Abstract
Objectives
The soluble bacterial pattern recognition receptor, sCD14 augments inflammatory responses in oral cavity. The aim of the study was to investigate whether patients with geographic tongue (GT) with and without fissured tongue (FT) have impaired inflammatory regulation, manifesting as increased levels of sCD14 in the saliva.
Material and Methods
An enzyme-linked immunosorbent assay was used to measure the amount of sCD14 in whole and parotid saliva of patients diagnosed with GT (GT whole, n = 21; GT parotid, n = 23) and control subjects (GT whole, n = 25; GT parotid, n = 18). The levels of sCD14 were also evaluated according to our previous clinical assessment of GT based on the number of lesions detected on the tongue, as ‘mild’ (a single lesion), ‘moderate’ (2–5 lesions), or ‘severe’ (≥6 lesions). Diagnosis of FT was established when multiple grooves or fissures were observed on the dorsal and lateral surfaces of the tongue.
Results
GT patients had significantly higher sCD14 levels in whole (p<.05) and parotid saliva (p<.001), compared with controls. GT patients with FT had significantly increased sCD14 levels only in parotid saliva. A gradual increase in sCD14 levels in parotid and unstimulated saliva was seen in GT patients with multiple tongue lesions compared with single lesions.
Conclusions
GT patients had increased sCD14 in both parotid and unstimulated saliva. sCD14 seems to increase local inflammatory responses, which suggests its involvement in the pathophysiology of GT.
Introduction
Geographic tongue (GT) is a common oral mucosal lesion that affects the dorsal and lateral surfaces of the tongue and is characterised by atrophy of the filiform papillae, resulting in denuded areas with whitish peripheral zones that migrate across the tongue by healing at one site while extending into another [Citation1]. The concomitant occurrence of GT and fissure tongue (FT) has been reported in several studies [Citation2,Citation3]. Furthermore, it has been suggested that GT and FT represent different reaction patterns of the same inflammatory disease of the tongue [Citation4]. It has been proposed that GT is transforming into FT [Citation5].
The innate immune system is the first line of defence against microbial infections. It employs pattern recognition receptors that recognize diverse molecular structures, including viruses and bacteria [Citation6]. The family of Toll-like receptors (TLRs) are essential molecules for microbial recognition in the oral cavity in building and maintaining host-microbe homeostasis [Citation7–10]. Saliva is considered to modulate bacteria and lipopolysaccharide (LPS) induced immune responses via immunomodulatory glycoproteins that function as antimicrobial agents, including secretory IgA, mucins, agglutinin, statherins, sTLR2, and sCD14 [Citation7,Citation11]. The concerted action of these agents provides multifunctional protection against microorganisms. CD14 is primarily well-known as an LPS receptor [Citation12]. Although LPS is considered its main ligand, CD14 also recognizes other pathogen-associated molecular patterns [Citation6]. sCD14 mediates proinflammatory cytokines induction via TLRs [Citation13]. The most prominent function of sCD14 is to the augment epithelial cell responsiveness to low concentrations of LPS [Citation12,Citation14]. Increased levels of sCD14 in saliva are reported in several oral pathologies, including periodontitis [Citation15] and oral lichen planus [Citation16]. Moreover, CD14 has been shown to upregulate IL-8 production in epithelial cells in the presence of pathogenic bacteria [Citation17]. We have previously shown that IL-8 and calprotectin levels are increased in unstimulated saliva of geographic tongue patients and are correlated with the clinical severity [Citation18,Citation19]. Histologically, subepithelial infiltrates accumulated in neutrophil pustules are prominent in GT lesions [Citation3]. Accordingly, the augmented local inflammatory response shown by previous studies suggest an increase of sCD14 in the oral cavity of patients with GT. The expression of sCD14 has been shown in human major salivary glands and saliva, implicating a local source of secretion into the oral cavity [Citation20]. To the best of our knowledge, sCD14 was not studied before in patients with GT. The aim of this study was to investigate whether GT patients with and without FT have increased salivary levels of sCD14 which correlate with the severity of the condition.
Materials and methods
Participants
This is a case-control study that included patients diagnosed with GT by oral medicine specialists. The inclusion criteria for patients were the clinical diagnosis of GT with or without FT, absence of any other oral mucosal lesions, periodontitis, and dental caries. Controls were included in absence of any other oral mucosal lesions, periodontitis, and dental caries. Exclusion criteria for patients and controls were age <18 years, and presence of other oral mucosal lesions. Patients and controls were recruited from the Clinic of Oral Medicine, Public Dental Health Service, Gothenburg, and from a private dental clinic in Borås, Sweden between August 2014 and April 2015.
The Central Ethical Review Board in Gothenburg, Sweden, approved the study (permit number DNR 032-12). Written informed consent was obtained from the patients, and the study was performed in accordance with the Declaration of Helsinki.
Clinical assessment of patients with geographic tongue (GT) with and without fissure tongue (FT)
GT was diagnosed when erythematous depapillated areas surrounded by a red, white or yellow border was observed [Citation5,Citation18]. The patients with GT were evaluated and grouped according to a clinical assessment, based on the number of lesions detected on the tongue, into ‘mild’ (a single lesion), ‘moderate’ (2–5 lesions), or ‘severe’ (≥6 lesions) [Citation5]. Diagnosis of GT with FT was established when multiple grooves or fissures were observed on the dorsal and lateral surfaces of the tongue [Citation5].
Collection of saliva samples
All the participants were asked to refrain from eating, drinking and performing oral hygiene procedures for at least 1 h prior to saliva collection. For each individual, the volume of the saliva was measured and calculated as the secretory flow rate (ml/min.).
Unstimulated whole saliva collection
Spitting method was used for the collection of unstimulated saliva, the subjects were asked to expectorate once a minute for 10 min. into a pre-weighed 50-ml tube that was kept on ice [Citation21].
Parotid saliva collection
For the collection of parotid saliva, a Lashley cup was fitted over the Stenson’s duct of the parotid gland and secretion was stimulated by placing drops of citric acid (2%) on the tongue once every minute over a 5-minute period. The parotid saliva was collected in a pre-weighed 10-ml tube that was kept on ice.
All samples were stored at −80 °C until analysis at the Department of Oral Microbiology and Immunology, School of Dentistry, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden. These samples are identified at the Biobank of Sahlgrenska Academy with a permit no. 890.
Handling of saliva samples
For one millilitre of unstimulated saliva and parotid saliva, twenty-five microlitres of protease inhibitor cocktail (Sigma-Aldrich, S8830; one tablet diluted in 4 ml distilled water) was immediately added to minimize protein degradation.). 40 microlitres of EDTA (Sigma-Aldrich, 2 mM) was added to decrease viscosity of unstimulated saliva samples.
All samples were then divided into 250-microlitre aliquots to reduce the risk of the fluid undergoing cycles of freezing and thawing, and immediately stored at −80 °C until analysis. The samples were prevented from undergoing any form of centrifugation. The samples did not undergo centrifugation, as this would have led to the loss of proteins [Citation21].
Enzyme-linked immunosorbent assay (ELISA)
Sandwich ELISA was used according to the manufacturer’s instructions (R&D systems, USA) to measure sCD14 concentration in the whole and parotid saliva samples, except in the case of pre-treatment of whole saliva samples with the anionic detergent sodium dodecyl sulphate (SDS, Sigma-Aldrich, 0.02%). SDS was used to dissociate the mucin particles, as described in our previous study for quantitative detection of cytokines in whole saliva [Citation21]. Whole saliva samples were incubated for 20 min in SDS (50 μl for 200 μl saliva) and placed on a 96-microwell plate in a duplicate dilution series (from 1/2 to 1/4). Parotid saliva lacks mucins therefore, SDS pre-treatment did only applied for whole saliva samples. The amount of sCD14 was determined in picograms/millilitre (pg/ml) by measurement of optical density (OD) on a plate reader using a wavelength of 450 nm. A standard curve generated from the OD values of standards provided by the manufacturer was used to determine the concentration of sCD14. The variation between duplicates was less than 5%. The level of sCD14 (pg/ml) in the saliva was determined using a standard curve of known concentrations of recombinant human CD14. The data presented in the study are given as secretory output (pg/min) to overcome individual variations of the salivary flow rate. The secretory output (pg/min) of sCD14 was calculated by multiplying the flow rate (ml/min) for the respective individual with the concentration of sCD14 (pg/ml) calculated from the standard curve [Citation21].
Statistical analysis
Univariate analysis, including an unpaired t-test and ordinary one-way ANOVA, was performed using GraphPad Prism, version 9.0.0 for Mac OS, GraphPad Software, La Jolla, CA, USA) to compare the CD14 secretory output levels of the patients with those of the controls. A p value <.05 was regarded as being statistically significant.
Results
Participants and salivary flow rate (FR)
shows the demographic data of the study population. Regarding the medical history, all patients and control subjects were free from systemic diseases and taking no medications. Parotid (n = 23) and unstimulated (n = 21) saliva from patients with GT were analysed for sCD14 and compared with parotid (n = 18) and unstimulated (n = 25) saliva from controls. No significant differences in age, gender or salivary flow rate were found between the patients with GT (with and without FT) and the controls.
Table 1. Demographic data of the study population.
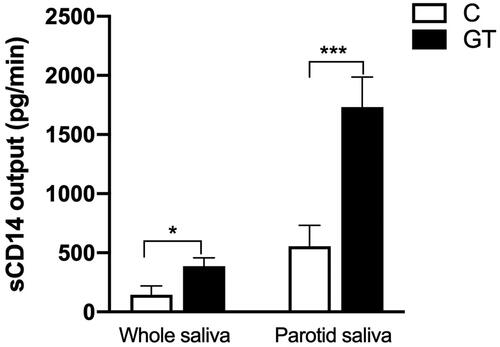
sCD14 output increases in unstimulated and parotid saliva of GT
In parotid saliva of patients with GT, the CD14 output (1731.4 ± 255.8 pg/min, n = 23) was significantly higher compared with controls (554.4 ± 164.9 pg/min, n = 18; p= .001). Similarly, unstimulated saliva of patients with GT showed significantly higher secretory output of CD14 (385.6 ± 70.9 pg/min, n = 21) compared with controls (144.4 ± 74.3 pg/min, n = 25; p= .02), as shown in .
sCD14 output increases with disease severity
shows that the sCD14 output for unstimulated saliva in GT patients with severe lesions (n = 7, 1230 ± 563.9 pg/min) was significantly increased compared with controls (n = 25, 144.4 ± 74.3 pg/min, p<.01). Although moderate GT lesions had higher levels of CD14 output (n = 11, 399.1 ± 108.4 pg/min) in unstimulated saliva, this difference did not reach a statistically significant level (p= .06). A slight increase in CD14 output was found among mild GT lesions in unstimulated saliva (n = 3, 262.8 ± 167.3 pg/min); however, without significant changes compared with controls (p= .6).
Figure 2. The effect of the clinical classification (mild, moderate, severe) on secretory output (pg/min) of sCD14 (mean + SEM) in controls (C) and patients with GT in (a) whole and (b) parotid saliva. Comparisons between the groups were analysed by unpaired t-test, **p<.01, ***p<.001.
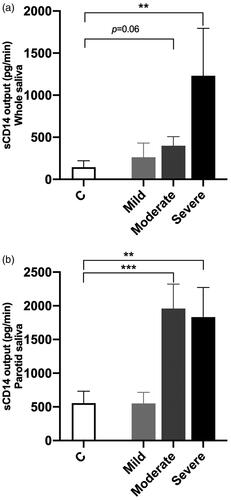
shows that the sCD14 output for parotid saliva in GT patients with moderate (n = 12, 1959 ± 361.8 pg/min, p < .001) and severe (n = 8, 1833 ± 438.8 pg/min, p < .01) lesions was significantly elevated when compared with controls (n = 18, 554.4 ± 164.9 pg/min), whereas mild GT lesions (n = 3, 550.4 ± 164.4 pg/min) showed no significant changes in sCD14 output in parotid saliva compared with controls ().
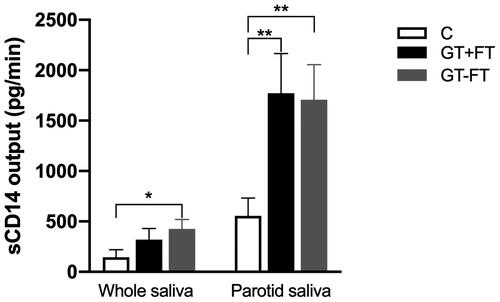
sCD14 output in GT patients with fissured tongue (FT)
shows a significant increase in sCD14 output in both parotid and unstimulated saliva samples from GT patients in the absence of FT (GT-FT), compared with the corresponding controls. Accordingly, output of parotid (Pa) GT + FT (n = 9, 1773 ± 392.3) and Pa GT-FT (n = 14, 1705 ± 347.7) were compared with output of Pa controls (n = 18, 554.4 ± 164.9) and showed a statistically significant difference (p < .01). Output of unstimulated saliva for GT-FT (n = 13, 426.0 ± 94.0) showed higher levels which was statistically significant compared with output of unstimulated saliva from controls (n = 25, 144.4 ± 74.3, p < .05). Although, sCD14 output in unstimulated saliva for GT + FT (n = 8, 320.1 ± 110.1) showed higher levels than that of controls, it did not reach any significance level (p = .2).
Discussion
The present study showed an increased sCD14 output in patients with GT with and without FT, which indicates augmented activity of the soluble pattern recognition receptor sCD14. Moreover, the higher levels of sCD14 in patients with multiple tongue lesions than in patients with single lesions point to the role of sCD14 in the pathogenesis of GT. In our previous study, a positive correlation was also found between the amount of IL-8 in saliva and what we defined as ‘more severe disease’, which is dependent on neutrophil chemotaxis [Citation18]. Recruitment of neutrophils in the subepithelial infiltrates of GT lesions has been shown to play a role in the pathogenesis of GT [Citation3]. However, the factors that cause the augmented inflammatory response and neutrophil chemotaxis in GT remain to be identified. CD14 has been shown to augment responses to many pathogen-derived ligands, including Gram-positive and Gram-negative bacterial peptidoglycan and spirochaetal lipoproteins [Citation12–15,Citation17]. Moreover, saliva-derived sCD14 has been shown to upregulate IL-8 production in oral epithelial cell cultures in the presence of pathogenic periodontal bacteria [Citation17]. The elevated levels of sCD14 observed in this study are likely to enhance the tissue inflammatory response to local microbiota, implicating sCD14 as one of the causative factors taking part in the pathogenesis of GT. CD14 has been shown to enhance cell activation through TLRs in oral epithelial cells [Citation10,Citation13]. The interactions of CD14 with TLRs are important for host in regulation of immune response to pathogens and affect the susceptibility to a variety of diseases [Citation6]. This is in line with current results, where the severity of GT lesions is positively correlated with the increased levels of sCD14.
The role of CD14 in the host defense against viral and bacterial infections has shown opposing results. A protective role of CD14 has been shown in intestinal infections, while the opposite effect has been described in pulmonary infections, depending on the infectious agent [Citation22]. In periodontitis, higher levels of CD14 were reported in gingival crevicular fluid without an increase in serum levels, indicating local production of CD14 in response to bacterial challenge [Citation23]. Furthermore, high levels of CD14 in the gingival crevicular fluid were associated with fewer and shallower gingival pockets, which contributed to a protective role by enhancing the phagocytosis of pathogenic plaque bacteria [Citation24]. The high levels of sCD14 found in patients with GT may be due to an altered microbial composition of the tongue mucosa. We have previously shown a shift in the lingual bacterial ecology in GT patients [Citation25]. GT lesions are characterised by atrophy of the filiform papilla on the dorsum of the tongue. Due to the presence of such lesions, the lingual microbiota might upregulate the sCD14 levels. Interestingly, upregulation of sCD14 levels in blood has been also reported in the gut, in the presence of sustained dysbiosis or epithelial barrier damage [Citation26]. It is suggested that if this condition is sustained, it may result in systemic immune activation, which ultimately leads to the loss of immune regulation and becomes a chronic condition [Citation26]. It remains unknown if the shift in the lingual microbiota in GT is a consequence of the lesions or dependent on other immunological or genetic factors that are associated with the initiation of the condition. Collectively, our data support a possible role for the host-microbial interactions in the GT pathogenesis. However, a limitation of the present study is the relatively small sample size, which limits the possibility to draw a robust conclusion.
The present study showed that the amount of sCD14 was five times greater in the parotid saliva of GT patients and four times greater in the saliva of controls than the amounts found in unstimulated saliva. The more prominent increase in sCD14 in parotid saliva may be explained by the fact that the secretion of sCD14 is mainly confined to serous cells, which are a rich source of sCD14 [Citation20]. Moreover, sCD14 can be absorbed by oral bacteria, mainly S salivarius, which further supports the result of the study that sCD14 in unstimulated saliva mainly attach to oral bacteria after reaching the mucosal and dental surfaces [Citation20].
CD14 is predominantly expressed by immune cells such as monocytes, macrophages, and antigen-presenting cells, but also by non-immune cells, including epithelial cells [Citation22]. Intriguingly, it appears that the cell type expressing CD14 specifies its functional activity [Citation14]. sCD14 increases the macrophage and neutrophil response to lipopolysaccharide (LPS) 50 to100 times, compared with LPS alone [Citation12]. sCD14 binds to LPS-binding protein (LBP) in plasma, thereby controlling the activation of neutrophils [Citation27]. However, the LBP levels in saliva are below the detection level, and elevated levels of sCD14 may therefore activate the neutrophil and inflammatory response in the oral mucosa [Citation17].
Recently, mutations in the IL-36RN gene for GT have been reported, where the mutation caused over-expression of IL-36 in the lingual mucosa [Citation28]. Elevated levels of IL-36 have been shown to upregulate the expression of CD14 in psoriasis and thereby enhancing the inflammatory response of cells to the local microbiota [Citation29,Citation30]. The genetic and environmental determinants of sCD14 are largely unknown, although its expression appears to be regulated at the genetic level in a cell type-specific manner [Citation31].
In conclusion, the present study showed increased salivary levels of sCD14 in patients with GT. Further studies are needed to elucidate whether the increased levels of sCD14 have any association with papillary atrophy and neutrophil infiltration in GT.
Author contributions
H. Cevik-Aras contributed to the design of the study, laboratory work, data analysis, and writing of the manuscript. A. Dafar took part in the sampling of the research material, data analysis, and critically revised the manuscript.
Acknowledgments
The study was financed by the Västra Götaland Region, Sweden.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Varoni E, Decani S. Geographic tongue. N Engl J Med. 2016;374(7):670–670.
- Assimakopoulos D, Patrikakos G, Fotika C, et al. Benign migratory glossitis or geographic tongue: an enigmatic oral lesion. Am J Med. 2002;113(9):751–755.
- Marks R, Radden BG. Geographic tongue: a clinico-pathological review. Australas J Dermatol. 1981;22(2):75–79.
- Kullaa-Mikkonen A, Penttilä I, Kotilainen R, et al. Haematological and immunological features of patients with fissured tongue syndrome. Br J Oral Maxillofac Surg. 1987;25(6):481–487.
- Dafar A, Cevik-Aras H, Robledo-Sierra J, et al. Factors associated with geographic tongue and fissured tongue. Acta Odontol Scand. 2016;74(3):210–216.
- Hajishengallis G, Russell MW. Innate humoral defense factors. Mucosal Immunology. 2015;15:251–270.
- Kuroishi T, Tanaka Y, Sakai A, et al. Human parotid saliva contains soluble toll-like receptor (TLR) 2 and modulates TLR2-mediated interleukin-8 production by monocytic cells. Mol Immunol. 2007;44(8):1969–1976.
- Ligtenberg AJ, Veerman EC, Nieuw Amerongen AV, et al. Salivary agglutinin/glycoprotein-340/DMBT1: a single molecule with variable composition and with different functions in infection, inflammation and cancer. Biol Chem. 2007;388(12):1275–1289.
- Nylund KM, Ruokonen H, Sorsa T, et al. Association of the salivary triggering receptor expressed on myeloid cells/its ligand peptidoglycan recognition protein 1 axis with oral inflammation in kidney disease. J Periodontol. 2018;89(1):117–129.
- Shang L, Deng D, Buskermolen JK, et al. Commensal and pathogenic biofilms alter toll-like receptor signaling in reconstructed human gingiva. Front Cell Infect Microbiol. 2019;9:282.
- van 't Hof W, Veerman EC, Nieuw Amerongen AV, et al. Antimicrobial defense systems in saliva. Monogr Oral Sci. 2014;24:40–51.
- Funda DP, Tuckova L, Farre MA, et al. CD14 is expressed and released as soluble CD14 by human intestinal epithelial cells in vitro: lipopolysaccharide activation of epithelial cells revisited. Infect Immun. 2001;69(6):3772–3781.
- Hajishengallis G, Martin M, Sojar HT, et al. Dependence of bacterial protein adhesins on toll-like receptors for proinflammatory cytokine induction. Clin Diagn Lab Immunol. 2002;9(2):403–411.
- Di Gioia M, Zanoni I. Toll-like receptor co-receptors as master regulators of the immune response. Mol Immunol. 2015;63(2):143–152.
- AlQallaf H, Hamada Y, Blanchard S, et al. Differential profiles of soluble and cellular toll like receptor (TLR)-2 and 4 in chronic periodontitis. PLoS One. 2018;13(12):e0200231.
- Srinivasan M, Kodumudi KN, Zunt SL. Soluble CD14 and toll-like receptor-2 are potential salivary biomarkers for oral lichen planus and burning mouth syndrome. Clin Immunol. 2008;126(1):31–37.
- Takayama A, Satoh A, Ngai T, et al. Augmentation of actinobacillus actinomycetemcomitans invasion of human oral epithelial cells and up-regulation of interleukin-8 production by saliva CD14. Infect Immun. 2003;71(10):5598–5604.
- Dafar A, Bankvall M, Garsjo V, et al. Salivary levels of interleukin-8 and growth factors are modulated in patients with geographic tongue. Oral Dis. 2017;23(6):757–762.
- Garsjo V, Dafar A, Jontell M, et al. Increased levels of calprotectin in the saliva of patients with geographic tongue. Oral Dis. 2020;26(3):558–565.
- Uehara A, Sugawara S, Watanabe K, et al. Constitutive expression of a bacterial pattern recognition receptor, CD14, in human salivary glands and secretion as a soluble form in saliva. Clin Diagn Lab Immunol. 2003;10(2):286–292.
- Dafar A, Rico P, Işık A, et al. Quantitative detection of epidermal growth factor and interleukin-8 in whole saliva of healthy individuals. J Immunol Methods. 2014;408:46–51.
- Zanoni I, Granucci F. Role of CD14 in host protection against infections and in metabolism regulation. Front Cell Infect Microbiol. 2013;3:32.
- Jin L, Ren L, Leung WK, et al. The in vivo expression of membrane-bound CD14 in periodontal health and disease. J Periodontol. 2004;75(4):578–585.
- Jin L, Darveau RP. Soluble CD14 levels in gingival crevicular fluid of subjects with untreated adult periodontitis. J Periodontol. 2001;72(5):634–640.
- Dafar A, Bankvall M, Cevik-Aras H, et al. Lingual microbiota profiles of patients with geographic tongue. J Oral Microbiol. 2017;9(1):1355206.
- Brenchley JM, Douek DC. Microbial translocation across the GI tract. Annu Rev Immunol. 2012;30:149–173.
- Hailman E, Vasselon T, Kelley M, et al. Stimulation of macrophages and neutrophils by complexes of lipopolysaccharide and soluble CD14. J Immunol. 1996;156(11):4384–4390.
- Liang J, Huang P, Li H, et al. Mutations in IL36RN are associated with geographic tongue. Hum Genet. 2017;136(2):241–252.
- D'Erme AM, Wilsmann-Theis D, Wagenpfeil J, et al. IL-36gamma (IL-1F9) is a biomarker for psoriasis skin lesions. J Invest Dermatol. 2015;135(4):1025–1032.
- Madonna S, Girolomoni G, Dinarello CA, et al. The significance of IL-36 hyperactivation and IL-36R targeting in psoriasis. Int J Mol Sci. 2019;20(13):3318.
- Reiner AP, Lange EM, Jenny NS, et al. Soluble CD14: genomewide association analysis and relationship to cardiovascular risk and mortality in older adults. Arterioscler Thromb Vasc Biol. 2013; 33(1):158–164.