘Effect of smoking on periodontal health: A 4-year follow-up study in Finnish adults’ completed at University of Eastern Finland, Kuopio, Finland which can be accessed at https://core.ac.uk/download/pdf/32429324.pdf.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes
* Many of the quitters and occasional smokers (n = 56) did not respond to question about amount of daily smoking (number of cigarettes/day)
Abbreviation: SD, Standard Deviation.
References
- Hugoson A, Rolandsson M. Periodontal disease in relation to smoking and the use of Swedish snus: epidemiological studies covering 20 years (1983-2003). J Clin Periodontol. 2011;38(9):809–816.
- Mdala I, Olsen I, Haffajee AD, et al. Comparing clinical attachment level and pocket depth for predicting periodontal disease progression in healthy sites of patients with chronic periodontitis using multi-state Markov models. J Clin Periodontol. 2014;41(9):837–845.
- Jang AY, Lee JK, Shin JY, et al. Association between smoking and periodontal disease in Korean adults: The Fifth Korea National Health and Nutrition Examination Survey (2010 and 2012). Korean J Fam Med. 2016;37(2):117–122.
- Ravidà A, Troiano G, Qazi M, et al. Dose-dependent effect of smoking and smoking cessation on periodontitis-related tooth loss during 10–47 years periodontal maintenance-a retrospective study in compliant cohort. J Clin Periodontol. 2020;47(9):1132–1143.
- Karasneh JA, Al Habashneh RA, Marzouka NAS, et al. Effect of cigarette smoking on subgingival bacteria in healthy subjects and patients with chronic periodontitis. BMC Oral Health. 2017;17(1):64.
- Jiang Y, Zhou X, Cheng L, et al. The impact of smoking on subgingival microflora: from periodontal health to disease. Front Microbiol. 2020;11:66.
- Calsina G, Ramon JM, Echeverria JJ. Effects of smoking on periodontal tissues. J Clin Periodontol. 2002;29(8):771–776.
- Ojima M, Hanioka T, Tanaka K, et al. Relationship between smoking status and periodontal conditions: findings from national databases in Japan. J Periodontal Res. 2006;41(6):573–9.
- Thomson WM, Broadbent JM, Welch D, et al. Cigarette smoking and periodontal disease among 32-year-olds: a prospective study of a representative birth cohort.J Clin Periodontol. 2007;34(10):828–834.
- Khemiss M, Ben Fekih D, Ben Khelifa M, et al. Comparison of periodontal status between male exclusive Narghile smokers and male exclusive cigarette smokers. Am J Mens Health. 2019;13(2):1557988319839872.
- Etter, J.-F., Due, T. V., Perneger, T. V., Saliva cotinine levels in smokers and nonsmokers. Am J Epidemiol. 2000;151(3):251–258.
- Benowitz NL, Jacob P, 3rd. Daily intake of nicotine during cigarette smoking. Clin Pharmacol Ther. 1984;35(4):499–504.
- Benowitz NL, Jacob P, 3rd, Denaro C. Stable isotope studies of nicotine kinetics and bioavailability. Clin Pharmacol Ther. 1991;49(3):270–277.
- Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18(2):188–204.
- Stevens KR, Munoz LR. Cigarette smoking: Evidence to guide measurement.Res Nurs Health. 2004;27(4):281–292.
- Montalto NJ, Wells WO. Validation of self-reported smoking status using saliva cotinine: a rapid semiquantitative dipstick method. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1858–1862.
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159.
- Chen X, Wolff L, Aeppli D, et al. Cigarette smoking, salivary/gingival crevicular fluid cotinine and periodontal status. A 10-year longitudinal study. J Clin Periodontol. 2001;28(4):331–339.
- Nishida N, Yamamoto Y, Tanaka M, et al. Association between involuntary smoking and salivary markers related to periodontitis: a 2-year longitudinal study. J Periodontol. 2008;79(12):2233–2240.
- Aromaa A, Koskinen S. Health and functional capacity in Finland: baseline results of the Health 2000 health examination survey. Helsinki: Publications of the National Public Health Institute; 2004.
- Suominen-Taipale AL, Nordblad A, Vehkalahti M, et al. Oral health in the Finnish adult population. Health 2000 survey. Helsinki: Publications of the National Public Health Institute;2008.
- Kiiskinen U, Suominen-Taipale AL, Aromaa A, et al. Self-reported oral health and dental care utilisation during the dental care reform. Preliminary results from the dental care reform assessment study. Helsinki: Publications of the National Public Health Institute;2005.
- WHO. Guidelines for controlling and monitoring the tobacco epidemic. Geneva: World Health Organization;1998.
- Helakorpi S, Uutela A, Prättälä R, et al. Health behaviour and health among Finnish adult population, spring 2000. Helsinki: Publications of the National Public Health Institute;2000.
- Keskitalo K, Broms U, Heliövaara M, et al. Association of serum cotinine level with a cluster of three nicotinic acetylcholine receptor genes (CHRNA3/CHRNA5/CHRNB4) on chromosome 15. Hum Mol Genet. 2009;18(20):4007–4012.
- Kramer IR, Pindborg JJ, Bezroukov V, et al. Guide to epidemiology and diagnosis of oral mucosal diseases and conditions. World Health Organization. Community Dent Oral Epidemiol. 1980;8(1):1–26.
- WHO. Oral health surveys: basic methods. 4th ed. Geneva: World Health Organization;1997.
- Suominen-Taipale AL, Nordblad A, Vehkalahti M, et al. Suomalaisten aikuisten suunterveys, terveys 2000–tutkimus. Helsinki: Publications of the National Public Health Institute;2004.
- Silness J, Loe H. Periodontal Disease in Pregnancy. II. Correlation between Oral Hygiene and Periodontal Condtion. Acta Odontol Scand. 1964;22:121–135.
- Bergstrom J, Eliasson S, Dock J. A 10-year prospective study of tobacco smoking and periodontal health. J Periodontol. 2000;71(8):1338–1347.
- ALHarthi SSY, Natto ZS, Midle JB, et al. Association between time since quitting smoking and periodontitis in former smokers in the National Health and Nutrition Examination Surveys (NHANES) 2009 to 2012. J Periodontol. 2019;90(1):16–25.
- Machtei EE, Dunford R, Hausmann E, et al. Longitudinal study of prognostic factors in established periodontitis patients. J Clin Periodontol. 1997;24(2):102–109.
- Okamoto Y, Tsuboi S, Suzuki S, et al. Effects of smoking and drinking habits on the incidence of periodontal disease and tooth loss among Japanese males: a 4-yr longitudinal study. J Periodontal Res. 2006;41(6):560–566.
- Leite FRM, Nascimento GG, Baake S, et al. Impact of Smoking Cessation on Periodontitis: A Systematic Review and Meta-analysis of Prospective Longitudinal Observational and Interventional Studies. Nicotine Tob Res. 2019;21(12):1600–1608.
- Fendrich M, Mackesy-Amiti ME, Johnson TP, et al. Tobacco-reporting validity in an epidemiological drug-use survey. Addict Behav. 2005;30(1):175–181.
- Duque A, Martinez PJ, Giraldo A, et al. Accuracy of cotinine serum test to detect the smoking habit and its association with periodontal disease in a multicenter study. Med Oral Patol Oral Cir Bucal. 2017;22(4):e425–e431.
- Tanaka K, Matsuse R, Miyake Y, et al. Salivary cotinine concentrations and prevalence of periodontal disease in young Japanese women: the Kyushu Okinawa maternal and child health study. J Periodontol. 2013;84(12):1724–1729.
- Ebersole JL, Steffen MJ, Thomas MV, et al. Smoking-related cotinine levels and host responses in chronic periodontitis. J Periodont Res. 2014;49(5):642–651.
- Kawachi I, Colditz GA. Invited commentary: confounding, measurement error, and publication bias in studies of passive smoking. Am J Epidemiol. 1996;144(10):909–915.
- Wells AJ, English PB, Posner SF, et al. Misclassification rates for current smokers misclassified as nonsmokers. Am J Public Health. 1998;88(10):1503–1509.
- Fang SC, Chen S, Trachtenberg F, et al. Validity of Self-Reported Tobacco Smoke Exposure among Non-Smoking Adult Public Housing Residents. PLoS One. 2016;11(5):e0155024.
- Sutton JD, Ranney LM, Wilder RS, et al. Environmental tobacco smoke and periodontitis in U.S. non-smokers. J Dent Hyg. 2012;86(3):185–194.
- Benowitz NL, Bernert JT, Caraballo RS, et al. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169(2):236–248.
- Benowitz NL. Biomarkers of environmental tobacco smoke exposure. Environ Health Perspect. 1999;107(Suppl 2):349–355.
- Vartiainen E, Seppala T, Lillsunde P, et al. Validation of self reported smoking by serum cotinine measurement in a community-based study. J Epidemiol Community Health. 2002;56(3):167–170.
- Vasankari T, Jousilahti P, Knekt P, et al. Serum cotinine predicts bronchial obstruction regardless of self-reported smoking history. Scand J Public Health. 2011;39(5):547–552.
- Connor Gorber S, Schofield-Hurwitz S, Hardt J, et al. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res. 2009;11(1):12–24.
- Lewis SJ, Cherry NM, Mc LNR, et al. Cotinine levels and self-reported smoking status in patients attending a bronchoscopy clinic. Biomarkers. 2003;8(3–4):218–228.
- Huang IC, Klosky JL, Young C, et al. Accuracy of self-reported smoking status in adult survivors of childhood cancer: a report from St. Jude Lifetime Cohort study.J Clin Oncol. 2017;35(15_suppl):10570.