Abstract
Objectives
The paper reports the demographic characteristics of patients with lichenoid lesions affecting only the upper labial mucosa, with or without associated lesions in the maxillary anterior gingiva, alongside the lesions’ clinical and histopathological features, treatment, follow-up and prognosis. Also, a new case with lengthy follow-up is presented.
Materials and methods
A systematic review was performed in line with PRISMA guidelines. The literature search sources were PubMed, Scopus and Web of Science.
Results
In all, 26 patients (21 women, 5 men) were included in the review. 80.8% (n = 21) of the labial lesions were clinically erythematous and 19.2% (n = 5) were accompanied by white striations. The gingiva was affected in 46.2% of cases. All patients (100%, n = 24) reported symptoms. All of the lesions presented histological features of lichenoid inflammation. Granulomas were noted in 65.4% (n = 17) of the lesions. Topical corticosteroid was the most frequent therapy (89.5%, n = 17).
Conclusions
Lichenoid lesions found solely in the upper labial mucosa, with or without adjacent gingival lesions, are rarely reported in the literature, and the reporting is often incomplete. A definitive aetiology could not be established for the lesions. Likewise, there is little information about this condition’s long-term prognosis.
Introduction
The first description of lichenoid tissue reaction (LTR), from 1973, characterizes it as a cascade of histological events that leads to epidermal basal cell damage and clinical presentation of lichen-like changes in the skin and mucosa [Citation1]. Lichenoid tissue reaction (or interface dermatitis) is seen in diverse mucocutaneous disorders, such as lichen planus (LP), lupus erythematosus and graft-versus-host disease (GVHD) [Citation2]. Of these, LP is considered the prototypic and most common, with global prevalence of 0.2–1% [Citation3,Citation4]. A chronic inflammatory disorder with unknown aetiology [Citation5], LP can also affect the oral mucosa [Citation6,Citation7]. Typically, oral lichen planus (OLP) presents as bilateral reticular buccal lesions in a middle-aged female patient. In addition to reticular type, the clinical forms of OLP include atrophic, erosive, papular, plaque and bullous. Frequently, these forms appear in combination [Citation6,Citation7].
Several criteria for a diagnosis of OLP have been proposed [Citation8–10], but no uniform, globally accepted criteria exist. The criteria employed thus far include both clinical and histopathological features of OLP, with proposals that lesions not compatible with the OLP criteria should be called oral lichenoid lesions (OLL) [Citation8–10]. Since OLL represent a wide range of chronic inflammatory changes to the oral mucosa wherein the clinicopathological features are similar to (and sometimes indistinguishable from) OLP [Citation10], differential diagnosis of these two conditions can be challenging. Although lichen planus is a condition with unknown aetiology, some lichenoid lesions may be caused by, for instance, medications, contact with dental restorative materials and GVHD [Citation6].
Although lichenoid lesions occasionally occur in the labial mucosa as part of OLP or OLL, their appearance solely in the upper labial mucosa area is rare. The first report specifically on upper labial mucosal lichenoid lesions, in association with anterior composite restorations in 12 patients, was published in 1996 [Citation11]. For 10 patients, a biopsy of the lesions was performed. The authors hypothesized that hypersensitivity to composite restorations, trauma from fillings, lip parafunction, increased lip pressure or the effect of microbes (Candida in particular) might contribute to the development of these lesions [Citation11]. A later report, by an overlapping group, described 25 patients with lichenoid reaction of the upper labial mucosa, some of them with erythema of the upper anterior attached gingiva [Citation12]. For most patients, diagnosis was performed only clinically. All patients except one had upper anterior buccal resin restorations. Based on the observation that most lesions healed after treatment with chlorhexidine, the authors suspected an association between microbial plaque and the lichenoid lesions. Also, hyposalivation was posited to be a co-factor in the lesions’ development [Citation12]. In 2006, a study describing six cases of lichenoid lesions of the upper labial mucosa and, in some patients, also the adjacent anterior gingiva found that, histopathologically, they displayed features of both lichenoid and granulomatous inflammation [Citation13]. The authors suggested the name ‘lichenoid and granulomatous stomatitis’ for the condition. Two of their patients may have experienced unusual drug reactions [Citation13]. Although a lichenoid reaction with granulomatous stomatitis has appeared also in other locations of the oral mucosa [Citation14], a retrospective study of 24 cases of lichenoid lesions of the upper labial mucosa with or without lichenoid lesions of the adjacent gingiva, where a biopsy was performed in four cases, found microscopic features consistent with LP or lichenoid inflammation without granuloma formation [Citation15]. Most patients in the latter study were receiving medication for cardiovascular disease, and presence of dental plaque and calculus was common [Citation15]. Still, the small number of cases precluded definitive conclusions as to the pathogenesis of the lesions.
The above-mentioned studies and a few conference abstracts on similar cases [Citation16–18] constitute the bulk of the work in the field. In light of this, a systematic literature review was conducted to reveal the demographic characteristics of patients with lichenoid lesions affecting only the upper labial mucosa, with or without associated lesions of the maxillary anterior gingiva. We also present the clinical and histopathological features, treatment, follow-up and prognosis of the lesions. In addition, the report describes a new case, with many years of follow-up.
Materials and methods
For a systematic review of the material available via PubMed, Scopus and Web of Science, we applied a literature search strategy with a combination of MeSH and free-text terms in English related to lichenoid reaction, granulomatous reaction, the upper lip and the labial mucosa ( presents the full set of search terms). An information specialist was involved in the search, which encompassed all material indexed as of 6 February 2021.
Figure 1. Flow diagram of the systematic review process, including searches of databases and other sources.
For inclusion, a report had to deal with cases of lichenoid lesions located in the upper labial mucosa either solely or also in the upper anterior gingiva, with clinical and histopathological examination. Another inclusion criterion was English-language reporting. Reports on cases lacking clinical or histopathological confirmation of the lesions were excluded.
We manually searched the reference lists of the works retrieved. Also, grey literature was searched, to uncover unpublished or ongoing trials. All articles retrieved, whether via the search strategy or from additional sources, were collected in RefWorks. After manual removal of duplicates, two of the authors (MH and MS) screened the titles and abstracts to identify those studies potentially meeting the inclusion criteria. Titles and abstracts not matching the criteria were excluded. Then, the full text of the remaining articles was assessed, and again those cases not meeting the inclusion criteria were excluded from consideration. Whenever there was insufficient information of the cases regarding meeting the inclusion criteria, the authors were contacted in efforts to obtain the relevant information.
The data from the cases fulfilling the inclusion criteria were compiled in an Excel spreadsheet, for data analysis following descriptive methods.
Assessment of the risk of bias: Having two reviewers assess the studies against the inclusion criteria was the main mechanism for ensuring quality. Any study not meeting the predefined criteria was not accepted for review. Formal quality-assessment tools were not relevant in this project.
The patient representing the case newly reported upon here gave informed consent. We conducted the review in accordance with the PRISMA statement guidelines. A diagram of the process’s flow is presented as .
Case report
A 56-year-old female was referred to the Oral and Maxillofacial Diseases Clinic of Kuopio University Hospital due to a smarting sensation of the oral mucosa. The patient’s medical history included obstructive sleep apnoea (OSA), lactose intolerance and medication with glucosamine for joint pain and with hormone replacement therapy (HRT). She was a non-smoker. Before the onset of symptoms, the patient was using analgesic drugs (e.g. etoricoxib).
Intraoral examination revealed natural dentition in good condition and erythema both of the upper labial mucosa and of the upper anterior labial gingiva. The patient reported experiencing a smarting and burning sensation affecting the upper lip, with difficulties in eating and talking. She had used 0.1% triamcinolone acetonide cream and amphotericin B lozenges for the condition, with no clear response.
A biopsy of the upper labial mucosa was taken, and the histopathological diagnosis was oral lichen planus (). Although the biopsy did not identify fungal hyphae, an oral yeast culture revealed candidiasis. The patient was prescribed local antifungal treatment (amphotericin B) and 0.05% clobetasol cream. After treatment, some erythema remained, and the patient still suffered from a smarting sensation affecting the upper lip, accompanied by a taste of metal in the mouth. Later, 0.03% tacrolimus ointment for application two times a day was prescribed.
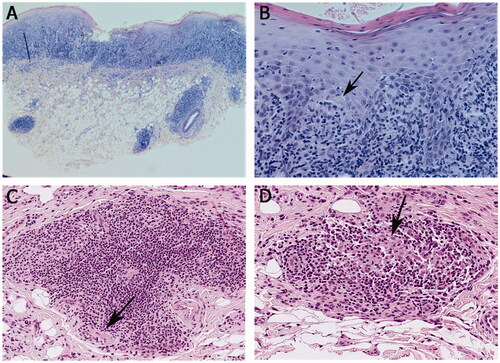
Figure 2. Histopathological findings from labial mucosal biopsies. (A, B) Hyperparakeratotic stratified squamous epithelium with saw-toothed rete ridges in some areas, basal cell degeneration, apoptotic basal keratinocytes (arrow in B) and inflammatory cell exocytosis. A dense band-like lymphocytic infiltration in the lamina propria and patchy inflammatory cell infiltrates in the submucosa. (C) An aggregate of monocytic inflammatory cells consisting mainly of lymphocytes, visible also perineurally (marked by the arrow). (D) Possible histiocytes visible in the infiltrate (indicated by the arrow). Haematoxylin and eosin stain, original magnification × 25 (A), × 200 (B), × 400 (C), × 400 (D).
Oral appliance therapy for OSA was initiated, after which the patient reported significant improvement in the oral symptoms. Clinically, slight erythema was visible locally in the marginal gingiva of the right canine. Sometime after treatment with clobetasol and tacrolimus ended, the smarting sensation returned. The patient was advised to use tacrolimus ointment again, in addition to which sodium-lauryl-sulphate-free toothpaste and avoidance of irritating food were recommended.
The patient was seen regularly every 1–24 months for 13 years. At the follow-up visits, the lesions varied clinically from obvious changes to only slight erythema yet always remained present (). The upper labial mucosa and upper anterior gingiva were variably erythematous at every visit. Occasionally, the upper labial mucosa presented slight reticular white striation, erosion and small whitish papules. On a few visits, the upper lip was slightly swollen. Candidiasis was diagnosed on a few occasions by oral yeast culture.
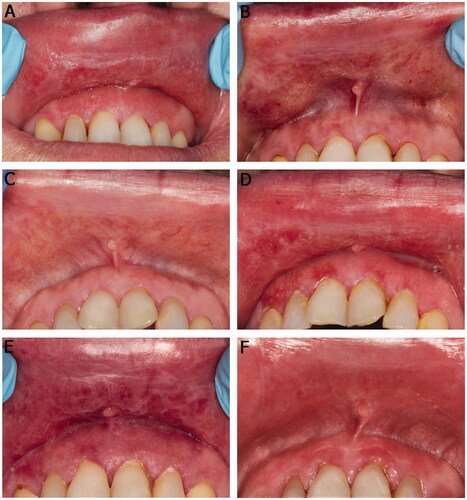
Figure 3. Clinical presentation of the upper labial mucosa and upper anterior labial gingiva over a 10-year span (A, B, D, and E). White reticular striae or papular lesions with erythema were seen at most visits. (C) A faint white stria to the labial mucosa after treatment with intralesional methylprednisolone (40 mg/ml) injection. (F) Slight erythema of the labial mucosa and gingiva on the last follow-up visit. The patient used tacrolimus when needed.
In laboratory tests performed to exclude other causes for the lesions and symptoms, complete blood count, red blood cell folate, serum B12, fasting plasma glucose, and serum zinc levels were normal. A swab test for herpes simplex virus was negative. Epicutaneous test for dental materials and prick tests for various vegetables and spices was negative. Likewise, tests for serum antinuclear antibodies and serum-extractable nuclear antigen antibodies were negative. Over the course of follow-up, the patient presented slowly progressing myopathy for which no specific aetiology was found. A new biopsy of the upper labial mucosa was taken four years after the first. This specimen too was histopathologically diagnosed as representing oral lichen planus (). The patient continued to experience smarting, a burning sensation and pain of variable intensity in the upper lip.
Multiple treatments to control the – sometimes extremely painful – symptoms were prescribed, by several clinicians. Among these were 0.1% betamethasone valerate cream, 0.05% clobetasol cream, 0.03 and 0.1% tacrolimus ointment, intralesional methylprednisolone (40 mg/ml) injection, systemic prednisolone, systemic acitretin, low-level biostimulative laser therapy and a chlorhexidine gel spray and mouth rinse. The candidiasis was treated with nystatin oral suspension, amphotericin B sucking tablets or systemic fluconazole. Regular scaling was performed. In addition, clinicians advocated rigorous oral hygiene. The patient suspended HRT treatment for a while, without any noticeable effect on the oral symptoms. Ultimately, the most effective short-term response of the lesions and symptoms to treatment was achieved with intralesional methylprednisolone, but relapse was inevitably seen.
Over the last two years of follow-up, the patient was applying 0.1% tacrolimus ointment nearly daily, using chlorhexidine mouth rinse occasionally, and visiting a dental hygienist regularly. She was not on any other medication. At the latest visit, the upper labial mucosa presented slight erythema and striation, and the upper anterior gingiva was erythematous (). Her symptoms were under rather good control.
Results
The literature review yielded 25 cases of clinically and histopathologically identified lichenoid lesions situated solely in the upper labial mucosa, with or without upper anterior labial gingival involvement. With the case detailed above, the total number of patients comes to 26. presents the demographic and clinical characteristics of all cases.
Table 1. Details of the demographic and clinical characteristics of the patients.
Mean age among the patients was 58.5 years, with a range of 33–72 years (). Most patients were female (80.8%, n = 21). For 20 patients, no information was available on the presence of any other medical conditions, while the remaining six had reported asthma (n = 1), multiple sclerosis (n = 1), osteoarthritis (n = 1), prostate hyperplasia (n = 1), hypertension (n = 1), hypercholesterolaemia (n = 1) and obstructive sleep apnoea (n = 1). For 69.2% of the cases (n = 18), no data pertaining to medication were available. Four patients (50%) were not on any systemic medication, while four did use medication: drugs for cardiovascular diseases (n = 3), nonsteroidal anti-inflammatory drugs (n = 2), HRT (n = 1) and glucosamine (n = 1). One patient was reported to suffer from allergies (to elastoplast), one was cited as having no allergies, and for the rest of the group (n = 24) there was no information on whether any allergies existed. Except for the patient introduced above, who was a non-smoker, no information on smoking status was available (n = 25). No data were available on drinking or family medical history for the patients.
In most cases (80.8%, n = 21), the labial mucosal lesions were clinically erythematous and occasionally accompanied by white striations (n = 5). Five patients’ lesions were described as ‘erythroleukoplakic, red, or white’, without more specific clinical description of each case. Ulceration was present in one patient. For five, the size of the lesions was reported; it averaged 12.6 mm (range: 5–20 mm). Alongside the lesions of the upper labial mucosa, gingival involvement was present in 46.2% of cases (n = 12), with the gingival lesions being erythematous in 92% of cases (n = 11). One case was part of the patient group displaying an ‘erythroleukoplakic, red, or white’ clinical appearance, without more specific information on the case in question. All patients for whom symptom data were available (n = 24) reported some symptoms: soreness, pain, swelling, burning and difficulties with mastication and oral hygiene; for two patients, no symptom data were available.
In 80.8% of cases (n = 21), no data related to dental plaque or calculus affecting the maxillary anterior teeth were reported. Dental plaque and calculus near the lesions were noticed in three patients. Composite resin fillings in the upper anterior teeth adjacent to the lesions were present in two cases, while no information about dental fillings in those teeth was reported for 80.8% of the patients (n = 21). In three cases, the datum provided for dental fillings was ‘No/Unknown’.
In the previously published cases, the biopsy site (labial mucosa or gingiva) was not reported in those patients where both sites were affected. In the present case, both biopsies were taken from the labial mucosa. presents the histopathological findings from each case. In 80.8% of cases (n = 21), an inflammatory infiltrate was found subepithelially. In the remaining patients (n = 5), one was found but with its location unspecified. In 61.5% of the biopsies (n = 16), the inflammatory infiltrate was band-like and described as lymphohistiocytic (73.1%, n = 19), lymphocytic (23.1%, n = 6) or lymphoplasmacytic (3.8%, n = 1). Other reported features of the lichenoid inflammation present were basal cell degeneration (42.3%, n = 11), apoptotic bodies (26.9%, n = 7), hyperkeratosis (23.1%, n = 6), epithelial hyperplasia (7.7%, n = 2) and saw-toothed rete ridges (3.8%, n = 1). Granulomas were noted in 65.4% of the lesions (n = 17). In addition, histiocytic aggregates (n = 3) and multinucleated giant cells (n = 1) were visible. Perivascular (73.1%, n = 19) or perineural (57.7%, n = 15) distribution of the inflammatory infiltrate was a common finding. In addition to the perineural inflammatory infiltrate, the patient introduced above presented inflammatory infiltrates around salivary gland ducts. No foreign materials were reported from any biopsies. Oral candidiasis was diagnosed in 28% of the cases (n = 7: from biopsy in six cases and from yeast culture in one). In 15 cases, PAS staining of the biopsy was done and 14 of these were negative for fungal hyphae. In other cases (n = 10), the staining method for detecting fungal hyphae in the biopsies was not reported. In one case, information about possible candidal organisms in the biopsy sample was not available. Ziehl–Neelsen (ZN) staining was performed in 23.1% of the cases (n = 6), with negative results in every one, in addition to which one case was described as ‘infectious origin negative’ and another as ‘bacterial infection negative’, without information on ZN staining. Immunohistochemical characterization of the inflammatory infiltrate was performed in 53.8% of cases (n = 14). The most commonly used marker was CD68 (n = 13), with nine biopsies showing positive staining and the staining result not being reported in four cases. Lymphocyte markers CD3, CD4 and CD20 were next most common among the immunohistochemical markers used.
Table 2. Histopathological features of the lesions.
For seven cases, the authors did not report whether or not the patients received treatment. Of those patients for which this was reported, all (n = 19) received some treatment for the lesions. The most frequently used therapy was topical corticosteroid treatment (89.5%, n = 17). In 36.8% of cases (n = 7), the patient received antifungal therapy, always combined with topical steroid application or other methods as described in the present case. In four cases (21.1%), chlorhexidine was used, as the only treatment (n = 2) or simultaneously with topical corticosteroids (n = 2). The less commonly chosen treatments were systemic corticosteroids (n = 2), a locally acting nonsteroidal anti-inflammatory drug (n = 1), tacrolimus (n = 1), oral acitretin (n = 1), biostimulative laser therapy (n = 1) and intralesional methylprednisolone acetate (n = 1). There were no data on the outcome of treatment in 34.6% of the patients (n = 9). Complete resolution of the lesions was reported for only one patient, occurring after three months’ topical corticosteroid treatment [Citation16]. Other patients experienced periods of exacerbation and quiescence (76.5%, n = 13), or their lesions/symptoms improved significantly yet were not eliminated entirely (17.6%, n = 3). The average duration reported for the lesions and/or follow-up on the patients was 37.1 months (range: 1–157); for 15 of them (57.7%), there was no information about follow-up or on the lesions’ persistence. No cases of malignant transformation were reported.
Discussion
Lichenoid lesions located solely in the upper labial mucosa, with or without upper anterior labial gingival involvement, are rare. In addition to the 26 cases included in this systematic review, there are some reports describing similar clinical findings without histopathological confirmation [Citation11,Citation12,Citation15,Citation19].
Most of the patients were middle-aged females, as is typical also for OLL and OLP [Citation20]. A previous review of oral lichenoid lesions of the upper lip and gingiva identified a similar pattern [Citation21]. No data were available on medication or medical history in most cases. Where medications were reported, the commonly used drugs were for cardiovascular diseases [Citation12,Citation13,Citation15]. Indeed, an association between these lesions and cardiovascular medications has been suspected [Citation13]. However, as cardiovascular diseases are common in this age group [Citation22] and since the number of cases is small, arriving at any definitive conclusions about such an association is impossible.
The most common clinical differential diagnosis for the lesions was OLP. The suggested criteria for OLP state that lesions incompatible with the OLP criteria should be called oral lichenoid lesions [Citation8–10]. Differential diagnosis of OLP and OLL can be challenging, but if clinical-feature information is supplemented with biopsy and histopathological examination, often these conditions can be differentiated. Biopsy is often recommended for OLP-related diagnostics [Citation8–10]. Clinically, the lesions were erythematous and sometimes combined with white striation, resembling OLP, but presence solely in the upper labial mucosa and attached gingiva is unusual for OLP. Also, in some cases, dental restorations were present adjacent to the lesions, which could suggest oral lichenoid contact reaction rather than OLP [Citation10]. The lesions’ histopathological features were largely consistent with OLP but often accompanied by findings such as a perivascular inflammatory infiltrate and granulomas, which would exclude a diagnosis of OLP [Citation10].
The symptoms most frequently associated with the lesions were pain and soreness. Many authors have reported the treatment outcomes with reference only to clinical evaluation of the lesions, without giving information about the symptoms’ response. Also, the follow-up periods were short. In the case introduced here, the disease has persisted for more than a decade and the symptoms have varied significantly over time. Occasionally, various treatments have relieved them. Still, pain and smarting sensation recur. Regrettably, the literature contains very few data on treatment efficacy or the long-term prognosis related to these lesions.
The etiopathogenesis of lichenoid lesions of the upper labial mucosa and the factors provoking them have been subject to speculation from several investigators. Blomgren et al. [Citation11] hypothesized that hypersensitivity to composite restorations, trauma from fillings, lip parafunction, increased lip pressure, or microbial effects (especially Candida-connected) could contribute to the development of these lesions [Citation11]. Backman and Jontell [Citation12], in turn, reported improvement or healing in most cases, wherein chlorhexidine was the only treatment, and they speculated that microbial irritation might initiate such lesions. In addition, they pointed to a possible association between medication- or mouth breathing-associated hyposalivation and development of the lesions [Citation12]. The review we conducted, however, yielded insufficient data on any provoking factors that could consistently explain them in the cases reported. Our patient visited an oral hygienist regularly, maintained good oral hygiene, used chlorhexidine periodically to eliminate irritation from dental plaque and avoided irritating food, all with no significant improvement to the lesions or the symptoms. Oral fungal infection or candidiasis seems to be an uncommon finding in these patients, though it was present in our case, in cases presented by Mainville et al. [Citation17], and in one case from Robinson et al.’s [Citation13] work.
The presence of granulomatous inflammation in cases of lichenoid lesions of the upper labial mucosa was first reported by Robinson and colleagues [Citation13]. These cases displayed both lichenoid and granulomatous histopathological features. The authors pointed out the difficulty of determining the primary disease process and speculated about the existence of an entity in which both lichenoid and granulomatous inflammations are present. Some cases, with similar clinical presentation, have not shown granulomatous inflammation in biopsies [Citation15] (in other cases, no biopsies were taken [Citation12,Citation15,Citation19]). In most cases reviewed here, the lesions displayed histopathological features of lichenoid inflammation accompanied by granulomas. Still, some were histopathologically solely lichenoid lesions.
Lesions with lichenoid and granulomatous histopathological features have been described in the literature before. Ferguson et al. [Citation23] reported a patient with OLP and concomitant granulomatous cheilitis. Studying lichenoid and granulomatous dermatitis (LGD), Magro and Crowson [Citation24], Breza and Magro [Citation25], and Braswell et al. [Citation26] found perineural inflammation in some LGD cases [Citation25,Citation26], especially associated with non-drug-induced hypersensitivity (reaction to tattoos and post-herpetic dermatitis) [Citation26]. Most lesions in our review were found to show perineural inflammation, and the patients experienced pain or discomfort at their location. Since immune-cell infiltration may be implicated in post-herpetic neuralgia [Citation27], a similar mechanism might explain the sometimes significant pain associated with upper-lip lichenoid lesions.
Some reports identify an association with mycobacterial infection in LGD [Citation24,Citation25]. Breza and Magro [Citation25] have stated that a lichenoid and granulomatous reaction pattern in tissue should highlight the possibility of atypical mycobacterial infection especially if accompanied by perineural inflammation. Although perineural inflammation was a common finding in our review, bacteria were not present in the cases in which ZN staining was done. Still, many reports lacked information on ZN staining or other possible infection-related features. In addition, Hakeem et al. excluded cases with a positive result for micro-organisms [Citation14].
Topical corticosteroids, commonly in ointment and suspension form, are the first line of treatment for symptomatic oral lichenoid lesions [Citation20]. Alternatively, topical calcineurin inhibitors or retinoids may be used. When topical therapy proves ineffective and the symptoms are stubborn, systemic corticosteroids are recommended. The treatments identified in our review were mainly in line with this recommendation, in that most of the cases were treated with topical corticosteroids. Complete [Citation16] or nearly full [Citation18] resolution of the lesions was reported only in patients treated with topical corticosteroids. However, the short follow-up times for these cases are worth noting in evaluation of the results. In most cases, complete resolution was not achieved. Calcineurin inhibitors and retinoids were reported only in our patient’s treatment. In our case, tacrolimus ointment applied regularly seemed to provide the best long-term response of the symptoms. Of note, topical tacrolimus is indicated for atopic dermatitis but it may be used off-label for other immunologically mediated mucocutaneous diseases [Citation28]. To date, no objective evidence suggests that the use of topical tacrolimus in the oral mucosa increases the risk of oral cancer [Citation28,Citation29].
In reports of similar cases not included in our systematic review, other treatment methods, such as chlorhexidine [Citation12] and combining the replacement of dental-filling materials with antifungal treatment [Citation11], produced good results. Our review revealed that, although antifungal treatment has often been combined with other treatment methods, outcomes did not differ from those in patients not receiving antifungal treatment. Finally, it is worth noting a promising result that has not yet been replicated: Georgakopoulou and Achtari [Citation19] combined clarithromycin with prednisolone for a short course in three patients suffering from lichenoid lesions of the upper lip. At six-month follow-up, the patients were free of lesions.
Conclusions
Lichenoid lesions of the upper labial mucosa with or without involvement of the upper anterior labial gingiva are rare. Reporting on these cases is often incomplete. For example, medical history is seldom reported, biopsies are not always taken, and sometimes the report does not present the histopathological findings. Also commonplace are poor description of the treatment outcome and short follow-up. Therefore, one cannot draw definitive conclusions about the aetiological factors, natural progression or the best treatment options in cases of lichenoid lesions of the upper lip.
Based on the present review, biopsy is recommended for the diagnosis of lichenoid lesions of the upper labial mucosa with or without involvement of the upper anterior labial gingiva. Uniform definition and diagnostic criteria, including clinical and histopathological features of these lesions, would aid in recognizing the condition and forming a foundation for future research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Pinkus H. Lichenoid tissue reactions. A speculative review of the clinical spectrum of epidermal basal cell damage with special reference to erythema dyschromicum perstans. Arch Dermatol. 1973;107(6):840–846.
- Sontheimer RD. Lichenoid tissue reaction/interface dermatitis: clinical and histological perspectives. J Invest Dermatol. 2009;129(5):1088–1099.
- González-Moles MÁ, Warnakulasuriya S, González-Ruiz I, et al. Worldwide prevalence of oral lichen planus: a systematic review and meta-analysis. Oral Dis. 2021;27(4):813–828.
- Boch K, Langan EA, Kridin K, et al. Lichen planus. Front Med. 2021;8:737813.
- Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84(1):53–60.
- Alrashdan MS, Cirillo N, McCullough M. Oral lichen planus: a literature review and update. Arch Dermatol Res. 2016;308(8):539–551.
- Warnakulasuriya S, Kujan O, Aguirre-Urizar JM, et al. Oral potentially malignant disorders: a consensus report from an international seminar on nomenclature and classification, convened by the WHO collaborating centre for oral cancer. Oral Dis. 2021;27(8):1862–1880.
- Kramer IR, Lucas RB, Pindborg JJ, et al. Definition of leukoplakia and related lesions: an aid to studies on oral precancer. Oral Surg Oral Med Oral Pathol. 1978;46(4):518–539.
- van der Meij EH, van der Waal I. Lack of clinicopathologic correlation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications. J Oral Pathol Med. 2003;32(9):507–512.
- Cheng Y-SL, Gould A, Kurago Z, et al. Diagnosis of oral lichen planus: a position paper of the american academy of oral and maxillofacial pathology. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(3):332–354.
- Blomgren J, Axell T, Sandahl O, et al. Adverse reactions in the oral mucosa associated with anterior composite restorations. J Oral Pathol Med. 1996;25(6):311–313.
- Backman K, Jontell M. Microbial-associated oral lichenoid reactions. Oral Dis. 2007;13(4):402–406.
- Robinson CM, Oxley JD, Weir J, et al. Lichenoid and granulomatous stomatitis: an entity or a non-specific inflammatory process? J Oral Pathol Med. 2006;35(5):262–267.
- Hakeem A, Bhattacharyya I, Aljabri M, et al. Lichenoid reaction with granulomatous stomatitis: a retrospective histologic study of 47 patients. J Oral Pathol Med. 2019;48(9):846–854.
- Katsoulas N, Tosios K, Sklavounou-Andrikopoulou A. Lichenoid lesions of the upper lip: a retrospective study of 24 cases. Med Oral. 2018;23(3):0–307.
- Ferrisse TM, Bufalino A, Massucato EMS, et al. Lichenoid and granulomatous stomatitis: comparative immunohistochemical between oral lichen planus and lichenoid lesions. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124(2):e85–86.
- Mainville G, Sadeghi S, Rawal Y, et al. Lichenoid and granulomatous stomatitis: 8 new cases and a decade of hindsight. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124(3):e226–227.
- Rodrigues GA, De Campos Neves M, León JE, et al. OP – clinical and histological features of lichenoid and granulomatous mucositis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123(2):e36.
- Georgakopoulou EA, Achtari MD. Oral lichenoid lesions of the upper lip. J Dermatol Case Rep. 2017;11(1):16–19.
- Carrozzo M, Porter S, Mercadante V, et al. Oral lichen planus: a disease or a spectrum of tissue reactions? Types, causes, diagnostic algorhythms, prognosis, management strategies. Periodontol 2000. 2019;80(1):105–125.
- Georgakopoulou EA, Malamos D, Achtari MD. Oral lichenoid lesions of the upper lip and gingiva: what we know so far. Oral Dis. 2018;24(1-2):135–137.
- Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):146–603.
- Ferguson A, Golden S, Morrison L. New-onset oral lichen planus and granulomatous cheilitis in a 66-year-old woman. JAAD Case Rep. 2016;2(2):177–180.
- Magro CM, Crowson AN. Lichenoid and granulomatous dermatitis. Int J Dermatol. 2000;39(2):126–133.
- S Breza T Jr, Magro CM. Lichenoid and granulomatous dermatitis associated with atypical mycobacterium infections. J Cutan Pathol. 2006;33(7):512–515.
- Braswell DS, Hakeem A, Walker A, et al. Lichenoid granulomatous dermatitis revisited: a retrospective case series. J Am Acad Dermatol. 2019;81(5):1157–1164.
- Sutherland JP, Steain M, Buckland ME, et al. Persistence of a T cell infiltrate in human ganglia years after herpes zoster and during post-herpetic neuralgia. Front Microbiol. 2019;10:2117.
- Al Johani KA, Hegarty AM, Porter SR, et al. Calcineurin inhibitors in oral medicine. J Am Acad Dermatol. 2009;61(5):829–840.
- Su Z, Hu J, Cheng B, et al. Efficacy and safety of topical administration of tacrolimus in oral lichen planus: an updated systematic review and meta-analysis of randomized controlled trials. J Oral Pathol Med. 2022;51(1):63–73.
Appendix
The authors wish to provide some supplemental information on the review’s exclusion and inclusion of several cases presented in the literature. In a study comprising 12 patients [Citation11], three patients presented lesions associated with amalgam restorations. We received information from the first author, Dr Blomgren, that those lesions were in the buccal mucosa. These three cases could not be identified from among the other patients’, so we had to exclude all 12 patients from our review. In another case series, of 24 patients, four had a biopsy taken [Citation15]. Upon contacting the first author, Dr Katsoulas, we were able to identify these four patients and include them in the review. In a recent study by Hakeem et al. [Citation14], the lesion location was described as ‘upper lip’ (without specification of the mucosal vs. vermilion side). When contacted, Dr Hakeem stated that these cases were likely entirely mucosal in nature, so we decided to include them in the review. Finally, eight cases from Mainville et al.’s [Citation17] work are presented as a group of patients since we could not obtain more specific information about each patient.
