Abstract
Objective
To explore oral health by increasing degree of obesity and the influence of modifying factors.
Materials and methods
A cross-sectional design was used. Swedish females (n = 118; 18–35 years) with morbid obesity were recruited from the BAriatric SUbstitution and Nutrition study (BASUN). Body mass index (BMI) was used as continuous and categorized into 35–39.9 kg/m2/40–44.9 kg/m2/≥45 kg/m2. Oral examinations assessed dental caries using the ICDAS system, periodontal status and saliva characteristics. Information on sociodemographics, oral health behaviour and symptoms was collected via a questionnaire.
Results
Mean BMI was 42.2 kg/m3 (SD 4.0; range 35.0–63.7). Significantly higher frequencies of dentine caries (p = .001) and total caries (p = .046) were found with higher BMI with an increase in total caries by 0.59 tooth surface (p = .025) for each increasing BMI degree. There were consistent associations between obesity and dentine caries for the group with the highest BMI (≥45), adjusted RR 2.08 (95% CI 1.20–3.61), and all stages of caries, adjusted RR 1.41 (95% CI 1.02–1.96). High scores were found for dental plaque (50.2%) and gingivitis (34.5%).
Conclusion
Young obese women exhibited poor oral health with higher caries levels by higher BMI. Dental care should adapt the prevention efforts for obese individuals. Trial Registration: The trial was prospectively registered on March 03; 2015; NCT03152617
Keywords:
The manuscript is structured in accordance with the STROBE guidelines (von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4:e296).
Introduction
The prevalence of overweight and obesity in both industrialized and developing countries has increased steadily in recent decades and the proportion classified as obese in the adult population worldwide was estimated in 2016 to be 13% (650 million people) [Citation1]. In Sweden, the corresponding share is about 16% [Citation2]. Obesity is generally defined by body mass index (BMI, kg/m2), with a BMI ≥30–34.99 classified as obesity class I, BMI 35–39.99 classified as class II, and BMI ≥40 classified as Class III or morbid obesity [Citation3], a classification widely used in scientific studies [Citation4,Citation5]. Additional classifications have been proposed for morbid obesity, particularly based on the trends of higher BMI rates [Citation1,Citation5]. Obesity rates differ between men and women, e.g. men being heavier in industrialized countries [Citation6] however, women seek obesity treatment to a greater extent than men [Citation7–9].
Obesity is a strong risk factor for developing several non-communicable diseases, such as type 2 diabetes, cardiovascular disease, and some cancer types [Citation10–12]. The effect of obesity on oral health is less studied. Associations have been observed between obesity and periodontal disease as well as tooth loss, but the causal relationships are unclear [Citation13,Citation14]. Relationships between obesity and dental caries have been found in the primary dentition [Citation15], but not among older children and adolescents [Citation16]. A few studies have examined the association between obesity and caries in adults with contradictory results; some showing an association [Citation17,Citation18] while others found no association [Citation19] or even an inverse relationship [Citation20].
The causal and behavioural patterns of general and oral health share similarities [Citation21]. The risk indicators for both obesity and oral disease include health habits; for instance, smoking [Citation21,Citation22]. There are also shared modifying factors such as age and socioeconomics; for instance, educational level [Citation23,Citation24].
Comorbidities and use of medication are common in obese individuals [Citation25], which may influence oral health; for instance, diminished saliva flow rates due to medication have been shown [Citation26]. In turn, this is a known risk factor for the development of carious lesions, as are other saliva characteristics, such as low buffering capacity and high microbial counts [Citation27,Citation28].
With obesity not only increasing worldwide but also those classified with obesity growing heavier with higher BMI rates [Citation1], there is a need to explore the relationship between obesity and oral health in greater detail. So far, the described studies in the field have used the common definition of obesity, that is a BMI ≥30 kg/m2 [14, 29]; hence, how different degrees of increasing obesity above this threshold are associated with or affect the oral health remains to be studied. In addition, the contradictory results regarding the link between obesity and caries need to be further investigated. Based on the above-described rationale, it was hypothesized that the risk for dental caries would be greater with increasing degrees of obesity. Therefore, the objective of the study was to describe oral health in individuals with increasing degrees of obesity and investigate the effect of modifying factors on the associations between obesity and dental caries.
Materials and methods
Study design and setting
The study used a cross-sectional design and the setting for data collection was Region Västra Götaland, Sweden.
Participants
Study participants were recruited from the BAriatric SUbstitution and Nutrition study (BASUN) cohort, a prospective non-randomized trial aiming to observe long-term outcomes of surgical and medical obesity treatment [Citation25]. The BASUN study enrolled individuals referred to the Regional Obesity Centre of Region Västra Götaland, Sweden, between May 2015 and November 2017. Treatment eligibility criteria for subjects with BMI 35–40 kg/m2 was dependent on the presence of obesity-related comorbidities, while all subjects with BMI ≥40 qualified for inclusion. Understanding verbal and written information in the Swedish language was required. In total, 971 obese subjects were included in the BASUN cohort. At inclusion, all female subjects <35 years of age were asked to participate in the current sub-study on oral health and bone health (the latter to be presented elsewhere). The age and gender restrictions were based on the predominance of women undergoing bariatric surgery (ca 80%) [Citation9], with planned follow-ups over ten years before menopausal onset. This paper reports on data on oral health prior to obesity treatment.
The study was approved by the Regional Ethical Review Board of Gothenburg (reg. no 673-14) and the Swedish Radiation Protection Committee (reg. no 14-39). Written informed consent was obtained from all participants.
Data sources and variables
The data sources were selected information from the main study BASUN and information from oral examinations at dentist visits.
The main predictor variable was BMI (kg/m2). Data about BMI and current pharmaceutical treatment (glucose-lowering treatment, blood pressure treatment, lipid-lowering treatment, treatment for anxiety/depression, treatment with antipsychotics, pain medication, hypothyroidism treatment, ADHD treatment) were obtained from the main BASUN study [Citation25]. For the analyses, BMI was used both as a continuous variable and categorized as 35–39.9 kg/m2, 40–44.9 kg/m2 and ≥45 kg/m2. Pharmaceutical treatment was categorized into medication vs. no medication.
The data collection at the dental visit was conducted in the following order: saliva sampling, filling in a self-report questionnaire, registration of periodontal status, and registration of dental caries. All participants had refrained from eating, drinking, chewing gum and tooth brushing for one hour prior to the visit. Two experienced dentists conducted the clinical examinations: NT (main examiner) and ALÖ. Wisdom teeth were excluded from all recordings.
The outcome variable was dental caries registered according to the International Caries Detection and Assessment System, ICDAS-II [Citation30]. The two dentists completed a calibration programme for caries registration prior to data collection. This included repeated sessions of the online ICDAS training programme until deemed accuracy (https://www.iccms-web.com/content/icdas), followed by discussions and separate examination of 45 extracted teeth (225 tooth surfaces). The inter-examiner reliability reached 0.77 (95% CI 0.69–0.84), calculated by linear weighted Cohen’s kappa (WK). Re-examination after six weeks produced an intra-examiner reliability of 0.75 (95% CI 0.65–0.85) (NT) and 0.70 (95% CI 0.62–0.79) (ALÖ), respectively. Five volunteers with altogether 630 tooth surfaces were examined clinically, with an inter-examiner WK of 0.88 (95% CI 0.85–0.92). The main examiner (NT) re-examined two persons after two months, intra-examiner WK 0.66 (95% CI 0.65–0.78). Four digital bitewing radiographs from each of seven randomly selected patients (420 tooth surfaces) in the main study were checked for reliability of radiographic diagnostics (NT and PL), producing an inter-examiner WK of 0.73 (95% CI 0.66–0.80) and an intra-examiner WK for both examiners of 0.89 (95% CI 0.84–0.94).
The registration of dental caries in the participants was carried out by clinical examination completed with radiographs. Before the visual examination, all teeth were professionally cleaned with prophy paste RDA 170 and a prophylaxis cup, flossed and then carefully air-dried. When needed, the visual examination was facilitated/aided by a ball-ended explorer to check surface shape or lesser cavities cautiously [Citation31]. All tooth surfaces (buccal, lingual, mesial, distal, occlusal) were scored according to the ICDAS II system from 0 (sound surface) to 6 (distinct excessive cavity with visible dentine). The radiographic examination comprised four digital bitewing radiographs, exposed in a standardized manner and examined under dimmed lighting on an Olorin Vista Line® monitor (Olorin AB, Kungsbacka, Sweden). Three tooth surfaces were radiographically scored (mesial, occlusal, distal) for lesion depth and type of restoration according to the ICDAS II coding system (Supplemental Figure 1). The clinical and radiographic recordings were weighted together for the mesial, occlusal and distal surfaces. If the difference between the clinical and the radiological valuation was maximally one step, the highest value was used. If a greater discrepancy was noted, the radiographs were re-examined. Noted differences occurred on surfaces where decay may be difficult to detect clinically, i.e. approximal surfaces and under dental fillings. For the analyses, dental caries was categorized as enamel caries (ICDAS codes 1–2), dentine caries (ICDAS codes 3–6) and total caries (ICDAS codes 1–6). Sealants, fillings, and missing teeth were recorded according to ICDAS protocols.
Variables to describe the cohort and to account for potential confounding completed the registrations. Dental plaque scores were registered on four surfaces (mesial, buccal, distal, lingual) of six index teeth according to Ramfjord, 1967 (coded 0/1) [Citation32]. Gingivitis was assessed by the presence of bleeding on probing (BOP) at two sites per tooth (mesiobuccal, distobuccal; coded 0/1). Periodontal pocket depth (PPD) was measured at the same two sites on each tooth from the gingival margin to the bottom of the gingival crevice [Citation33]. Periodontitis was considered present when the pocket depth was ≥3.5 mm (coded 0/1). The examiners underwent training and calibration sessions for the periodontal examination with an experienced dental hygienist.
Saliva sampling included the collection of unstimulated and stimulated saliva. Unstimulated whole saliva was collected during 15 min with the participants in a relaxed position, drooling passively into a measuring cylinder while maintaining minimal movements of the lips, tongue and facial muscles. The participants then chewed on a paraffin pellet until soft for one minute, followed by swallowing all the saliva in the mouth. Stimulated whole saliva was then collected with the participant spitting actively while chewing the paraffin pellet for five minutes. The secretion rates for unstimulated and stimulated saliva, respectively, were calculated from each volume (ml/min). The limit for a very low flow rate of unstimulated saliva was set to ≤0.1 ml/min [Citation34]. For stimulated saliva, the limit for a low flow rate was set at <1.0 ml/min and for a very low flow rate at less than 0.7 ml/min [Citation34]. The buffer capacity (low/medium/high) of the stimulated saliva was determined chairside (CRT buffer®, Ivoclar Vivadent, Lichtenstein). Laboratory analyses to obtain total mutans streptococci and lactobacilli counts per colony-forming unit (CFU)/ml of stimulated saliva were performed at the Department of Cariology, Institute of Odontology, University of Gothenburg, Sweden. Mutans streptococci were cultivated on mitis salivarius bacitracin and lactobacilli on rogosa agar under anaerobic conditions at 37 °C for two and three days, respectively. Mutans streptococci and lactobacilli were dichotomized in counts >1,000,000 CFU/ml and >100,000 CFU/ml, respectively.
The self-report questionnaire included questions on age (dichotomized into 18–29 years/30–35 years), educational level (post-secondary schooling >12 years vs. maximum secondary school ≤12 years) and marital status (cohabiting vs. not cohabiting). Oral health habits were represented by tooth brushing (twice daily vs. once a day/a few times a week/seldom or never), interdental cleaning (daily/3–5 times a week vs. 1–2 times a week/never), smoking (never smoked/stopped smoking ≥1 year ago vs. smoke occasionally/smoke daily), visits to the dentist in the last five years (once a year/3–4 appointments vs. 1–2 appointments/never), and reason for the last dental appointment (routine visit vs. pain/other problems).
Data management and statistical methods
Data were controlled and managed in Microsoft Excel® (Microsoft Corporation, WA, USA). Statistical analyses were carried out using the SPSS® statistical software (Statistical Package for Social Sciences) version 26 (IBM Corporation, NY, USA).
For the analyses, categorized and dichotomized variables as described above were used. The analyses comprised descriptive statistics including numbers, percentages, means, standard deviation (SD), median values and range, when indicated. Chi-square tests were applied to analyse differences between BMI categories and categorical variables: sociodemographic characteristics, medication and oral health habits. One-way ANOVA with the post-hoc Tukey test was used to evaluate the difference of means in caries severity between BMI categories. Tests of linear trends with contrast were carried out. Graphical presentation and linear regression models described the relation between continuous BMI and dental caries. To explore the impact of possible confounders, the caries experience by BMI categories was analysed by generalized linear models using Poisson distribution. To account for possible overdispersion, robust estimation was used. Crude and univariate analyses were followed by full models, including possible confounders as covariates, and using the least obese group as reference (BMI 35–39.9). For the regression models, the numbers of microbiota were transferred into logarithms to achieve normal distributions. Spearman’s correlation coefficient was used to calculate the correlation between clinically and radiologically registered caries (ICDAS0 r = 0.959, ICDAS1–2 r = 0.922, ICDAS3–6 r = 0.930).
Results
A total of 118 participants were included in the study (Supplemental Figure 2). Their mean BMI was 42.2 kg/m2 (SD 4.0, median 41.8, range 34.9–63.7) with mean age 27.8 years (SD 4.9).
Descriptive data on socioeconomic characteristics and oral health habits of the cohort in total and by BMI group are presented in . Most of them were cohabiting (55.1%) and four out of five had an education ≤12 years. Pharmaceutical treatment was common (46.6%) and one out of five reported smoking daily or occasionally, with younger participants (<30 years) smoking more frequently, 30.3% vs. 11.5% among older participants (p = .001, not in tables). Poor oral hygiene habits were common, with one-third of the total group reporting toothbrushing less than twice daily and two-thirds reporting no regular interdental cleaning. More than half of the individuals in the highest BMI group (BMI ≥ 45) reported infrequent visits to the dentist (p = .015). Likewise, oral problems or pain as the reason for the last visit was most common in this group (p = .037).
Table 1. Sociodemographic characteristics and oral health habits of the cohort in total and by BMI group.
The outcome in dental caries and other clinical characteristics of the total cohort and by BMI group are presented in . There was a significantly lower mean number of tooth surfaces with no evidence of caries (ICDAS0) with higher BMI group (p = .045). There were corresponding higher mean numbers of tooth surfaces with dentine caries (ICDAS3–6) and total caries (ICDAS1–6) with higher BMI group (p = .001 and p = .046, respectively) and the differences were consistent between the group with BMI ≥ 45 vs. the other groups (not in tables). Moreover, parallel significant linear trends were seen for ICDAS0 (p = .017), ICDAS3–6 (p = .001) and for ICDAS1–6 (p = .017). No such differences or trends were seen for enamel caries (ICDAS1–2). Most missing teeth had been extracted due to orthodontic treatment according to reports by the participants; still, 31% of missing teeth were reported as extracted due to caries (not in tables).
Table 2. Clinical characteristics of the total cohort and by BMI group.
The mean plaque score and overall mean gingivitis score were high in the total sample (50.3% and 34.5%, respectively) with no statistically significant differences between the BMI groups. The mean number of sites with PPD, defined as ≥3.5 mm, was 2.3 (SD 5.5).
shows the salivary characteristics of the sample. The mean stimulated flow rate was 1.5 ml/min; however, nearly one-quarter of the participants produced less than 1.0 ml/min and one out of ten less than 0.7 ml/min (not in table). The mean unstimulated flow rate was 0.2 ml/min with more than a third (36.4%) having a flow rate ≤0.1 ml/min. There were no statistically significant differences in flow rates between the BMI groups. Pharmaceutical treatment was not related to an unstimulated saliva flow rate <0.1 ml/min (p = .455) or to a stimulated saliva flow rate <0.7 ml/min (p = .581). However, among those being on pharmaceutical treatment there were more individuals with a stimulated saliva flow rate <1.0 ml/min than individuals with a higher flow rate (64.3% vs. 41.1%, p = .033).
Table 3. Salivary characteristics of the cohort in the total group and by BMI group.
The microbiota exhibited moderate mean numbers of mutans streptococci while the mean numbers of lactobacilli were higher with one-fifth of the total sample having numbers exceeding 100,000 CFU/ml. The only statistically significant difference in microbiota between the BMI groups was having mutans streptococci exceeding 1,000,000 CFU/ml; however, few participants exhibited such numbers.
Linear regression models using continuous BMI as the predictor showed a B 0.29 (p = .144) for ICDAS1–2, B 0.29 (p = .007) for ICDAS3–6 and B 0.59 (p = .025) for ICDAS1–6. The B coefficient shows how much the dependent variable (surfaces with caries) will increase in average if the independent variable increases by one step (BMI). displays a graphical presentation for ICDAS1–6. Poisson regression models () using the trichotomous BMI as the independent factor and caries as the dependent variable (ICDAS1–2, ICDAS3–6, ICDAS1–6) with the least obese group as reference (BMI 35–39.9) revealed consistent statistically significant associations regarding dentine caries and total caries for the group with BMI ≥ 45, in both crude analyses and in multivariate models with adjustments for age and other covariates (socioeconomy, general health, oral health habits, saliva characteristics). The strongest crude association was shown for dentine caries (ICDAS3–6) in the group with BMI ≥ 45: RR 2.43 (95% CI 1.35–4.37), modified in the fully adjusted model to RR 2.08 (1.20–3.61). For enamel caries and for the group with a BMI between 40 and 44.9, most associations were not statistically significant.
Figure 1. Scatter-plot with linear correlation between Body Mass Index (BMI) and total dental caries (International Caries Detection and Assessment System, ICDAS1–6).
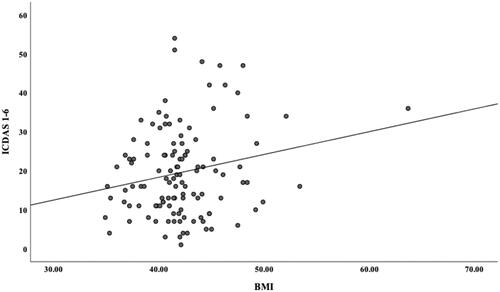
Table 4. Associations between obesity (predictor variable) and dental caries (outcome variable), crude and adjusted as indicated.
Discussion
The key results in this study of young obese women were the significant trends of a higher frequency of dental caries lesions and fewer tooth surfaces with no caries with higher BMI. The associations were strongest for the group with BMI ≥ 45 in dentine caries and independent of differences in socio-economy, medication, health habits and saliva characteristics. The periodontal health status indicated unfavourable oral health, and low flow rates for both stimulated and unstimulated saliva were common.
The study has some limitations. The cross-sectional design enabled investigation of associations in the regression models but no causations which must be considered in the interpretation. For that purpose, longitudinal designs are needed. A limitation was that the sample size was controlled by the main BASUN study. However, the number of participants in the present sub-study is well comparable with that in similar studies [Citation16,Citation29]. It can be seen as another limitation that individuals with a BMI <35 were not included but since our goal was to study different degrees of obesity, those with the lowest degree of morbid obesity constituted the reference group. On the other hand, the wide range in BMI mirrored the growing obesity epidemic in the world [Citation1]. This made it possible to investigate higher degrees of obesity in relation to oral health, completing earlier studies, most of which only dichotomized body weight into obese/not obese [Citation14].
Possible bias might have been at hand in the sampling of only women, however this reflects the greater number of women undergoing obesity treatment [Citation7,Citation8,Citation25]. The BASUN study also did not include people with language barriers or reluctance to participate [Citation25].
Measures were taken to ensure the quality of the clinical data collection. Thus, the clinical examinations were based on a broad protocol and performed under standardized conditions and repeated calibration sessions showed good intra- and interrater reliability. The saliva collection was supervised so that no mouth or cheek movements were made during the collection of the unstimulated saliva [Citation34]. It would have been ideal if the saliva collection had been performed at the same time of day for all individuals to take circadian rhythm into account [Citation35], but this was impossible for logistical reasons. Another limitation is that the present study did not include dietary data or information on fluoride supplements that could possibly affect the outcome. For the caries recording, the seven-level ICDAS criteria [Citation30] were used to assess accurately the status of tooth surfaces and quantify the prevalence of dental caries. The system has been increasingly used in recent years, especially in epidemiological studies, and shows good validity and reliability [Citation36]. Mostly, the ICDAS criteria for visual examination have been used while radiographic information has been recognized as an important addition, particularly to diagnose interproximal caries [Citation37]. Together with the careful calibrations, this probably ensured good sensitivity of the caries registration [Citation30,Citation36,Citation38].
As mentioned above, most studies exploring associations between oral health and obesity have hitherto used the definition of obesity by the WHO; that is, a BMI of 30 or higher, with diverging results [Citation14,Citation29]. However, the growing obesity epidemic over recent decades, both in Sweden and internationally [Citation1,Citation2] implies a need to study the oral health of individuals with more extreme obesity. Thus, we included candidates for bariatric surgery in our sample and the results showed that individuals with the most severe obesity condition were those at risk of dental caries. Moreover, we found a significant linear trend of increasing caries frequency with higher BMI. These findings should be considered when planning preventative dental efforts for obese individuals.
The caries prevalence in the study needs to be seen in its context; however, so far, this has been sparsely studied in the general adult Swedish population. One recent study from southern Sweden revealed lower numbers of decayed surfaces in corresponding ages than in our study (dentine caries, mean 3.7 vs. 4.5 surfaces) [Citation39]. Thus, the caries frequency in the least obese group (BMI 35–39.9, mean 3.2 surfaces) in our study was close to the population means in the study referred to. This might support the reasoning of an increased risk of caries only at higher BMI levels. Internationally, few studies have reported on the caries prevalence in adults and the data collection methods differ [Citation40]. However, Sweden has been recognized as having among the lowest caries prevalence rates in adults [Citation41].
The participants’ periodontal health condition was generally poor, irrespective of BMI group. In the earlier mentioned Swedish study of a general population, the mean plaque score was lower, 11.6% in 20-year olds and 19.2 in 30-year olds [Citation39], to be compared with 50.3% in our study with similar differences for gingivitis. However, in a recent study, young Swedish adults scored more like the obese participants in our study [Citation42]. The authors discussed their findings in terms of inadequate preventative programs, although such programs are the standard in Swedish dental care for young people. For obese individuals, the challenges and stress of struggling with their excessive weight may leave them with less energy to maintain good oral hygiene [Citation43]. Many participants in the study also reported poor oral health behaviour, and it is especially notable that individuals in the highest BMI group reported regular dental visits less often than individuals in the other BMI groups. Obese individuals may be ashamed of their situation and experience stigmatization [Citation44–45], which, in turn, may lead to avoiding dental care [Citation46].
The associations between dental caries and obesity in the study remained statistically significant and strong for the highest BMI group after accounting for potential modifying factors. Low socioeconomic status has been identified as being associated with both obesity [Citation23,Citation47] and poor oral health [Citation24]. However, in a study of Swedish women, the associations between dental health and cardiovascular disease were independent of socioeconomic status, which is in line with our findings [Citation48]. Another possible modifying factor is smoking, which may have been used as a weight control strategy as smoking is believed to suppress appetite [Citation49]. Still, it did not affect the association between obesity and caries. Smoking is linked to oral disease in general [Citation21], but no clear association with caries has been established [Citation50].
Obesity has been recognized to affect both the flow and composition of the saliva [Citation51]. This is in line with our findings, as low flow rates were common for both unstimulated and stimulated saliva using generally accepted definitions [Citation34]. Moreover, the flow rates in the study were well below the mean values with respect to age and gender found in a Swedish population study [Citation52]. Pharmaceutical treatment is a known factor for hyposalivation [Citation27] and was common in all BMI groups in the study; however, we found inconsistent associations between such treatment and hyposalivation. Possibly, since the individuals in the sample were relatively young, the time of pharmaceutical use may have been too short to produce an effect on the saliva flow.
Regarding cariogenic microorganisms, higher numbers of mutans streptococci could be expected given the caries scores. On the other hand, the lactobacilli counts were high, and the caries process involves both mutans streptococci and lactobacilli [Citation53] and other bacteria [Citation54]. The unexpected lower mutans streptococcus counts by higher BMI in our results might possibly be seen as a chance variation. High bacterial counts mirror the consumption of carbohydrates and future studies should include dietary aspects [Citation21,Citation55]. Likewise, both saliva flow rates and saliva composition should be investigated further in obese subjects, including the full variety of substances in saliva, e.g. electrolytes. However, in our study, the chosen saliva factors (flow rate and microbiota) had no significant influence on the associations between obesity and dental caries.
Finally, the external validity of the results should be considered. Based on the sampling framework, and compared to other studies, the results should be reasonably generalizable to similar Western contexts.
To conclude, young obese women in the study exhibited poor oral health and higher caries levels with higher BMI. The associations were independent of modifying factors. Dental care should adapt the prevention efforts for obese individuals.
Author contributions
NT conceptualization, formal analyses, writing-original draft preparation, funding acquisition. PL methodology – odontological aspects, resources, writing – review and editing. KM methodology – medical aspects, writing – review and editing. LF methodology – medical aspects, writing – review and editing. BE methodology – medical aspects, writing – review and editing. ALÖ: conceptualization, methodology, data curation, formal analyses, funding acquisition, supervision, writing-original draft preparation.
Supplemental Material
Download Zip (118.7 KB)Acknowledgements
The authors express their gratitude to the clinics where the data collection was carried out.
Disclosure statement
No potential competing interest related to the current work was reported by any of the authors.
Data availability statement
The data that support the findings of this study are not openly available due to reasons of sensitivity but available from the corresponding author upon reasonable request.
Additional information
Funding
References
- World Health Organization [Internet]. Geneva: WHO; c2019. Obesity and overweight. 2021 [cited 2021 Apr 12]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- Public Health Agency of Sweden [Internet]. [Overweight and obesity]. 2021 [cited 2021 May 16]. Available from: https://www.folkhalsomyndigheten.se/livsvillkor-levnadsvanor/fysisk-aktivitet-och-matvanor/overvikt-och-fetma/. Swedish.
- World Health Organization. Report of a WHO consultation on obesity. Obesity: preventing and managing the global epidemic. Geneva (Switzerland): World Health Organization; 2000.
- Sturm R. Increases in morbid obesity in the USA: 2000–2005. Public Health. 2007;121(7):492–496.
- Sturm R, Hattori A. Morbid obesity rates continue to rise rapidly in the United States. Int J Obes. 2013;37(6):889–891.
- Kanter R, Caballero B. Global gender disparities in obesity: a review. Adv Nutr. 2012;3(4):491–498.
- Guerra ME, Jean RA, Chiu AS, et al. The effect of sociodemographic factors on outcomes and time to discharge after bariatric operations. Am J Surg. 2020;219(4):571–577.
- Holmberg D, Santoni G, Xie S, et al. Gastric bypass surgery in the treatment of gastro-oesophagal reflux symptoms. Aliment Pharmacol Ther. 2019;50(2):159–166.
- Scandinavian Obesity Surgery Registry (SOReg) [Internet]. [cited 2021 May 16]. Available from: http://www.ucr.uu.se/soreg. Swedish.
- Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371(9612):569–578.
- Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 2009;9:88.
- Afshin A, Forouzanfar MH, Reitsma MB, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27.
- Keller A, Rohde JF, Raymond K, et al. Association between periodontal disease and overweight and obesity: a systematic review. J Periodontol. 2015;86(6):766–776.
- Nascimento GG, Leite FR, Conceicao DA, et al. Is there a relationship between obesity and tooth loss and edentulism? A systematic review and meta-analysis. Obes Rev. 2016;17(7):587–598.
- Manohar N, Hayen A, Fahey P, et al. Obesity and dental caries in early childhood: a systematic review and meta-analyses. Obes Rev. 2020;21(3):e12960.
- Shivakumar S, Srivastava A, Shivakumar G. Body mass index and dental caries: a systematic review. Int J Clin Pediatr Dent. 2018;11(3):228–232.
- Hamasha AA, Alsolaihim AA, Alturki HA, et al. The relationship between body mass index and oral health status among Saudi adults: a cross-sectional study. Community Dent Health. 2019;36(1):217–222.
- Akarsu S, Karademir SA. Association between body mass index and dental caries in a Turkish subpopulation of adults: a cross-sectional study. Oral Health Prev Dent. 2020;18:85–89.
- Idrees M, Hammad M, Faden A, et al. Influence of body mass index on severity of dental caries: cross-sectional study in healthy adults. Ann Saudi Med. 2017;37(6):444–448.
- Song IS, Han K, Ryu JJ, et al. Obesity is inversely related to the risks of dental caries in Korean adults. Oral Dis. 2017;23(8):1080–1086.
- Sheiham A, Watt RG. The common risk factor approach: a rational basis for promoting oral health. Community Dent Oral Epidemiol. 2000;28(6):399–406.
- Moynihan PJ, Kelly SAM. Effect on caries of restricting sugars intake: systematic review to inform WHO guidelines. J Dent Res. 2014;93(1):8–18.
- Norberg M, Lindvall K, Stenlund H, et al. The obesity epidemic slows among the middle-aged population in Sweden while the socioeconomic gap widens. Glob Health Action. 2010;10:3.
- Schwendicke F, Dörfer CE, Schlattmann P, et al. Socioeconomic inequality and caries: a systematic review and meta-analysis. J Dent Res. 2015;94(1):10–18.
- Höskuldsdóttir G, Mossberg K, Wallenius V, et al. Design and baseline data in the BAriatic surgery SUbstitution and Nutrition study (BASUN): a 10-year prospective cohort study. BMC Endocr Disord. 2020;20(1):23.
- Närhi TO, Vehkalahti MM, Siukosaari P, et al. Salivary findings, daily medication and root caries in the old elderly. Caries Res. 1998;32(1):5–9.
- Närhi TO, Meurman JH, Ainamo A, et al. Association between salivary flow rate and the use of systemic medication among 76-, 81-, and 86-year-old inhabitants in Helsinki, Finland. J Dent Res. 1992;71(12):1875–1880.
- Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet 2007;369(9555):51–59.
- Ribeiro-Silva AE, Baptista Menezes AM, Demarco FF, et al. Obesity and dental caries: systematic review. Rev Saúde Pública. 2013;47(4):799–812.
- Ismail AI, Sohn W, Tellez M, et al. The international caries detection and assessment system (ICDAS): an integrated system for measuring dental caries. Community Dent Oral Epidemiol. 2007;35(3):170–178.
- Topping GVA, Pitts NB. Clinical visual caries detection. In: Pitts NB, editor. Detection, assessment, diagnosis and monitoring of caries. Basel: Karger; 2009. p. 15–41.
- Ramfjord SP. The periodontal disease index (PDI). J Periodontol. 1967;38(6 Part II)Suppl:602–610.
- Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38(6)Suppl:610–616.
- Nauntofte B, Tenovuo J, Lagerlöf F. Secretion and composition of saliva. In: Fejerskov O, Kidd EAM, editors. Dental caries: the disease and its clinical management. Oxford: Bleckwell Munksgaard; 2003. p. 7–27.
- Humphrey SP, Williamson RT. A review of saliva: normal composition, flow, and function. J Prosthet Dent. 2001;85(2):162–169.
- Ekstrand KR, Gimenez T, Ferreira FR, et al. The international caries detection and assessment system – ICDAS: a systematic review. Caries Res. 2018;52(5):406–419.
- Walsh T, Macey R, Riley P, et al. Imaging modalities to inform the detection and diagnosis of early caries. Cochrane Database Syst Rev. 2021;15:3.
- Agustsdottir H, Gudmundsdottir H, Eggertsson H, et al. Caries prevalence of permanent teeth: a national survey of children in Iceland using ICDAS. Community Dent Oral Epidemiol. 2010;38(4):299–309.
- Norderyd O, Koch G, Papias A, et al. Oral health of individuals aged 3-80 years in Jönköping, Sweden during 40 years (1973–2013). II. Review of clinical and radiographic findings. Swed Dent J. 2015;39(2):69–86.
- Crocombe LA, Mejia GC, Koster CR, et al. Comparison of adult oral health in Australia, the USA, Germany and the UK. Aust Dent J. 2009;54(2):147–153.
- Kassebaum NJ, Bernabé E, Dahiya M, et al. Global burden of untreated caries: a systematic review and metaregression. J Dent Res. 2015;94(5):650–658.
- Ericsson JS, Abrahamsson KH, Östberg AL, et al. Periodontal health status in Swedish adolescents: an epidemiological, cross-sectional study. Swed Dent J. 2009;33(3):131–139.
- Salleh MR. Life event, stress and illness. review article. Malays J Med Sc. 2008;15:9–18.
- Homer CV, Tod AM, Thompson AR, et al. Expectations and patients’ experiences of obesity prior to bariatric surgery: a qualitative study. BMJ Open 2016;6(2):e009389.
- Duarte C, Matos M, Stubbs RJ, et al. The impact of shame, self-criticism and social rank on eating behaviours in overweight and obese women participating in a weight management programme. PLoS One 2017;12(1):e0167571.
- van der Zande MM, Exley C, Wilson SA, et al. Disentangling a web of causation: an ethnographic study of interlinked patient barriers to planned dental visiting, and strategies to overcome them. Community Dent Oral Epidemiol. 2021;49(2):144–157.
- Lahmann PH, Lissner L, Gullberg B, et al. Sociodemographic factors associated with long-term weight gain, current body fatness and central adiposity in Swedish women. Int J Obes Relat Metab Disord. 2000;24(6):685–694.
- Cabrera C, Hakeberg M, Ahlqwist M, et al. Can the relation between tooth loss and chronic disease be explained by socio-economic status? A 24-year follow-up from the population study of women in Gothenburg, Sweden. Eur J Epidemiol. 2005;20(3):229–236.
- Audrain-McGovern J, Benowitz NL. Cigarette smoking, nicotine, and body weight. Clin Pharmacol Ther. 2011;90(1):164–168.
- Benedetti G, Campus G, Strohmenger L, et al. Tobacco and dental caries: a systematic review. Acta Odontol Scand. 2013;71(3–4):363–371.
- Roa I, Del Sol M. Obesity, salivary glands and oral pathology. Colomb Med. 2018;49(4):280–287.
- Flink H, Bergdahl M, Tegelberg A, et al. Prevalence of hyposalivation in relation to general health, body mass index and remaining teeth in different age groups of adults. Community Dent Oral Epidemiol. 2008;36(6):523–531.
- Tanzer JM, Livingston J, Thompson AM. The microbiology of primary dental caries in humans. J Dent Educ. 2001;65(10):1028–1037.
- Takahashi N, Nyvad B. The role of bacteria in the caries process: ecological perspectives. J Dent Res. 2011;90(3):294–303. 201
- Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ 2012;346:e7492.
