?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
To explore whether plaque and gingival bleeding are more frequently experienced by adolescents with juvenile idiopathic arthritis (JIA) compared to matched controls without JIA; explore whether surface- and site-specific periodontal outcomes vary between the two groups; and for participants with JIA, investigate associations between disease-specific features and periodontal outcomes.
Material and methods
In this comparative cross-sectional study, selected surfaces, and sites of index teeth in 10–16-year-olds with JIA and matched controls were examined by modified versions of Simplified Oral Hygiene Index (OHI-S) and Gingival Bleeding Index (GBI). Mixed-effects logistic regressions, reporting odds ratios (OR) with 95% confidence interval (CI), were applied. Intra-class correlation coefficients (ICCs) were calculated to quantify the degree of dependency of measures within the same individual.
Results
144 and 159 adolescents with JIA were evaluated according to OHI-S and GBI; corresponding numbers of controls were 154 and 161. Plaque and gingival bleeding were more frequent in individuals with JIA than controls. Adjusted analyses showed association between JIA status and OHI-S > 0 (OR = 2.33, 95% CI: 1.47 − 3.67, ICC = 0.45) and GBI > 0 (OR = 1.54, 95% CI: 1.10 − 2.16, ICC = 0.41 and 0.30). Surface-specific distribution of plaque varied among the two groups.
Conclusions
Our results highlight the importance of increased awareness of oral health care in patients with JIA and that surface- and site-specific differences in periodontal outcomes exist between individuals with JIA and controls. Few JIA disease-specific variables associated with plaque or gingival bleeding.
Introduction
Juvenile idiopathic arthritis (JIA) is the most prevalent chronic arthritis condition in childhood and adolescence [Citation1]. The likelihood of dental clinicians to meet young individuals with JIA in their practice is high, especially in the Nordic countries where relatively high incidence rates of JIA have been reported [Citation2] and regular free oral health care to children and adolescents is often provided by the Public Dental Service (PDS) [Citation3]. In Norway, an incidence rate of JIA in 23/100,000 children per year has been demonstrated [Citation2,Citation4].
Historically, a high burden of dental caries has been reported in children and adolescents with JIA [Citation5–Citation8]. More recently, however, the reported prevalence has decreased and is now on a level with that of the general population [Citation9,Citation10]. Nevertheless, a recent systematic review, suggested that plaque, gingivitis, and periodontitis were more common amongst children and adolescents with JIA as compared to individuals without JIA [Citation9]. However, the included studies varied, being different in size, design, and statistical approach, thus, the results should be interpreted with caution.
As the dentition involves multiple teeth, clustering of measurements is common in dental research [Citation11], with teeth clustered within the patient. Units within a cluster may be exposed to identical external factors or share related characteristics [Citation12]. Hence, as periodontium is the supporting structure of the dentition, it is likely that oral hygiene and gingival bleeding are more comparable around teeth within the same patient (cluster), than between different patients. The dependency between teeth within the same patient requires adjustment, since ignoring this may lead to underestimated standard errors, too small p-values, and enhanced chance of incorrectly rejecting the null hypothesis (Type I error) [Citation13]. The epidemiologic nomenclature of clustering often refers to individuals nested within groups such as families and schools, while dental research often presents a special type of natural clustering [Citation14] with surfaces clustered within teeth and teeth clustered within individuals. Statistical t-tests are frequently used in publications in dental journals [Citation11] and are a commonly used approach on measures aggregated to patient level (e.g. GBI (Gingival Bleeding Index) or OHI (Oral Hygiene Index)), even if the measures originally were on tooth level. Hence, using simple statistical tests and aggregated data (at patient level), additional information on correlation (non-independency) within the patient is lost. We sought to overcome this problem by using multilevel modelling, an approach which, to the best of our knowledge, has not yet been applied in studies addressing periodontal health among young individuals with JIA.
The aims of this study were to explore whether plaque and gingival bleeding are more frequently experienced by adolescents with JIA as compared to matched controls without JIA. Additionally, we explored whether surface- and site-specific periodontal outcomes vary between the two groups. Finally, and specifically for participants with JIA, we investigated possible associations between disease-specific features and the periodontal outcome variables. We hypothesise that participants with JIA have more plaque and gingival bleeding, as well as more variance in surface- and site-specific distribution of the periodontal outcome variables than participants without JIA (controls). We also hypothesise that plaque and gingival bleeding vary according to the value on JIA specific features within the group of JIA patients.
Material and methods
Study design
Baseline data from a prospective longitudinal multicenter study, NorJIAFootnote1, are used in this comparative cross-sectional sub-study evaluating periodontal health of adolescents from 10–16 years. The multicenter study included children and adolescents (4–16 years old) diagnosed with JIA by a paediatric rheumatologist according to the criteria defined by the International League of Associations for Rheumatology (ILAR) [Citation15] and presented a written informed consent. There were no participants with major medical comorbidities such as congenital facial anomalies, skeletal dysplasia, or malignancies in the cohort. Data for the present sub-study were collected between April 2015 and August 2018. Baseline data on dental caries has recently been published with a similar methodological approach (sample size calculation included) [Citation10].
Participants
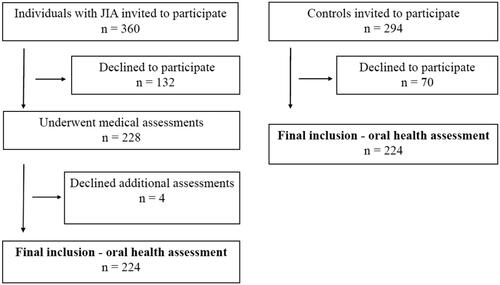
Three university hospitals, located in western, central, or northern Norway, were involved in the recruitment. Participant flow diagram are presented in . A total of 228 individuals were included in the medical examination, while four participants declined a further oral health assessment. Participants having an oral examination (n = 224) were matched 1:1 with controls corresponding to sex, age, centre site, and mothers’ country of origin (western or non-western). The controls were enrolled from seven Public Dental Service (PDS) clinics and did not have JIA or substantially no other chronic diseases (Additional file 1, Supplementary Table 1). Both rural and urban areas were represented. For the controls, data collection was combined with a planned regular dental examination and as an incentive for participation two cinema tickets were offered. For this sub-study only adolescents (10–16 years) were evaluated, hence 162 adolescents with JIA and 162 controls were enrolled.
Questionnaires and construction of variables
A questionnaire including socio-behavioral and subjective clinical information was given all participants (and/or caregivers, as appropriate) [Citation10]. Variables included in this sub-study were educational level of caregivers, number of caregivers in the household, mothers’ country of origin, frequency of toothbrushing, frequency of tooth flossing during the last 3 months, gingival bleeding during toothbrushing, pain or discomfort during toothbrushing, frequency of intraoral ulceration(s) and perception of dry mouth. Family name of the participant was evaluated if the item regarding mothers’ country of origin was missing (in Norway quite often the family name of a child includes both father’s and mother’s name). Finally, participants with JIA, were asked if they had received information about the importance of good oral health in relation to the JIA diagnosis, and if so, they were asked to specify the health profession of the communicator. The coding of these self-reported variables is presented in Additional file 2, Supplementary Table 1A. Specifically for participants with JIA, exploratory data analysis was facilitated by including the variables; JIA category, various blood tests, medication, age of disease onset, disease duration, activity/remission status, physician’s global assessment of disease activity visual analogue scale (MDgloVAS), patient/parent-reported global assessment of overall well-being visual analogue scale (PRgloVAS), Childhood Health Assessment Questionnaire (CHAQ) hygiene item tooth brushing. The coding is presented in Additional file 2, Supplementary Table 1B.
Examinations
The participants with JIA were examined by experienced paediatric rheumatologists at one of the three university hospitals. The disease-specific clinical background variables and blood tests are described in detail in Additional file 3 [Citation10] and Additional file 4, respectively. The oral assessments of selected index teeth, surfaces, and sites, carried out by one out of five dentists, are shown in Additional file 5, Supplementary Table 1. As the present study is part of a comprehensive oral health examination in the NorJIA project, involving multiple clinical variables, a full mouth protocol was not possible. Instead Simplified Oral Hygiene Index (OHI-S) by Greene and Vermillion [Citation16] was decided on, accordingly selected index teeth were first permanent molars and one incisor in the upper- and lower jaw. OHI-S consists of two components, the simplified Debris Index (DI-S) and the simplified Calculus Index (CI-S). However, registration of oral hygiene was modified as subgingival calculus was not recorded (supragingival calculus was recorded). Only fully erupted teeth were scored, and in case of fixed orthodontic appliances, the assessment was not done. Gingival bleeding was evaluated on the same index teeth represented in S-OHI, by using a modified Gingival Bleeding Index (GBI) introduced by Ainamo and Bay [Citation17]. This index was modified as no horizontal movement of the probe on the surface was performed, instead a vertical movement at three sites on the respective surface (mesial, medial, and distal) was implemented. Probing of the orifice of the gingival crevice was done with a World Health Organisation (WHO) periodontal measuring probe with a 0.5 mm ball tip. Additionally, fissure of lip and/or corner of lip, gingival ulcers with discontinuation of the epithelia of at least 3 mm, buccal ridging, tongue indentation, buccal gingival hyperplasia (buccal side of the lower and upper anterior teeth), and potential mouth dryness (by evaluating if dental mirror sticks to buccal mucosa) were recorded. The coding of these variables is presented in Additional file 2, Supplementary Table 1C. The coding of the clinical oral variables is presented in Additional file 2, Supplementary Table 1D.
Training process
Prior to the study, the dentists underwent theoretical courses in how to use the modified version of the OHI-S and the modified version of the GBI. Uncertainties about the procedures were discussed until clarity. The dentists were given a plastic-coated instruction sheet of the written descriptions to accompany the oral examinations as a guide. To illustrate the force to be applied on probing of gingival sulcus to determine gingival bleeding, the dentists practiced with a dental probe on a digital letter weight (Wedo Package Scale Paket 50 Plus). At two different sessions, three intervals with seven attempts were performed.
Statistical methods
Data were analysed using SPSS version 25.0 (IBM Corp. Released 2013, IBM SPSS Statistics for Windows, Armonk NY: IBM Corp) and STATA version 16 (Stata Corp LP, College Station, TX). To describe continuous demographic and clinical variables mean and standard deviations (SD) were applied. To evaluate differences in categorical variables between children with and without JIA chi-squared tests were applied. Linear regression models, with robust variance estimates correcting for matching, were used to explore differences in the aggregated indexes OHI-S and GBI, between the matching pairs. The data of the modified version of the OHI-S (OHI-S > 0) had a clustered 2-level hierarchical structure with surfaces/tooth (level 1) clustered within individuals (level 2) and GBI (GBI > 0) had a clustered 3-level hierarchical structure with sites/surfaces (level 1) clustered within teeth (level 2), and teeth clustered within individuals (level 3). Consequently, random intercept logistic models (RIM) were applied. The levels in the multilevel models with regard to the dichotomous periodontal outcome variables and background variables are illustrated in Additional file 6, Supplementary Tables 1A and 1B. The formulas estimated (using restricted maximum likelihood REML), for the 3-level data, using mixed-effects (random intercept) logistic regression were:
is the intra class correlation for the measurements clustered within teeth, while
is the intra class correlation for the measurements of the teeth clustered within individual. For the 2-level data, the mixed-effects logistic regression models were:
is the intra class correlation for the measurements of the teeth clustered within individual.
The multilevel models account for clustering of periodontal data for sites (k) within teeth (j) and within individuals (i). By applying separate mixed-effects logistic models, intra-class correlation coefficients (ICC) for the matching pairs were tested. Outcome measure was periodontal data as represented by OHI-S > 0 and GBI > 0 and the main exposure variable was JIA group status (JIA/control group). Possible confounders in the mixed-effects logistic regression analysis were identified consequent to socio-behavioral, and subjective clinical variables that were statistically significantly associated with JIA status and the respective periodontal outcome variables (OHI-S > 0 or GBI > 0). These possible confounding variables together with oral variables statistically associated with the periodontal outcome variables in unadjusted analysis and the main exposure variable (JIA/control group) were adjusted for in the mixed-effects logistic regression analysis. Specifically, for participants with JIA, age and gender were adjusted for in the mixed-effects logistic regression analysis. By calculating the ICCs the effect of dependency was evaluated at individual level (oral hygiene and gingival bleeding data), and at tooth level (gingival bleeding data). The ICC demonstrate variations between teeth and individuals as a proportion of the total variance. ICC varies between 0 (implying the respective periodontal health variable is independent within individuals/teeth) and 1 (implying no variation of the respective periodontal health variable within an individual, i.e. no variation between clusters). Scheffe post-hoc test was used to adjust significance levels in multiple comparisons in the mixed-effects logistics regressions. P-values below 0.05 were regarded as statistically significant.
Ethical approval
Approval was given regional ethics committee (2012/542/REC). Leaders of different County Dental Health Authorities, at different Oral Health Centre of Expertise, and at the three paediatric departments at the university hospitals also approved the study. Before participation written informed consent was signed. The NorJIA study is registered at ClinicalTrials.gov (No: NCT03904459).
Results
Sample characteristics
As previously described [Citation10], the response rates for the individuals with JIA and controls were 63.3% (228/360) and 76.2% (224/294), respectively (). The proportion of girls was less among the eligible individuals with JIA who declined participation, compared to the participants with JIA (58.3% vs 59.2%, p=.027) [Citation10]. Also, mean age among the individuals with JIA who declined participation was smaller compared to the cohort as a whole (10.5 (SD 3.5) years, p<.001) [Citation10].
The 224 participants with JIA who recieved an oral health examination were matched to a control [Citation10]. Mean age of both individuals with JIA and controls was 12.0 years (SD 3.2) (p=.974). Also 133 (59.4%) of the participants with JIA were girls, corresponding number in the control group were 134 (59.8%). Among the 224 pairs, 211 (94.2%) of the pairs were matched in relation to mother’s background of origin (for 10 individuals the family name was evaluated).
Oral hygiene and gingival bleeding were exclusively assessed on participants 10 years and older resulting in 162 individuals with JIA and 162 controls. For this sub-study, a total of 144 and 159 individuals with JIA were evaluated according to OHI-S and GBI, corresponding numbers for the control group were 154 and 161 (Additional file 5, Supplementary Table 1). The age group in this sub-study correspond to adolescence according to the World Health Organisation [Citation18].
Ninety-four mothers in the JIA group (62.3%) vs. 111 (76.6%) in the control group had higher education (p=.008) (). Corresponding figures for fathers were 62 (41.9%) and 86 (60.6%) (p=.001). A larger proportion of individuals with than without JIA, confirmed dry mouth on a regular basis (7.9% vs. 2.5%, p=.030). Forty-three responders with JIA (43/149, 28.9%) reported that they had received information about the importance of good oral health in relation to the JIA diagnosis. The information was mainly communicated by physicians and dentists (results not shown). Potential oral health risk factors (concomitant diagnoses and use of medication among the participants) are presented in Additional file 1, Supplementary Table 1.
Table 1. Socio-behavioral, and subjective clinical characteristics of 162 individuals with juvenile idiopathic arthritis and 162 controls, aged 10–16 years.
Experience of plaque and gingival bleeding in individuals with and without JIA
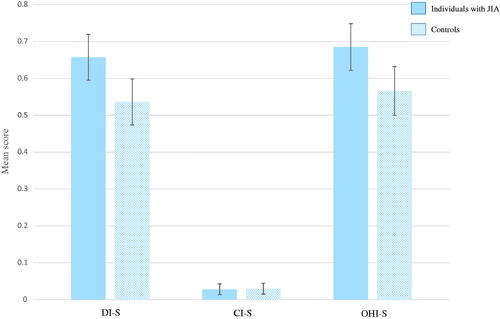
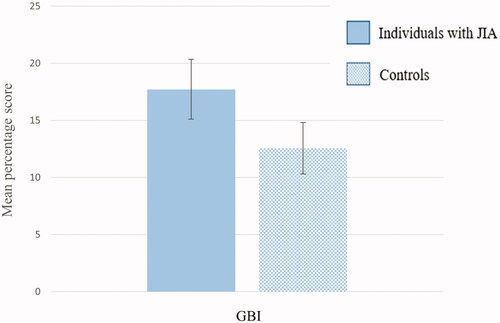
The two components of the modified OHI-S; DI-S and CI-S showed a mean score of 0.66 (SD 0.38) and 0.03 (SD 0.09) in individuals with JIA, and 0.54 (SD 0.40) and 0.03 (SD 0.09) in controls, respectively. Sixteen individuals with JIA and 16 controls had calculus (CI-S > 0). Mean OHI-S score altogether was 0.69 (SD 0.39) amongst individuals with JIA and 0.57 (SD 0.42) amongst the controls. All scores, except for the CI-S, showed statistically significant differences between the two groups (p<.012). The OHI-S score had a beta (mean difference between individuals with JIA and controls) of 0.12 (95% CI: 0.03 − 0.21) (p=.010) when correcting for matching pairs. demonstrates mean score with error bars of the modified DI-S, CI-S and OHI-S, separately for individuals with and without JIA. Mean percentage score of the modified GBI showed a statistically higher value in individuals with JIA than in controls (17.72 (SD 16.83) vs. 12.56 (SD 14.32), p=.004). The GBI score had a beta (mean difference between individuals with JIA and controls) of 5.16 (95% CI: 1.67 − 8.66) (p=.004) when correcting for matching pairs. demonstrates mean percentage score with error bars of the modified GBI, separately for individuals with and without JIA.
Multilevel analyses
The correlation between the matching pairs for the dichotomised outcome variables OHI-S > 0 and GBI > 0 were weak (ICC = 0.01 and ICC < 0.001, respectively), consequently the original matching in the data was not accounted for in the final multilevel models. Regressing OHI-S (presence of plaque (DI-S > 0) and/or calculus (CI-S > 0) on JIA status while adjusting for socio-behavioral, and clinical characteristics showed a statistically significant association between JIA status and OHI-S > 0 (Odds Ratio (OR) = 2.33, 95% Confidence Interval (CI): 1.47 − 3.67) (). Corresponding finding for gingival bleeding (GBI > 0) was OR = 1.54, 95% CI: 1.10 − 2.16 (p=.013) (). Independent of JIA status, molars were more likely to present with OHI-S > 0 compared to incisors (OR = 5.43, 95% CI: 3.73 − 7.91, p<.001), and lingual surfaces (36, 46) were more likely to present with OHI-S > 0 compared to buccal surfaces (11, 31, 16, 26) (OR = 2.81, 95% CI: 1.67 − 4.73, p<.001). Corresponding ORs for GBI > 0 were 1.78 (95% CI: 1.31 − 2.42, p<.001) and 1.24 (95% CI: 0.83 − 1.83, p=.291). In the multilevel model with oral hygiene (OHI-S > 0) as outcome variable, the ICC at individual level with no covariates was 0.32, demonstrating that 32% of the variance in the oral hygiene variable was between rather than within individuals. In the adjusted analysis, the ICC was 0.45. Corresponding ICC with gingival bleeding (GBI > 0) as outcome variable were 0.29 and 0.30, respectively. The ICC at tooth level with gingival bleeding (GBI > 0) as outcome variable was 0.43 with no covariates, demonstrating that 43% of the variance in the gingival bleeding variable was between rather than within teeth. In the adjusted analysis, the corresponding ICC was 0.41. All ICCs were statistically significant (p<.001).
Table 2. Group affiliation, socio-behavioral, and clinical characteristics in relation to oral hygiene (OHI-S > 0), and gingival bleeding (GBI > 0). Unadjusted and adjusted mixed-effects logistic regression.
Surface- and site-specific periodontal health by group affiliation
depicts the results from mixed-effects modelling regressing side-, jaw-, and surface-specific traits on OHI-S > 0 and side-, jaw-, surface- and site-specific traits on GBI > 0, separately for individuals with and without JIA. and demonstrate prevalence with error bars of OHI-S > 0 at surface level and GBI > 0 at site level, separately for individuals with and without JIA. There was a statistically significant interaction between surface and group affiliation (p<.001) on OHI-S > 0. Whereas the interaction between surface and group affiliation (p=.351), and site and group affiliation (p=.27) were nonsignificant on presence of gingival bleeding (GBI > 0). Among both individuals with JIA and controls, the likelihood of OHI-S > 0 was statistically significantly less on buccal surface of maxillary central (OR = 0.20, 95% CI: 0.12 − 0.34 and OR = 0.14, 95% CI: 0.08 − 0.26) and on buccal surface of mandibular central (OR = 0.17, 95% CI: 0.10 − 0.28 and OR = 0.18, 95% CI: 0.10 − 0.31) compared to buccal surface of maxillary 1. molars. Among the controls only, the ORs of OHI-S > 0 was statistically significantly less in maxilla compared to mandibula (OR = 0.39, 95% CI: 0.28 − 0.53) and statistically significantly larger on lingual surface of mandibular 1. molars than on the buccal surfaces of maxillary 1. molars (OR = 5.63, 95% CI: 3.57 − 8.88).
Figure 4. Percentage (95% confidence interval represented as error bars) of plaque or calculus (OHI-S > 0) at surface level among individuals with juvenile idiopathic arthritis (JIA) and controls.
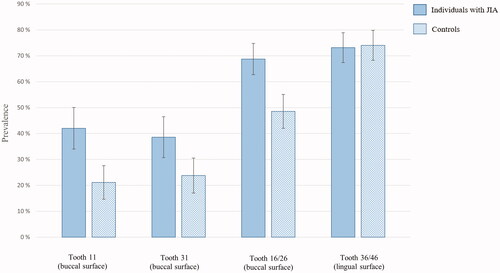
Figure 5. Percentage (95% confidence interval represented as error bars) of gingival bleeding (GBI > 0) at site level among individuals with juvenile idiopathic arthritis (JIA) and controls.
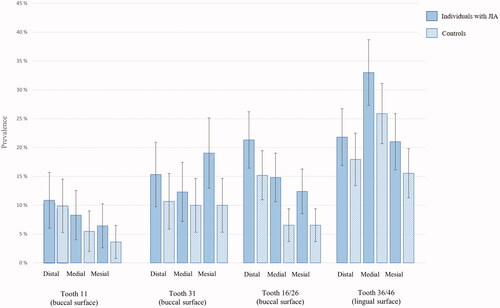
Table 3. Oral variables including side, jaw, and surfaces in relation to oral hygiene (OHI-S > 0), and side, jaw, surfaces, and sites in relation to gingival bleeding (GBI > 0) by group affiliation. Unadjusted mixed-effects logistic regression.
Disease-specific variables associated with the periodontal health variables
Adjusted mixed-effects logistic regressions showed a statistically significantly higher risk of OHI-S > 0 in individuals with systemic arthritis, compared to individuals with oligoarthritis persistent (OR = 5.20, 95% CI: 1.07 − 25.32) (). However, due to small number of participants in some of the categories no conclusions can be made. Individuals aged >6 years at JIA onset had a statistically significantly higher risk of plaque OHI-S > 0 in the adjusted analysis, compared to individuals aged ≤6 years at JIA onset (OR = 1.80, 95% CI: 1.08 − 3.00) (). The seven participants reporting difficulties with tooth brushing, according to CHAQ hygiene item Tooth brushing, had statistically significantly higher risk of gingival bleeding (GBI > 0), compared to participants reporting no difficulties (OR = 2.92, 95% CI: 1.14 − 7.45). For the multilevel model with covariates analysing disease-specific features in relation to oral hygiene (OHI-S > 0) the ICC at individual level was 0.29 (p<.001). In relation to gingival bleeding (GBI > 0), the respective ICC was 0.27 (p<.001), whilst the ICC at tooth level was 0.41 (p<.001).
Table 4. Disease-specific features and oral variables in relation to oral hygiene (OHI-S > 0) and gingival bleeding (GBI > 0) among participants with juvenile idiopathic arthritis (JIA). Unadjusted and adjusted mixed-effects logistic regression.
Other clinical characteristics of oral cavity independent of plaque and gingival bleeding
No statistically significant differences of other clinical characteristics were found between individuals with JIA and controls ().
Table 5. Other clinical characteristics of oral cavity among individuals with juvenile idiopathic arthritis (JIA) and controls ≥10 years.
Discussion
Plaque and gingival bleeding were more frequently experienced in individuals with JIA as compared to controls without JIA. Surface-specific distribution of plaque varied between the two groups. Individuals older than 6 years at JIA onset had higher risk of plaque, compared to individuals 6 years or younger at disease onset.
An important strength of the present study is the multilevel modelling, considering the clustered structure of the data and facilitating optimal utilisation of outcome data. In view of the statistically significant ICCs calculated in this study, the application of mixed-effects logistic regressions was essential. This also shows that periodontal outcome variables within the individuals (plaque and gingival bleeding) and within teeth (gingival bleeding) are highly dependent. A weak correlation between the matching pairs was observed, demonstrating that the background variables (sex, age, centre site, and background-origin) were weak matching variables for the periodontal outcome variables. Consequently, convergence problems occurred if matching was added to the mixed-effects models and the original matching in the data was not accounted for in the multilevel models. Anyhow, linear regression analyses correcting for matching were performed, demonstrating statistically significant mean difference in the aggregated indexes OHI-S and GBI between the matching pairs. Hormonal changes in puberty are known to exacerbate gingival inflammation (puberty gingivitis) and occurs at different ages for girls and boys [Citation21]. Although the individual’s pubertal stage varies with age and adolescents with chronic rheumatic conditions might experience delayed puberty [Citation22], the comparative design strengthened the results. Regarding representativeness of the Norwegian adolescent population of JIA, three out of four existing Norwegian regional paediatric rheumatology centres were involved in the recruitment, all JIA categories were included, and the sample size was relatively large in this field of research. However, potential non-response bias for individuals with JIA should not be disregarded [Citation23]. Other limitations in the present study were the use of index teeth, as a full mouth registration could potentially be more informative, also a full mouth protocol would have statistical advantage, however results demonstrate appropriate power. The present study did not consider that some participants had erupting premolars adjacent to index-teeth examined. Mixed dentition may have an impact on the oral environment, however the mesial surfaces being closest to erupting premolars were not scored. Also, the impact of non-steroidal anti-inflammatory drugs (NSAIDs) frequently used by individuals with JIA and folic acid supplementation, administered to prevent side effects of methotrexate [Citation24], might have been evaluated. NSAIDs and folic acid constitute potential confounders to gingival bleeding as NSAIDs are suggested to decrease inflammatory signs of gingivitis [Citation25], and it has been suggested that supplementation of folic acid improves gingiva’s resistance to local irritants, hence reduces gingival inflammation [Citation26]. Another limitation was the modification of the diagnostics tools, complicating comparisons with other epidemiological studies.
In our study we found that individuals with JIA were more likely to have plaque and gingival bleeding compared to their peers. This compares well with the study of Welbury et al. [Citation8], presenting the highest sample size in the previously mentioned systematic review [Citation9]. A recently published article from 2019 [Citation27], thus not included in the systematic review, with a relatively large sample size (85 individuals with JIA) found gingival inflammation as measured by bleeding on probing (BOP) to be increased in adolescents with JIA. In our study one might speculate that the higher level of education amongst caregivers in the control group as compared to the JIA group might have biased the results, as more frequent toothbrushing and lower plaque score are reported in adolescents with caregivers having higher educational level [Citation28]. However, we found no association between caregivers’ educational level and plaque or gingival bleeding in the adjusted analysis in the present study. Only two studies [Citation27,Citation29] focussing on gingival health among individuals with JIA have described socioeconomic background characteristics of the participants, and only Grevich et al. [Citation27] adjusted for socioeconomic covariates known to influence oral health [Citation30]. This could perhaps be a component in explaining the divergent findings in this field.
Accumulation of dental plaque, denoted as biofilm, is affected by the ability and motivation of oral hygiene procedures. Function ability, e.g. disability in upper limbs or temporomandibular joints (TMJs) has been highlighted as a risk factor for reduced oral hygiene among individuals with JIA [Citation8,Citation31,Citation32]. In the present study, there was no difference in the reported oral behaviours between individuals with and without JIA, but individuals with JIA reporting difficulties with tooth brushing, according to CHAQ hygiene item Tooth brushing, had higher risk of gingival bleeding, compared to individuals with JIA reporting no difficulties. This finding is most likely consistent with plaque-induced gingivitis. Nevertheless, other host factors, such as salivary composition and amount, diet, gingival inflammation, chewing effects and movements of the soft tissues, status of tooth surface, and the microbial composition of the biofilm itself may play a role in the amount of plaque accumulation [Citation33,Citation34]. In total absence of oral hygiene [Citation35] and if oral hygiene were performed, but without special instructions [Citation36], more plaque accumulation in molars compared to anterior teeth and in mandibular dentition compared to maxillary dentition, have been demonstrated [Citation35,Citation36]. In the present study, molars presented a higher risk of plaque as compared to anterior teeth, but only the control group had a significant increased risk of plaque in the mandible, compared to the maxilla. Microbial species have also shown to differ significantly across tooth types and location, with the highest mean total count at the lower molars [Citation34]. Significant difference between the two groups in the present study were notable for buccal surface of first permanent molars in the maxilla, presenting increased risk of plaque, and possibly an increased risk of gingival bleeding of mediobuccal site in the individuals with JIA as compared to the controls. Altered salivary composition and flow rate among individuals with JIA are reported [Citation7,Citation37–42], as saliva significantly influences dental biofilm [Citation43] the proximity of buccal surface of first molars to the exit of the parotid ducts may postulate a relation between increased risks of plaque on this surface among individuals with JIA. Considering the statistically significant interaction between surface and group affiliation on presence of plaque in the present study, biological host variations point towards a role in the variation in distribution and amount of plaque between individuals with JIA and controls, however the nature of plaque are fluctuating, and only longitudinal studies can detect such relations.
Grevich et al. [Citation27] suggested microbiota as a potential contributing factor to the disease pathogenesis of JIA and gingivitis. A recent NorJIA-based study by Frid et al. [Citation44] evaluated salivary oral microbiome, plaque, and gingival bleeding score of some of the individuals with JIA (n = 59) and controls (n = 34), also included in the present study. Among the individuals with JIA, a higher abundance of microbiota associated with chronic inflammation was found, and dysbiosis of the salivary microbiome in individuals with JIA was suggested to trigger local immune response, including gingival bleeding. Different systemic factors may modify the immune-inflammatory response, such as medication [Citation45] and nutrition [Citation46,Citation47], and perhaps some individuals with JIA are more prone to gingival bleeding due to susceptibility of disrupted symbiosis between the biofilm and host immune-inflammatory response, known to cause gingivitis [Citation48]. To our knowledge, only one other study [Citation27] has explored the association between disease-specific features and periodontal health outcomes in young individuals with JIA by use of regression analyses. In the present study no JIA disease-specific feature were found to be associated with gingival bleeding, except the CHAQ hygiene item Tooth brushing. Age at JIA onset was found to be associated with increased risk of plaque in the present study. Some researchers suggest mechanisms of JIA disease vary with age at onset [Citation49], but it is difficult to make any presumptions regarding this finding.
This study clearly demonstrates the need for improved oral hygiene among individuals with JIA. A meta-analysis from 2011 [Citation50] “showed that plaque accumulation and gingival inflammation scores significantly increased the prevalence of bacteraemia following toothbrushing”. Considering the immunosuppressant therapy used by many of the young individuals with JIA, optimal oral health and professional maintenance in this group is emphasised.
JIA encompasses a heterogenous group disease categories requiring individualised and optimised treat-to target strategies. While remission on medication is an accessible goal for many, more focus will switch to treatment tolerance, adverse events and risk of infections. Future research should address longitudinal studies with adequate sample sizes in more homogeneous JIA groups with special focus on disease activity, drug exposure, and its relation to oral health status. Furthermore, to increase knowledge of plausible susceptibility to periodontal disease among individuals with JIA, studies specifically targeting host variation with regard to immune response, microbial diversity, salivary gland involvement, and nutrition are needed.
Conclusions
Plaque and gingival bleeding were more frequently experienced in individuals with JIA as compared to controls without JIA. Multilevel analyses showed an interaction between surface and group affiliation on the presence of plaque. Few JIA disease-specific variables were associated with plaque or gingival bleeding, however, results suggest that certain features may increase individual’s susceptibility. Our results underscore the importance of increased awareness of oral health care in patients with JIA amongst health care providers.
Ethics approval and consent to participate
The study was approved by the Regional Committees for Medical and Health Research Ethics (2012/542/REC), Rogaland, Vestland (West). Written informed consents were obtained from the caregivers and the adolescents as appropriate. The study was registered at ClinicalTrials.gov (No: NCT03904459). All procedures were performed in accordance with relevant guidelines.
Author contributions
EGG: Contributed to the design and data collection of this sub-study, performed statistical analysis, and wrote the manuscript in consultation with MSS, ANÅ and SAL. MSS: Conceived and designed this sub-study. ANÅ: Conceived the idea of performing multilevel analysis. SAL: Performed statistical analysis. MR: Aided in interpretation and writing of the manuscript and to the design and data collection of the NorJIA study. JF, JH, PF, KT, KR: Contributed to data collection and provided valuable comments. AR, AB, KL, XS, LC: Provided valuable comments. All authors have read and approved the manuscript.
Supplementary_information.docx
Download MS Word (53.1 KB)Acknowledgements
This study is part of the multicenter NorJIA Study (The Norwegian JIA Study – Temporo-mandibular Involvement, Oral Health, Uveitis, Bone Health and Quality of Life in Children with Juvenile Idiopathic Arthritis (JIA)). NorJIA is a collaboration among universities (University of Bergen, Norwegian University of Science and Technology, The Arctic University of Norway), university hospitals (Haukeland University Hospital, St. Olav’s Hospital, University Hospital of North Norway) and oral health centers (Oral Health Centre of Expertise in Western Norway-Vestland, Center for Oral health Services and Research, Trondheim, Public Dental Health Service Competence Centre of Northern Norway) in Bergen, Trondheim and Tromsø. Represented by Karen Rosendahl MD PhD (PI), Marit Slåttelid Skeie DDS PhD, Marite Rygg MD PhD, Ellen Nordal MD PhD, Anne N. Åstrøm DDS PhD, Karin Tylleskär MD, Annika Rosén DDS PhD, Elisabeth Grut Gil DDS, Johannes Maria Fischer DDS, Xieqi Shi DDS PhD, Oskar Angenete MD, Lena Cetrelli DDS, Gunnar Lyngstad DDS, Marie Sager DDS, Astrid J Feuerheim PhD, Anette Lundestad MD, Thomas Augdal MD, Paula Frid DDS, Veronika Rypdal MD, Josefine Halbig DDS, Athanasia Bletsa DDS PhD, Marit Midtbø DDS PhD, Larissa von Wangenheim Marti DDS and Mats Säll DDS. We are indebted to radiographers Marianne Lothe Vollan and Erik Haro, and the study nurses Tone Kvinnsland Amdal, Susanne Irene Tobiesen Eidset, Line Rapp Simonsen, Marte Grimsmo Teige, Brita Lena Hansen, and Lisbeth Aune. Finally, we are thankful to all the children and their caregivers who participated in the study.
Disclosure statement
The authors declare that they have no competing interests.
Data availability statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
Notes
1 The Norwegian JIA Study – Temporo-mandibular Involvement, Oral Health, Uveitis, Bone Health and Quality of Life in Children with Juvenile Idiopathic Arthritis (JIA).
References
- Petty RL, Lindsley CB. Wedderburn LR Textbook of Pediatric Rheumatology, 7th edn. Section two. Chapter 15. Juvenile Idiophatic Arthris: 2016. p.188–224.
- Berntson L, Gare BA, Fasth A, et al. Incidence of juvenile idiopathic arthritis in the Nordic countries. A population based study with special reference to the validity of the ILAR and EULAR criteria. J Rheumatol. 2003;30(10):2275–2282.
- Widström EA, Byrkeflot LI, Pälvärinne R, et al. Systems for provision of oral health care in the Nordic countries. Tandlaegebladet. 2015;119(9):702–711.
- Moe N, Rygg M. Epidemiology of juvenile chronic arthritis in Northern Norway: a ten-year retrospective study. Clin Exp Rheumatol. 1998;16(1):99–101.
- Storhaug K. Caries experience in disabled pre-school children. Acta Odontol Scand. 1985;43(4):241–248.
- Storhaug K, Holst D. Caries experience of disabled school-age children. Community Dent Oral Epidemiol. 1987;15(3):144–149.
- Siamopoulou A, Mavridis AK, Vasakos S, et al. Sialochemistry in juvenile chronic arthritis. Br J Rheumatol. 1989;28(5):383–385.
- Welbury RR, Thomason JM, Fitzgerald JL, et al. Increased prevalence of dental caries and poor oral hygiene in juvenile idiopathic arthritis. Rheumatology (Oxford). 2003;42(12):1445–1451.
- Skeie MS, Gil EG, Cetrelli L, et al. Oral health in children and adolescents with juvenile idiopathic arthritis - a systematic review and meta-analysis. BMC Oral Health. 2019;19(1):285.
- Gil EG, Astrom AN, Lie SA, et al. Dental caries in children and adolescents with juvenile idiopathic arthritis and controls: a multilevel analysis. BMC Oral Health. 2021;21(1):417.
- Fleming PS, Koletsi D, Polychronopoulou A, et al. Are clustering effects accounted for in statistical analysis in leading dental specialty journals? J Dent. 2013;41(3):265–270.
- Rutterford C, Copas A, Eldridge S. Methods for sample size determination in cluster randomized trials. Int J Epidemiol. 2015;44(3):1051–1067.
- Hannigan A, Lynch CD. Statistical methodology in oral and dental research: pitfalls and recommendations. J Dent. 2013;41(5):385–392.
- Masood M, Masood Y, Newton JT. The clustering effects of surfaces within the tooth and teeth within individuals. J Dent Res. 2015;94(2):281–288.
- Petty RE, Southwood TR, Manners P, et al. International league of associations for rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–392.
- Greene JC, Vermillion JR. The simplified oral hygiene index. J Am Dent Assoc. 1964;68(1):7–13.
- Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975;25(4):229–235.
- Age limits and adolescents. Paediatr Child Health. 2003;8(9):577–578.
- Wallace CA, Ruperto N, Giannini E, et al. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol. 2004;31(11):2290–4.
- Wallace CA, Giannini EH, Huang B, et al. American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res (Hoboken). 2011;63(7):929–36.
- Pari A, Ilango P, Subbareddy V, et al. Gingival diseases in childhood - a review. J Clin Diagn Res. 2014;8(10):ZE01–4.
- Kao KT, Denker M, Zacharin M, et al. Pubertal abnormalities in adolescents with chronic disease. Best Pract Res Clin Endocrinol Metab. 2019;33(3):101275.
- Locker D. Response and nonresponse bias in oral health surveys. J Public Health Dent. 2000;60(2):72–81.
- Ferrara G, Mastrangelo G, Barone P, et al. Methotrexate in juvenile idiopathic arthritis: advice and recommendations from the MARAJIA expert consensus meeting. Pediatr Rheumatol Online J. 2018;16(1):46.
- Polak D, Martin C, Sanz-Sanchez I, et al. Are anti-inflammatory agents effective in treating gingivitis as solo or adjunct therapies? A systematic review. J Clin Periodontol. 2015;42(Suppl 16):S139–S51.
- Vogel RI, Fink RA, Schneider LC, et al. The effect of folic acid on gingival health. J Periodontol. 1976;47(11):667–668.
- Grevich S, Lee P, Leroux B, et al. Oral health and plaque microbial profile in juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2019;17(1):81.
- Honkala E, Freeman R. Oral hygiene behavior and periodontal status in European adolescents: an overview. Community Dent Oral Epidemiol. 1988;16(4):194–198.
- Santos D, Silva C, Silva M. Oral health and quality of life of children and adolescents with juvenile idiopathic arthritis according to their caregivers' perceptions. Spec Care Dentist. 2015;35(6):272–278.
- Fisher-Owens SG, Platt LJ, Weintraub JA, et al. Influences on children's oral health: a conceptual model. Pediatrics. 2007;120(3):e510–e520. PMID: 17766495.
- Leksell E, Ernberg M, Magnusson B, et al. Intraoral condition in children with juvenile idiopathic arthritis compared to controls. Int J Paediatr Dent. 2008;18(6):423–433.
- Pugliese C, van der Vinne RT, Campos LM, et al. Juvenile idiopathic arthritis activity and function ability: deleterious effects in periodontal disease? Clin Rheumatol. 2016;35(1):81–91.
- Haffajee AD, Teles RP, Patel MR, et al. Factors affecting human supragingival biofilm composition. I. Plaque mass. J Periodontal Res. 2009;44(4):511–519.
- Haffajee AD, Teles RP, Patel MR, et al. Factors affecting human supragingival biofilm composition. II. Tooth position. J Periodontal Res. 2009;44(4):520–528.
- Furuichi Y, Lindhe J, Ramberg P, et al. Patterns of de novo plaque formation in the human dentition. J Clin Periodontol. 1992;19(6):423–433.
- Cumming BR, Loe H. Consistency of plaque distribution in individuals without special home care instruction. J Periodontal Res. 1973;8(2):94–100.
- Feres de Melo AR, Ferreira de Souza A, de Oliveira Perestrelo B, et al. Clinical oral and salivary parameters of children with juvenile idiopathic arthritis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(1):75–80.
- Kobus A, Kierklo A, Zalewska A, et al. Unstimulated salivary flow, pH, proteins and oral health in patients with juvenile idiopathic Arthritis. BMC Oral Health. 2017;17(1):94.
- Walton AG, Welbury RR, Foster HE, et al. Sialochemistry in juvenile idiopathic arthritis. Oral Dis. 2002;8(6):287–290.
- Brik R, Rosen I, Savulescu D, et al. Salivary antioxidants and metalloproteinases in juvenile idiopathic arthritis. Mol Med. 2010;16(3-4):122–128.
- Brik R, Livnat G, Pollack S, et al. Salivary gland involvement and oxidative stress in juvenile idiopathic arthritis: novel observation in oligoarticular-type patients. J Rheumatol. 2006;33(12):2532–2537.
- de Oliveira Perestrelo B, Feres de Melo AR, de Sant'Anna GR, et al. Compromised salivary parameters of children with juvenile idiopathic arthritis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121(3):262–268.
- Marsh PD, Do T, Beighton D, et al. Influence of saliva on the oral microbiota. Periodontol 2000. 2016;70(1):80–92.
- Frid P, Baraniya D, Halbig J, et al. Salivary oral microbiome of children with juvenile idiopathic arthritis: a Norwegian cross-sectional study. Front Cell Infect Microbiol. 2020;10:602239.
- Kinane DF. Periodontitis modified by systemic factors. Ann Periodontol. 1999;4(1):54–64. PMID: 10863375.
- Zmora N, Bashiardes S, Levy M, et al. The role of the immune system in metabolic health and disease. Cell Metab. 2017;25(3):506–521.
- Pludowski P, Holick MF, Pilz S, et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun Rev. 2013;12(10):976–989.
- Murakami S, Mealey BL, Mariotti A, et al. Dental plaque-induced gingival conditions. J Periodontol. 2018;89(Suppl 1):S17–S27.
- Barnes MG, Grom AA, Thompson SD, et al. Biologic similarities based on age at onset in oligoarticular and polyarticular subtypes of juvenile idiopathic arthritis. Arthritis Rheum. 2010;62(11):3249–3258.
- Tomas I, Diz P, Tobias A, et al. Periodontal health status and bacteraemia from daily oral activities: systematic review/meta-analysis. J Clin Periodontol. 2012;39(3):213–228.