Abstract
Background
Smoking is associated with the deteriorating health of the gingiva and periodontium. The long-term beneficial effects of smoking cessation on oral health are well known. However, the effects of short-term smoking cessation on gingival bleeding and periodontal pocket depth are unknown. The purpose of the present study was to determine the effects of short-term smoking cessation on gingival bleeding and periodontal pocket depth.
Methods
Dentate smokers with a mean age of 56.9 ± 14.4 years at an outpatient smoking cessation clinic participated in this study. A professional dentist checked the periodontal pocket depth and gingival bleeding. Patients visited the smoking cessation clinic on their first visit and 2, 4, 8, and 12 weeks (three months). The gingival assessment was re-performed in those who succeeded in smoking cessation 3 months after the baseline.
Results
The baseline data of 83 patients showed that an increase in pocket depth was associated with increasing age and the amount of smoking. A significant increase in gingival bleeding (p = .031) and increase in pocket depth (p = .046) were observed 3 months after the baseline in patients who successfully quit smoking (n = 14).
Conclusion
Short-term smoking cessation increased periodontal pocket depth and gingival bleeding. These findings may reflect healing processes that occur in the healthy gingiva.
Implications
Study findings will be useful to advise patients during smoking cessation programs. Dentists can inform patients that an initial increase in gingival bleeding and pocket depth could be associated with smoking cessation. Such advice will prevent patients from any apprehension that may cause them to recommence smoking.
Introduction
Tobacco smoking is a preventable risk factor for various diseases in the human body, such as different types of cancers, respiratory tract infections, cardiac issues, and liver problems. Smoking is also associated with the health of the oral cavity and causes gingivitis, periodontitis, oral cancers, and many other problems [Citation1]. The periodontal ligament and the supporting alveolar bone, which holds the teeth, are damaged by the inflammation caused by smoking, which ultimately leads to tooth loss. Based on observational studies included in a systemic review by Leite et al. [Citation2], smokers have an 80% higher risk of periodontitis than quitters and never-smokers. Smoking also detrimentally affects neutrophils and macrophages, which are essential as immunocompetent gingival cells. Many studies assert that smokers have less gingival bleeding on probing than do non-smokers [Citation3,Citation4]. Preber et al. [Citation4] proposed that a possible explanation for decreased gingival bleeding in smokers is vasoconstriction of the peripheral blood vessels caused by nicotine. However, heavy smokers have a severe periodontal breakdown and bleeding compared to infrequent smokers and non-smokers [Citation5]. Thus, smoking has chronic and time-dependent effects on gingival health.
The periodontal pocket, defined as a pathologically deepened gingival sulcus, is an essential clinical feature of periodontal disease. Periodontal pockets are chronic inflammatory lesions. Complete healing does not occur because of the persistence of the bacterial attack, which continues to stimulate an inflammatory response. Chronic periodontitis results in inflammation within the supporting tissues of the teeth, progressive attachment loss, and bone loss. Smokers with chronic periodontitis have more attachment loss, which leads to deeper pockets [Citation6].
Based on interventional studies, periodontal treatment resulted in pocket depth reduction and a 0.2 mm higher gain in the attachment level among quitters than nonquitters. Studies have shown that the response to quitting smoking is apparent within 12 months [Citation2]. Thus, quitting tobacco is very important for successful periodontal therapy.
Various studies have shown the long-term effects of smoking cessation on the gingiva and periodontal depth, like reduction in the attachment loss progression, pocket depth, and radiographic bone loss [Citation2,Citation7]. The follow-up time in these studies ranged from 4 to 32 years. Our study focuses on the short-term effects of smoking cessation on gingival and periodontal health.
Methods and materials
Study population
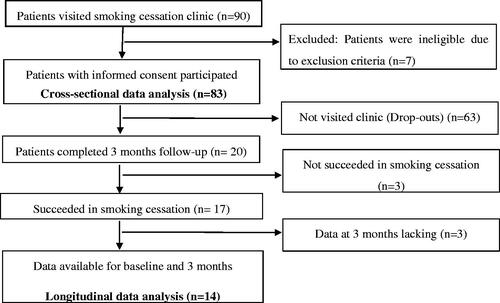
The study participants were the first-visit smokers who used conventional cigarettes, reported a desire for smoking cessation, and visited the outpatient clinic at the National Hospital Organisation Kyoto Medical Centre between January 18, 2017, and October 3. All patients who fulfilled these criteria were asked to participate in this study. Among these, those who provided written informed consent were included. The exclusion criterion was advanced cancer requiring palliative care and the patients in whom the pocket depth could not be measured because of missing teeth
Age, number of cigarettes per day, smoking years, Fagerstrom test of nicotine dependence (FTND) score, body mass index (BMI), waist circumference, blood pressure, and respiratory carbon monoxide (CO) levels were recorded. This study was approved by the Ethical Review Committee of the National Hospital Organisation Kyoto Medical Centre (Fushimi-ku, Kyoto, Japan).
Smoking cessation
The FTND is a standard instrument for assessing the intensity of physical addiction to nicotine. The items are summed to yield a total score of 0–10. The higher the FTND score, the more intense is the patient’s physical dependence on nicotine [Citation8].
Smoking cessation in this study was accomplished through nicotine patches or varenicline. Patients visited the smoking cessation clinic on their first visit and 2, 4, 8, and 12 weeks (three months) thereafter while being treated with transdermal nicotine patches or oral varenicline. On every visit, continuity in smoking cessation was assessed, and a nurse and a doctor provided specific advice regarding the continuation of the cessation treatment. At the end of the three-month anti-smoking treatment period, maintenance of smoking cessation was evaluated. A patient was judged to have succeeded in quitting smoking when presenting with an expiratory carbon monoxide (CO) concentration of ≤7 parts per million (ppm) and reporting that they had not smoked for more than 1 week since starting the treatment. An attempt to quit smoking was considered unsuccessful when the patient stopped visiting during the treatment period or continued visiting but failed to quit smoking. The gingival assessment was re-performed in those who succeeded in smoking cessation 3 months after the baseline.
Periodontal examinations
Clinical examination of the oral cavity was conducted by using a mouth mirror and a calibrated periodontal probe. A single experienced dentist recorded all the clinical parameters throughout the study, using the same instruments. The measurements were performed to the nearest millimetre for all teeth, except for the third molars, at six sites of every present tooth (i.e. mesiobuccal, mid-buccal, and distobuccal, mesiolingual, mid-lingual, and distolingual). The periodontal pocket depth was classified into three categories: Grade 0, pocket depth of 0–3 mm; Grade 1, pocket depth of 4–5 mm; and Grade 2, pocket depth of >6 mm. Bleeding on probing (BOP) was a dichotomous variable (i.e. present or absent). A calibrated periodontal probe was used to measure the depth and determine the configuration of the periodontal pocket [Citation9]. Gentle probing can be attained by running a probe around the teeth in the first 2 mm of the sulcus without applying any force apically. The WHO perio probe made by the Japanese company YDM Corporation was used to assess the periodontal status of individual patients, with the recommended probing force of 20–25 g to assess the periodontal status of each patient [Citation10].
Statistical analysis
A professional statistician conducted all statistical analyses using the Statistical Package for Social Sciences (SPSS) Statistics 17.0 statistical software package (SPSS Inc., Chicago, IL, USA). The prevalence and association between the aforementioned parameters were studied. The analysis included descriptive statistics, Fisher’s exact test, and an unpaired t-test. The two groups that presented as BOP-positive or BOP-negative at baseline were compared by using the mean, standard deviation, Fisher’s exact test, unpaired t-test, and Mann–Whitney U test. After 3 months, alterations between baseline and follow-up were calculated by using the McNemar test, paired t-test, and Wilcoxon signed-rank test.
Results
Cross-sectional study
The study participants consisted of 83 dentate smokers (19 women and 64 men) with an age (mean ± standard deviation) of 56.9 ± 14.4 years. The data in represent the baseline data. No smoking cessation treatment was implemented at this stage. For comparative purposes, patients were classified into three groups, based on the pocket depth; 33 patients in the Grade 0 group (i.e. pocket depth of 0–3 mm) 29 patients in the Grade 1 group (i.e. pocket depth of 4–5 mm), and 21 patients in the Grade 2 group (i.e. pocket depth of >6 mm).
Table 1. Patient characteristics are classified according to pocket depth.
Patients in the Grade 0 group, who had no or a shallow gingival pocket depth, were younger than patients in the Grade 2 group, who had a deep pocket (age [mean ± standard deviation]: 54.4 ± 15.8 years vs. 67.3 ± 10.8 years, p = .006; (). Patients in the Grade 1 group, who had a reduced gingival pocket depth, were similarly younger than patients in the Grade 2 group (age: 55.8 ± 10.4 years vs. 67.3 ± 10.8 years; p = .001). Patients in the Grade 0 group were younger than patients in the Grade 1 group, although this difference was not significant (age: 54.4 ± 15.8 years vs. 55.8 ± 10.4; p > .999. These findings indicated that pocket depth increased with increasing age.
The smoking amount was 16.1 ± 6.2 cigarettes/day in the Grade 0 group and 22.9 ± 14.8 cigarettes/day in the Grade 1 group. We observed an increasing trend in the smoking amount, although the difference was not significant (p = .059). The Grade 2 group, which had a deep gingival pocket depth, was associated with increased smoking (i.e. 26.4 ± 12.1 cigarettes/day). The Grade 0 and Grade 2 patient groups were significantly different (p < .001). These results suggested that the increase in pocket depth was associated with heavy smoking ().
For comparison purposes, smokers were divided into two groups based on BOP (). At the baseline visit, no significant difference existed in age (p = .445), smoking amount (p = .356), smoking years (p = .737), FTND score (p = .140), BMI (p = .298), and respiratory CO levels (p = .415) between 46 smokers who had a positive BOP result and 37 smokers who had a negative BOP result.
Table 2. Patient characteristics according to gingival bleeding.
Longitudinal study
Eighty-three patients underwent the smoking cessation treatment and were assessed at their first visit and 2, 4, 8, and 12 weeks. Seventeen patients were able to successfully quit smoking 3 months after the baseline. Other patients dropped out or were not successful in smoking cessation. The periodontal pocket was measured 3 months after the baseline visit. Data were missing for three of 17 patients for whom smoking cessation was successful. Therefore, the results of the baseline visit and the follow-up 3 months after starting the smoking cessation treatment were compared on the remaining 14 patients. These patients consisted of 3 (21.4%) women and 11 (78.6%) men with an average age of 60 ± 14 years. The smoking amount was 23.1 ± 18.5 cigarettes per day, and the smoking years were 38.8 ± 13 years.
We observed a significant increase in BMI after 3 months of smoking cessation treatment (p = .002) (). In addition, waist circumference increased significantly (p = .003), which may be associated with the increased BMI. The respiratory CO level significantly decreased after smoking cessation (p = .001). This finding confirmed that a patient had stopped smoking. An increase in body weight is a nicotine withdrawal symptom, which suggested that patients had quit smoking.
Table 3. Comparative data showing characteristics at baseline and 3 months after baseline: Systemic parameters.
The number of Grade 0 patients (i.e. no or shallow pocket depth) reduced from five patients (35.7%) at baseline to three patients (21.4%) at 3 months after starting the smoking cessation therapy. The number of Grade 1 patients did not change. The number of group 2 patients (i.e. deep pocket depth) significantly changed from two patients (14.3%) at baseline to four patients (28.6%) at 3 months. The overall increase in pocket depth from baseline to 3 months showed a significant p-value of .046 ().
Table 4. Comparative data showing characteristics at baseline and 3 months after baseline Gingival pocket depth.
At the baseline, five (35.7%) patients had BOP. Three months after starting the smoking cessation therapy, the number of patients increased to 11 (78.6%) patients with gingival BOP (). However, no change in systolic blood pressure or diastolic blood pressure occurred.
Table 5. Comparative data showing characteristics at baseline and 3 months after baseline Gingival bleeding (BOP).
Discussion
The purpose of the present study was to determine the effects of short-term smoking cessation on periodontal pocket depth and gingival bleeding. We found that short-term smoking cessation increased periodontal pocket depth and gingival bleeding. These findings may reflect the healing processes that occur in the healthy gingiva. We discuss our findings concerning other researchers’ findings below.
Periodontal pocket depth
Our study demonstrated that group 2 patients (i.e. deep pockets) were older than patients in group 1 and group 0 (i.e. shallow pocket and no pocket, respectively). These findings are compatible with those of other epidemiologic studies [Citation11–13], demonstrating more periodontal disease in older age groups than in younger age groups.
A previous study by Ragghianti et al. [Citation14] showed a significant association between age and periodontal conditions. In that study, the population age was ≥20 years. The investigators reported that pocket depth and the clinical attachment level increased with increasing age. They compared smokers and non-smokers and found that probing depth and the mean clinical attachment levels were higher in smokers in all age groups. Although our study participants were all smokers and much older, our findings were partially compatible with these results.
We observed that a greater number of cigarettes were associated with deep periodontal pockets, which led to periodontitis in heavy smokers. Our findings were compatible with those of Torrungruang et al. [Citation15], who also demonstrated a positive correlation between the level of cigarette consumption and the severity of periodontitis. They observed that the greater the number of cigarettes, the greater was the clinical attachment level. They also observed a dose-effect relationship between the level of cigarette consumption and the odds of having moderate and severe periodontitis. The adjusted odds ratio was 7.9 for heavy smokers who smoked more than 30 pack-years. This ratio was higher than that of light (<15 pack-years) and moderate smokers (>15pack-years).
Based on observational studies included in a systematic review by Leite et al. [Citation2], the risk for periodontitis incidence or progression among individuals who quit smoking was similar to the risk in never-smokers. However, continuing smokers have an 80% higher risk of periodontitis than do never-smokers or former smokers. In all studies cited in a systematic review by Leite et al. [Citation2], the observation period was 4–32 years of follow up found an increase in bone loss in smokers, compared to non-smokers, after a follow up of 10 years [Citation16–18]
A review by Heasman et al. [Citation19] reported that nonsurgical periodontal treatment reduces pocket depth in smokers and non-smokers. However, more significant probing depth reductions were obtained by nonsurgical periodontal therapy in non-smokers than in smokers.
A study by Preshaw et al. [Citation20] related to smoking cessation treatment reported the results after 1 year of smoking cessation. They conducted a clinical trial on 49 smokers with nonsurgical chronic periodontitis management. Nonsurgical management consisted of oral hygiene instructions, methodical root surface instrumentation using manual and ultrasonic instruments, and smoking counselling. Over 12-months, the investigators found no significant differences in the primary outcomes of the pocket probing depth, clinical attachment level, and BOP among the nonquitters, quitters, and oscillators who completed the trial with regular visits at 3 months, 6 months, and 12 months. However, they reported that the change in probing depth between baseline and 12 months indicates a significant reduction in favour of the quitters than in the rest of the participants.
We found an increase in the pocket depth among the quitters at 3 months from baseline without any intervention for oral health. This finding in our study is remarkable because no study, to the best of our knowledge, has shown the short-term (i.e. 3 months) effects of smoking cessation on the periodontal pocket depth. This effect may reflect the healing process, which is an increase in pocket depth and gingival bleeding initially after smoking cessation, followed by healthy gingiva with a decrease in pocket depth.
Gingival bleeding
A previous report by Neto et al. [Citation21] indicated that smoking might decrease gingival bleeding because of changes in the proportion of blood vessels in the periodontal tissues. The aforementioned study revealed that the gingival bleeding sites, the amount of gingival exudate, and the number of gingival sites with distinct redness were significantly lower in smokers than in non-smokers.
Gingival blood flow and the gingival crevicular fluid are well-known markers of gingival health. Nicotine causes vasoconstriction and interferes with healing during periodontal treatment, thereby delaying healing. Morozumi et al. [Citation22] have reported that gingival blood flow significantly increases within 3 days after smoking cessation. Gingival blood flow and the gingival crevicular fluid act in combination to promote periodontal health by improving gingival microcirculation.
In our study, 14 people were able to quit smoking successfully. We observed that gingival bleeding increased in smokers after they quit smoking (). Three months after the baseline, the gingival bleeding status was checked. This result was similar to that of the study by Nair et al. [Citation23], which showed a statistically significant increase in the mean proportion of tooth sites that exhibited bleeding after probing from the baseline to 4–6 weeks after quitting. However, they did not show any change in the probing depth or the number of sites probing greater than 2 mm between visits. Our results are also compatible with several studies that have reported [Citation21–23] that smoking decreases gingival blood flow while smoking cessation increases the blood flow.
Limitations
Our study showed all results for 3 months after the initial consultation at our smoking cessation clinic. First, the exact timeline of quitting smoking was not measured, but it was estimated as within the range of 1–8 weeks after the baseline visit. Second, gingival bleeding and periodontal pocket depths were not assessed during every patient visit. Third, the follow-up period in this study was only 3 months. More studies are required with long regular follow-ups to examine the effect of smoking cessation on the gingiva and periodontium. Finally, the number of patients included in our study was limited. For future studies, we recommend a greater number of patients to observe smoking cessation effects.
Conclusion
The pocket depth and BOP were significantly increased in patients who stopped smoking successfully. These short-term changes may reflect healing processes, which could be followed by healthy gingiva in the future. The study results can be used as evidence by healthcare providers to motivate smokers for smoking cessation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Fiorini T, Musskopf ML, Oppermann RV, et al. Is there a positive effect of smoking cessation on periodontal health? A systematic review. J. Periodontol. 2014;85(1):83–91.
- Leite FRM, Nascimento GG, Baake S, et al. Impact of smoking cessation on periodontitis: a systematic review and meta-analysis of prospective longitudinal observational and interventional studies. Nicotine Tob Res. 2019;21(12):1600–1608.
- Dietrich T, Bernimoulin JP, Glynn RJ. The effect of cigarette smoking on gingival bleeding. J. Periodontol. 2004;75(1):16–22.
- Preber H, Bergström J. Occurrence of gingival bleeding in smoker and non-smoker patients. Acta Odontol Scand. 1985;43(5):315–320.
- Vouros ID, Kalpidis CD, Chadjipantelis T, et al. Cigarette smoking associated with advanced periodontal destruction in a greek sample population of patients with periodontal disease. J Int Acad Periodontol. 2009;11:250–257.
- Newman MG, Takei H, Klokkevold PR, et al. Carranza's clinical periodontology. Philadelphia: W.B. Saunders Co; 2002.
- Warnakulasuriya S, Dietrich T, Bornstein MM, et al. Oral health risks of tobacco use and effects of cessation. Int Dent J. 2010;60(1):7–30.
- Heatherton TF, Kozlowski LT, Frecker RC, et al. The fagerström test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127.
- Glossary of periodontal terms. Chicago: American Academy of Periodontology; 2001.
- Al Shayeb KN, Turner W, Gillam DG. Periodontal probing: a review. Prim Dent J. 2014;1;3(3):25–29.
- Abdellatif HM, Burt BA. An epidemiological investigation into the relative importance of age and oral hygiene status as determinants of periodontitis. J Dent Res. 1987;66(1):13–18.
- Grossi SG, Genco EE, Machtei AW, et al. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. J Periodontol. 1994;65(3):260–267.
- Grossi SG, Genco RJ, Machtei EE, et al. Assessment of risk for periodontal disease. II. Risk indicators for alveolar bone loss. J Periodontol. 1995;66(1):23–29.
- Ragghianti MS, Greghi SL, Lauris JR, et al. Influence of age, sex, plaque and smoking on periodontal conditions in a population from Bauru, Brazil. J Appl Oral Sci. 2004;12(4):273–279.
- Torrungruang K, Nisapakultorn K, Sutdhibhisal S, et al. The effect of cigarette smoking on the severity of periodontal disease among older Thai adults. J. Periodontol. 2005;76(4):566–572.
- Okamoto Y, Tsuboi S, Suzuki S, et al. Effects of smoking and drinking habits on the incidence of periodontal disease and tooth loss among Japanese males: a 4-yr longitudinal study. J Periodontal Res. 2006;41(6):560–566.
- Baljoon M, Natto S, Bergstrom J. Long-term effect of smoking on vertical periodontal bone loss. J Clin Periodontol. 2005;32(7):789–797.
- Paulander J, Wennstrom JL, Axelsson P, et al. Some risk factors for periodontal bone loss in 50-year-old individuals. A 10-year cohort study. J Clin Periodontol. 2004;31(7):489–496.
- Heasman L, Stacey F, Preshaw PM, et al. The effect of smoking on periodontal treatment response: a review of clinical evidence. J Clin Periodontol. 2006;33(4):241–253.
- Preshaw PM, Heasman L, Stacey F, et al. The effect of quitting smoking on chronic periodontitis. J Clin Periodontol. 2005;32(8):869–879.
- Neto JB, Rosa EF, Pannuti CM, et al. Smoking and periodontal tissues: a review. Braz Oral Res. 2012;26(spe1):25–31.
- Morozumi T, Kubota T, Sato T, et al. Smoking cessation increases gingival blood flow and gingival crevicular fluid. J Clin Periodontol. 2004;31(4):267–272.
- Nair P, Sutherland G, Palmer RM, et al. Gingival bleeding on probing increases after quitting smoking. J Clin Periodontol. 2003;30(5):435–437.