Abstract
Objective
This study aimed to 1) investigate the relationships between hair cortisol concentration (HCC), insomnia symptoms, Health-Related Quality of Life (HRQoL) and Oral Health-Related Quality of Life (OHRQoL) in preschool children with severe early childhood caries, 2) compare HCC, insomnia symptoms, HRQoL and OHRQoL in preschool children with severe early childhood caries with these factors in children without clinical signs of dental caries, and 3) explore correlations between caries scores and HCC, insomnia symptoms, HRQoL and OHRQoL.
Material and Methods
A case-control pilot study, including 12 children with severe early childhood caries and 28 controls, aged 3-5 years. Dental examination was performed and hair samples for cortisol were taken. Parents filled out questionnaires about their child’s insomnia symptoms, HRQoL and OHRQoL. Interpreters were used in families with language difficulties.
Results
The key findings in this pilot study were tendencies that children with severe early childhood caries had more insomnia symptoms, and poorer OHRQoL than the controls. Caries scores was correlated with insomnia symptoms and OHRQoL.
Conclusions
Dentists should include questions about the child’s sleep when they see the child, as insomnia related to dental caries may lead to several physical, mental, and social problems.
Introduction
Dental caries is the most common preventable chronic disease worldwide and causes discomfort, acute and chronic pain, and tooth loss. The risk for dental caries incidence is predominantly most common among children [Citation1]. The National Board of Health and Welfare in Sweden states that the prevalence of dental caries is 4% in 3-year-old children. In 6-year-old children in Sweden, the incidence of dental caries increased from 21% to 27% between 2011 and 2019. Today, more than one in four children in Sweden has dental caries at six years of age. Children with parents who have immigrated from countries outside the Nordic countries and western Europe have twice the risk of developing dental caries than children with parents born in Sweden [Citation2].
The human stress-response system, which comprises the autonomic nervous system and the hypothalamic-pituitary-adrenal (HPA) axis, has evolved such that it becomes rapidly activated and subsequently reverts to relaxation and recovery. Stress load over time causes an imbalance in the system and affects cortisol levels, which impairs sleep, the immune system, mood and learning capacity [Citation3]. Common problems in children with early childhood caries are stress, insomnia, irritation, frustration, difficulties in socializing, and poor school performance [Citation4,Citation5]. Consequently, dental caries has a negative impact on general health and the quality of life of children [Citation6]. Dental caries is correlated with cortisol in saliva, where children with early childhood caries have higher levels of cortisol than caries-free children [Citation7,Citation8]. After dental treatment, cortisol levels decrease [Citation7,Citation9]. However, measuring saliva cortisol only measures momentary and not prolonged stress. A novel method of measuring cortisol levels has been developed in recent years, in which prolonged stress levels are measured in human hair [Citation10]. The cortisol level in hair increases in children with poor health, and in those with increased exposure to a stressful social environment [Citation11], poor socioeconomic status in the family, and high BMI [Citation12]. The hypothesis in this study was that the HPA-axis is activated over longer time periods in children with dental caries, and the cortisol level in the hair increases. The relationship between hair cortisol and dental caries in children has not previously been reported. Analyzing cortisol concentrations in hair provides a retrospective and cumulative concentration of cortisol over time, just as the year rings in a tree. The sampling can be completed on one occasion and is non-invasive.
Sleep is essential for health and well-being, and 3-5-year-old children should sleep 10 to 13 h per 24 h (including naps) on a regular basis to promote health [Citation13]. Pain has a great impact on children with dental caries [Citation5], and is related to insomnia [Citation14,Citation15]. The general International Classification of Sleep Disorders, ICSD-2, criteria for insomnia include difficulty falling asleep (sleep-onset problems, SOP) and/or difficulty staying asleep and waking up earlier than desired (sleep-maintenance problems, SMP). These problems lead to impaired daytime functioning and several physical, mental, and social problems [Citation16]. The regulation of neurobiological behavioral and cognitive processes becomes affected, mainly through behavioral tendencies and neurological changes, leading to more negative and less positive emotions [Citation17,Citation18]. Moreover, executive functions are often impaired, which affects learning abilities and school performance negatively [Citation19]. Insomnia in young children is related to the health-related quality of life (HRQoL), which is an individual’s perceived physical and mental health over time. Insomnia affects psychological well-being, cognitive capacity and feelings about school, as well as social relationships with peers and friends [Citation20]. Moreover, severe early childhood caries has a negative effect on HRQoL and on oral HRQoL (OHRQoL) [Citation6,Citation21–23], whereas dental interventions in children with early childhood caries have a significant positive impact on parental ratings of their overall oral health and physical, mental, and social functioning [Citation24,Citation25]. OHRQoL includes the individual’s comfort when eating, sleeping and engaging in social interaction, their self-esteem, and their satisfaction with respect to their oral health.
The aims of this study were to 1) investigate the relationships between hair cortisol concentration (HCC), insomnia symptoms, HRQoL and OHRQoL in preschool children with severe early childhood caries, 2) compare HCC, insomnia symptoms, HRQoL and OHRQoL in preschool children with severe early childhood caries with these factors in children without clinical signs of dental caries, and 3) explore correlations between caries scores and HCC, insomnia symptoms, HRQoL and OHRQoL. Furthermore, this study aimed to assess the feasibility of using these measurements in a larger intervention study for preschool children with severe early childhood caries.
Materials and methods
Design
This study was a case-control pilot study that examined hair cortisol concentration (HCC), insomnia symptoms, HRQoL and OHRQoL in preschool children with severe early childhood caries, and compared these factors with those in a control group of children with no clinical signs of dental caries. The hypothesis was that children with severe early childhood caries have higher HCC, report insomnia symptoms more frequently, and have poorer HRQoL and poorer OHRQoL than children without clinical signs of dental caries. Since this study was a pilot study no power calculation was made.
Ethical considerations
The study was approved by the Regional Ethics Committee for Medical Research (Ref. No. 2018/175-31) and performed in accordance with the Helsinki Declaration [Citation26]. Oral and written information was given to both the children and their parents. An age-adjusted pictogram sheet that described the study procedure was used to inform the children [Citation27]. The children gave oral consent, and the parents gave written consent for their child’s participation in the study, in accordance with Section 18 of the Swedish Act concerning the Ethical Review of Research Involving Humans (2003:460). The act states that children under the age of 15 years should be informed about studies and should decide for themselves whether to participate or not.
Participants
Study group
The study group consisted of a consecutive sample of children aged 3–5 years with severe early childhood caries, referred for dental caries treatment under general anesthesia at the Department of Pediatric Dentistry, The Institute for Postgraduate Dental Education, Jönköping, Sweden.
Fifteen children (n = 15) and their parents received information about the study when visiting the dental clinic. Three parents in this group declined to participate: One parent was illiterate and stated that the questionnaire was too difficult to answer because of language difficulties (even though the use of an interpreter was offered), and two parents thought that answering the questionnaires would be too time-consuming.
Control group
The control group consisted of a convenience sample of children aged 3–5 years with no clinical signs of dental caries. This group was selected from one public dental service, in a small town in south-east Sweden.
Thirty-nine children and their parents received written information about the study by mail when they were invited for a regular dental examination. During the visit to the clinic, they received oral information from one of the authors (ALS). If the child had no clinical signs of dental caries when examined, they were eligible for the study and asked whether they were willing to provide written consent. Ten parents declined to participate; three parents stated that the questionnaire was too difficult to answer because of language difficulties (even though the use of an interpreter was offered), one child’s sister had recently died, and the other six parents gave no reason for declining to participate.
Exclusion criteria
Exclusion criteria were children with diagnosis of physiological or cognitive disorders.
Procedure
Clinical examination
Clinical examination was performed in a fully equipped dental chair with the aid of a mouth mirror, with drying by compressed air. Children in the study group underwent radiographic examination if they cooperated to the procedure. Four children in the study group cooperated to bite-wing radiographic. Dental examination was performed by the third author (ALS), who is pediatric dentist, with use of the Tell, Show, Do method [Citation28]. After the child had been examined, the parents completed a health declaration with demographics (age, sex, and health status), and questionnaires about the child’s sleep habits, health-related quality of life, and oral health-related quality of life. If a parent had difficulties with the Swedish language, a dental nurse read the questions out loud, and these were then repeated in the parent’s language by the interpreter, who also helped the parent to complete the questionnaires. Meanwhile, the dentist (ALS) took a hair sample for measurement of cortisol. If the child declined to give a hair sample, cortisol analysis was excluded. Afterwards, they received a small gift, which is a common routine for every child after a dental visit in Sweden and not exclusive for the study participants. Data were collected between August 2019 and March 2020.
Measures
Caries scores
Severe early childhood caries was defined as loss of tooth substance that had reached the stage of cavitation into the dentin on a tooth surface not previously restored. A proximal caries lesion was defined as a lesion seen clinically or in a radiograph that clearly extended into the dentin [Citation29]. Restored teeth and teeth extracted due to caries were also recorded. Extracted incisors and canines were registered as four decayed surfaces, and extracted molars as five surfaces. Caries data are presented as decayed, extracted and filled tooth surfaces (defs).
Hair cortisol concentration
Hair strands were cut close to the scalp. The hair samples collected were at least three centimeters long and least half a centimeter thick. Hair grows approximately one centimeter per month, so a sample of length 3 cm reflects the stress response during the preceding three months. The samples were stored in a dark locker at room temperature. Cortisol concentrations were determined by competitive radioimmunoassay, explained in detail by Karlén et al. [Citation30]. Measurements of HCC in children are reliable (ref. needed). The average HCC along the length of the hair increases up to 10 years of age. The reference value in children aged 4–5 years is 5.0 pg/mg, which is lower than the reference values for adults [Citation31].
Insomnia symptoms
The Swedish version of the six-item parent-proxy questionnaire PISI was used to assess insomnia symptoms [Citation32,Citation33]. The PISI measures two dimensions of sleep difficulties: sleep-onset problems (SOP), and sleep-maintenance problems (SMP). The PISI has one item (No. 6) that determines sleep duration by asking about the child’s total hours of sleep per night. The answers are scored using a simple 6-point Likert scale. The total mean score ranges from 0-30 points, where higher values indicate more insomnia symptoms over the last week. Items 1–5 (SOP and SMP) range from 0 = ‘Never/0 nights’ to 5 = ‘Always/7 nights’, with a maximum score of 10 points for SOP and 15 points for SMP. Item 6 (sleep duration) ranges from 0 = ‘11–13 h per night’ to 5 = ‘Less than 5 h per night’. The general ICSD-II criteria for insomnia have been used in the development of the measurement. The validity and reliability of the Swedish version of PISI have been tested, with good results [Citation33].
Health-related quality of life
The Swedish proxy-version of KIDSCREEN-27, which contains 27 items, was used to assess HRQoL over the preceding week [Citation34,Citation35]. The validity and reliability of this generic questionnaire have been tested and are good. It measures five dimensions of HRQoL: physical well-being (level of physical activity, energy, and fitness), psychological well-being (positive emotions and satisfaction with life), autonomy and parent relation (perceived level of autonomy, interaction between child-parent, and feeling loved and supported), social support and peers (interaction between child-peers), and school environment (perception of cognitive capacity, learning, concentration and feelings about school). The answers are scored using a 5-point Likert scale, ranging from ‘No agreement’ to ‘Total agreement’. Lower values indicate a poorer HRQoL. T-scores of 50 and sd ± 10 are regarded as normal [Citation34]. The copyright holder gave consent for the questionnaire to be used for the study.
Oral Health-Related quality of life
The Swedish version of the Child Oral Health-Related Quality-of-Life (COHQoL) questionnaire was used to explore OHRQoL and its impact on family life [Citation36]. The COHQoL consists of two components: the Parental-Caregivers Perception Questionnaire (P-CPQ) and the Family Impact Scale (FIS) [Citation37,Citation38].
The P-CPQ includes 33 items in four dimensions: oral symptoms (7 items), functional limitations (7 items), emotional well-being (9 items), and social well-being (10 items). The FIS includes 14 items in three dimensions: parental/family activity (5 items), parental emotions (4 items), family conflict (4 items), and financial burden (1 item). The answers to the P-CPQ and FIS components are scored using a 5-point Likert scale, ranging from 0 = ‘Never’ to 4 = ‘Very often’. The participants can also answer ‘Don’t know’. The Swedish version of P-CPQ has good construct consistency, acceptable internal consistency (Cronbach’s alpha 0.77), and moderate test-retest reliability (ICC 0.63) [Citation36].
Statistical analysis
Descriptive statistics were computed using the IBM-SPSS software, version 27 (Chicago IL). Rasch measurement analysis was used for all five dimensions of KIDSCREEN-27, the results of which were thereafter transformed to T-values [Citation34]. Mann Whitney U tests were performed to determine whether the descriptive data of the study group differed from those of the control group. Spearman’s rank correlation (ρ) test was used to analyze the correlations between outcomes in the different measurements.
One child in the study group had hair so short that it was not possible to take a sample. Data were missing from the PISI for one child. Data were missing from the psychological wellbeing dimension of KIDSCREEN-27 for one child, and from the autonomy and parent relation dimensions for another. Remaining data from these participants were included in the data analyses.
One child in the control group did not agree to hair sampling, and the hair of another was too short. Remaining data from these participants were included in the data analyses. One single outlier was found in the control group with high levels of HCC and insomnia symptoms. Further investigation showed that this child had been diagnosed with Lyme Borreliosis recently, and therefore data from this participant were excluded in all analyses.
The level of statistical significance was set at 5% (p ˂ .05).
Results
The total sample consisted of n = 40 children (for characteristics, see ).
Table 1. Characteristics of the children and their parents.
The study group consisted of n = 12 children (girls n = 5, boys n = 7), mean age 4.7 (sd ±1.1) years old. The caries scores mean in the study group was defs 16.8 (sd ± 9.3). The lowest caries score was defs 9 (n = 2) and the highest defs 39 (n = 1). One child in this group had food allergy and one child had primary adrenocortical insufficiency.
The control group consisted of n = 28 children (girls n = 9, boys n = 19), mean age 3.9 (sd ± 0.5) years old, without clinical signs of dental caries, neither cavitated nor early caries lesions (defs = 0). Three children in this group had been diagnosed with asthma (one of whom did not supply a hair sample for cortisol determination), and one had food allergy.
Hair cortisol concentration
The cortisol concentrations of the study group and control group did not significantly differ (). It became apparent during analysis that two children with high HCC in the control group had been diagnosed with asthma. The statistical analysis was repeated with the cortisol concentrations from these children excluded. This did not change the results, and thus these children were included in further analyses. HCC was not significantly correlated with insomnia symptoms, HRQoL or OHRQoL.
Table 2. Hair cortisol concentration, insomnia symptoms, health-related quality of life and oral health-related quality of life in the two groups.
Insomnia symptoms
The total PISI mean score differed significantly (p < .001) between the study group (mean 9.7, sd ± 3.5) and the control group (mean 4.3, sd ± 2.6). SMP was significantly higher in the study group (mean 4.6, sd ± 1.9, p < .001) than in the control group (mean 1.6, sd ± 1.6), and sleep duration (mean 1.8, sd ± 1.6, p < .05) was shorter. Differences in SOP between the groups were not significant (). Parents of ten children in the study group reported that other family members had their sleep disturbed due to their child’s oral health (FIS).
Health-related quality of life
presents data from KIDSCREEN-27. There were no statistically significant differences between the groups. The T-scores of both groups were above 50.
Oral health-related quality of life
The overall scale scores of the P-CPQ and the FIS were significantly higher (p < .001) in the study group (P-CPQ mean 31.1, sd ± 19.4, FIS mean 14.2, sd ± 7.5) than in the control group (P-CPQ mean 3.6, sd ± 5.7, FIS mean 0.4, sd ± 1,9). For scores of all dimensions, see . The total percentage of the answer ‘Don’t know’ in the OHRQoL was 2%. Eight children in the study group and one child in the control group reported pain in the teeth, lips, jaw or mouth often or very often (P-CPQ). One parent (study group) reported financial difficulties (FIS) during the preceding three months because of the child’s oral health.
Correlations between caries scores and HCC, insomnia symptoms, HRQoL and OHRQoL in all children
Caries scores (defs) were significantly correlated with OHRQoL (P-CPQ: ρ = .74, p = < .001; FIS: ρ = .89, p = < .001). Defs, P-CPQ and FIS were significantly correlated with sleep duration (, ) and SMP (, ). No significant correlations were found between defs and HCC or any of the dimensions of KIDSCREEN-27.
Figure 1. Multiple line chart of the mean number of decayed, extracted and filled tooth surfaces (defs), Family Impact Scale (FIS) and Parental-Caregivers Perception Questionnaire (P-CPQ) related to sleep duration, n = 39.
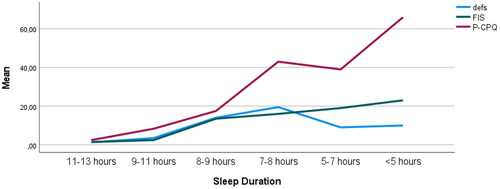
Figure 2. Multiple line chart of the mean number of decayed, extracted and filled tooth surfaces (defs), Family Impact Scale (FIS) and Parental-Caregivers Perception Questionnaire (P-CPQ) related to sleep maintenance problems (SMP), n = 39.
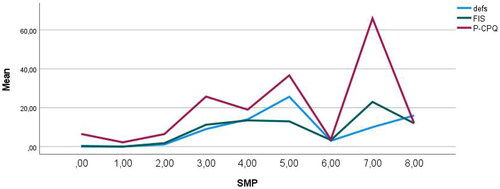
Table 3. Correlationsa between oral health and insomnia symptoms, n = 39.
Discussion
This study has examined the hypothesis that children with severe early childhood caries have higher HCC, more insomnia symptoms, poorer HRQoL and poorer OHRQoL than children without clinical signs of dental caries. The initial intention for the study had been to include 30 children with severe early childhood caries and 60 caries-free children from two different regions in Sweden. This was, however, not possible due to circumstances related to the outbreak of Covid-19, and the study was terminated before the intended number of participants had been included. Therefore, this study must be seen as a pilot study and conclusions from the results should be drawn with some caution.
The results of this study showed no significant differences in HCC or HRQoL. However, children with severe early childhood caries had more problems maintaining sleep, fewer hours of sleep per night and poorer OHRQoL. Caries scores was strongly correlated with SMP, sleep duration and OHRQoL, i.e. the greater the defs score, the more insomnia symptoms and poorer OHRQoL.
The results do not support our main hypothesis – that children with severe early childhood caries have higher HCC than children without clinical signs of dental caries. It was not possible to carry out deeper statistical analysis, including multivariate analysis, due to a lack of power, given the low number of study subjects. Surprisingly, the median HCC of the study group was higher than that of the control group. However, this difference is not statistically significant, probably because the sample is too small. Moreover, some outliers in the control group might have affected the analysis. HCC was not correlated with any other variable. This result is consistent with a recently published study of the correlations between HCC and objectively measured sleep and physical activity in 54 preschool children [Citation39]. A future study with a greater number of participants will be needed to give reliable results. The results from this study will be used for a power calculation for the new upcoming study.
The results supported our hypothesis that children with severe early childhood caries have more insomnia symptoms than healthy controls. Children in the study group slept eight to nine hours per night, to be compared with nine to eleven hours in the control group. Those in the study group also had more problems maintaining sleep during the night. The sleep duration for the children in the control group agrees with the general sleep recommendation [Citation13,Citation40], while it is lower than recommended for those in the study group. Caries scores were correlated with fewer hours of sleep per night and with SMP. Similar results have been reported in preschool children in China [Citation41]. It is possible that the reported sleep problems are an effect of dental pain. One child out of five has sleep problems in five-year-old children with dental pain (n = 549) [Citation42]. Moreover, dental pain is strongly correlated with trouble sleeping in children aged 8–18 years [Citation43]. Are insomnia symptoms and few hours of sleep per night risk factors for dental caries development, or do these factors cause the disease? Or both? Chen et al. [Citation44] showed that there is a correlation between an increased risk for dental caries development in deciduous teeth in Japanese children and three factors: late bedtime, short sleep duration, and irregular sleep. The risk of dental caries development was 30% higher in children with short sleep duration (shorter than 8 h) and high SMP than in a control group. In contrast, parents in another study reported less SMP in their children after dental caries treatment [Citation45,Citation46]. It is probable that both processes are occurring, and a vicious circle is established in these cases.
The results support our hypothesis that children with severe early childhood caries have a poorer oral health-related quality of life. The overall health-related quality of life of children, however, is not impaired. Our result confirms those of previously published studies, which showed that severe early childhood caries in 5-6-year-old children lowers the total score on OHRQoL and the scores in certain dimensions: parental/family activities, parental emotions, and financial burden [Citation47]. The impact of dental caries on OHRQoL increases with the severity of the disease [Citation48]. The negative impact of dental caries on OHRQoL has also been shown in a study that used the Early Childhood Oral Health Impact Scale (B-ECOHIS) [Citation6,Citation21,Citation48], and in one that used the Pediatric Quality of Life Inventory (PedsQL™) [Citation49]. OHRQoL questionnaires are better suited to distinguish between children with severe early childhood caries and caries-free children than generic HRQoL questionnaires [Citation50]. This is not surprising, since OHRQoL questionnaires have been designed to explore complications due to caries and other complaints of the mouth and teeth in detail. However, overall quality of life, as measured by the HRQoL questionnaire, may be affected by dental caries in the long term.
Dental caries is a slowly progressive disease. Discomfort and/or pain frequently develop gradually and might be interpreted as a normal condition. Additionally, young children often have difficulties verbally communicate chronic discomfort and/or pain. Dentists should ask not only direct questions about pain, but also wider questions about sleep, tooth brushing, and eating and drinking problems, to pick up signs of discomfort and pain from rotten teeth in young children. To prevent early childhood caries, it is important to inform and educate parents about the oral health of the child at an early stage. A recent study reported that receiving advice from the dentist or dental hygienist during pregnancy influenced the age at which mothers began brushing their children’s teeth. Furthermore, that study stated that education in oral health to impact parents’ habits and ensure favorable oral health for the child is important not only from dentists and dental hygienists but from pediatricians and gynecologists as well [Citation51]. We would like to add the importance of the child health care nurse, who meets the family regularly after the child’s birth.
This study has some limitations. The study was underpowered because of the impact of COVID-19 on recruitment and conclusions should be drawn with caution. The participating children were young and unable to answer the questionnaires themselves. Consequently, proxy versions were used. Measurements of the children’s insomnia symptoms and their quality of life are thus estimates made by their parents.
One strength of this study is that children and their parents were included even if they did not speak or understand the Swedish language, by offering authorized interpreters when needed. Many studies exclude participants if they cannot use the language used for the study. This not only increases the risk of losing valuable information (which affects the generalizability of findings), but also raises the question of health inequality and social justice [Citation52]. Most of the children with severe early childhood caries in our study had an immigrant background. It was considered important to include these families, as many studies have shown that the incidence of dental caries is high in this group of children [Citation52–54].
Three parents in the control group and one in the study group declined to participate due to language difficulties. The questionnaires were translated verbatim by the interpreters. There is no knowledge whether the use of an interpreter influenced the results, and if so, how and to what extent this was the case. Only limited guidance is available on how to use interpreters in research and to what extent the results are influenced by their use [Citation52]. Moreover, it is possible that displaced children and parents have had life experiences that confound the results of our study, especially those concerning quality of life, sleep, and stress.
All children in the study were clinically examined. Children with severe early childhood caries who could cooperate in the procedure were subject to radiographic examination. Children with no clinical signs of dental caries did not undergo bite-wing radiography. The dental caries prevalence might, therefore, have been underestimated. Since the purpose of this study was to investigate the correlations between severe early childhood caries and HCC, insomnia symptoms and quality of life, any underestimation of caries scores is of minor importance.
Conclusion
This study reports that severe early childhood caries are correlated with insomnia symptoms. A correct anamnesis in dental services should include questions about the child’s sleep duration as well as sleep-onset problems and sleep-maintenance problems. The study shows also that measuring oral health-related quality of life is more appropriate than measuring general health-related quality of life in preschool children with severe early childhood caries. Future studies are needed to investigate HCC, insomnia symptoms, and OHRQoL in a greater number of children with severe early childhood caries.
| Abbreviations | ||
| FIS | = | Family Impact Scale |
| HCC | = | Hair cortisol concentration |
| HRQoL | = | Health-Related Quality of Life |
| OHRQoL | = | Oral Health-Related Quality of Life |
| P-CPQ | = | Parental-Caregivers Perception Questionnaire |
| PISI | = | Pediatric Insomnia Severity Index |
| SOP | = | Sleep onset problems |
| SMP | = | Sleep maintenance problems |
Acknowledgements
The authors want to thank the children and their parents for participating in the study.
Disclosure statementt
The authors report there are no competing interests to declare.
Data availability statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Correa-Faria P, Paixao-Goncalves S, Paiva SM, et al. Dental caries, but not malocclusion or developmental defects, negatively impacts preschoolers’ quality of life. Int J Paediatr Dent. 2016;26(3):211–219.
- Socialstyrelsen. Karies bland barn och ungdomar [National Board of Health and Welfare. Caries in children and adolescents; Internet; Updated 2022. May 17; Cited 2022 October 17]. Available from: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/ovrigt/2022-5-7906.pdf. Swedish.
- Schenk HM, Jeronimus BF, van der Krieke L, et al. Associations of positive affect and negative affect with allostatic load: a lifelines cohort study. Psychosom Med. 2018;80(2):160–166.
- Bonecker M, Abanto J, Tello G, et al. Impact of dental caries on preschool children’s quality of life: an update. Braz Oral Res. 2012;26(spe1):103–107.
- Gilchrist F, Marshman Z, Deery C, et al. The impact of dental caries on children and young people: what they have to say? Int J Paediatr Dent. 2015;25(5):327–338.
- Gomes MC, Pinto-Sarmento TC, Costa EM, et al. Impact of oral health conditions on the quality of life of preschool children and their families: a cross-sectional study. Health Qual Life Outcomes. 2014;12:55.
- Pani SC, Al Odhaib M. The impact of dental treatment on the salivary cortisol levels of children with severe early childhood caries. Eur Arch Paediatr Dent. 2013;14(5):307–312.
- Caruso S, Gatto R, Cinque B, et al. Association between salivary cortisol level and caries in early childhood. Eur J Paediatr Dent. 2018;19:10–15.
- Rai K, Hegde AM, Shetty S, et al. Estimation of salivary cortisol in children with rampant caries. J Clin Pediatr Dent. 2010;34(3):249–252.
- Manenschijn L, Koper JW, Lamberts SW, et al. Evaluation of a method to measure long term cortisol levels. Steroids. 2011;76(10-11):1032–1036.
- Karlen J, Ludvigsson J, Hedmark M, et al. Early psychosocial exposures, hair cortisol levels, and disease risk. Pediatrics. 2015;135(6):e1450–e1457.
- Rippe RC, Noppe G, Windhorst DA, et al. Splitting hair for cortisol? Associations of socio-economic status, ethnicity, hair color, gender and other child characteristics with hair cortisol and cortisone. Psychoneuroendocrinology. 2016;66:56–64.
- Paruthi S, Brooks LJ, D’Ambrosio C, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American academy of sleep medicine. J Clin Sleep Med. 2016;12(6):785–786.
- Klasser GD, Almoznino G, Fortuna G. Sleep and orofacial pain. Dent Clin North Am. 2018;62(4):629–656.
- Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539–1552.
- Chaput JP, Gray CE, Poitras VJ, et al. Systematic review of the relationships between sleep duration and health indicators in the early years (0–4 years). BMC Public Health. 2017;17(Suppl 5):855.
- Palmer CA, Alfano CA. Sleep and emotion regulation: an organizing, integrative review. Sleep Med Rev. 2017;31:6–16.
- Tempesta D, Socci V, De Gennaro L, et al. Sleep and emotional processing. Sleep Med Rev. 2018;40:183–195.
- Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;9:151–161.
- Sundell AL, Angelhoff C. Sleep and its relation to health-related quality of life in 3–10-year-old children. BMC Public Health. 2021;1:1043.
- Correa-Faria P, Daher A, Freire M, et al. Impact of untreated dental caries severity on the quality of life of preschool children and their families: a cross-sectional study. Qual Life Res. 2018;27(12):3191–3198.
- Martins-Junior PA, Vieira-Andrade RG, Correa-Faria P, et al. Impact of early childhood caries on the oral health-related quality of life of preschool children and their parents. Caries Res. 2013;47(3):211–218.
- Nora AD, da Silva Rodrigues C, de Oliveira Rocha R, et al. Is caries associated with negative impact on oral health-related quality of life of pre-school children? A systematic review and Meta-Analysis. Pediatr Dent. 2018;40:403–411.
- Ridell K, Borgström M, Lager E, et al. Oral health-related quality-of-life in Swedish children before and after dental treatment under general anesthesia. Acta Odontol Scand. 2015;73(1):1–7.
- World Medical Association World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194.
- DART. Kommunikationsstöd i vårdsituationer [Communication support in care situations; Internet; Updated 2019. May 14; Cited 2021 January 29]. Available from: https://bildstod.vgregion.se/. Swedish.
- Koch G, Poulsen S, Espelid I, et al. Pediatric dentistry. A clinical approach. Third ed. Wiley Blackwell; 2017.
- Koch G, Lindhe J. The effect of supervised oral hygiene on the gingiva of children. J Periodontal Res. 1967;2(1):64–69.
- Karlen J, Ludvigsson J, Frostell A, et al. Cortisol in hair measured in young adults – a biomarker of major life stressors? BMC Clinical Pathol. 2011;11:12.
- Noppe G, Van Rossum EF, Koper JW, et al. Validation and reference ranges of hair cortisol measurement in healthy children. Horm Res Paediatr. 2014;82(2):97–102.
- Byars KC, Simon SL, Peugh J, et al. Validation of a brief insomnia severity measure in youth clinically referred for sleep evaluation. J Ped Psych. 2017;42:466–475.
- Angelhoff C, Johansson P, Svensson E, et al. Swedish translation and validation of the pediatric insomnia severity index. BMC Pediatr. 2020;20(1):253.
- Ravens-Sieberer U, Gosch A, Erhart M, et al. The KIDSCREEN questionnaires. Quality of life questionnaire for children and adolescents. Handbook. Lengerich; Pabst Science. 2006.
- Ravens-Sieberer U, Gosch A, Rajmil L, et al. KIDSCREEN-52 quality-of-life measure for children and adolescents. Expert Rev Pharmacoecon Outcomes Res. 2005;5(3):353–364.
- Dimberg L, Lennartsson B, Bondemark L, et al. Validity and reliability of the Swedish versions of the short-form child perceptions questionnaire 11–14 and parental perceptions questionnaire. Acta Odontol Scand. 2019;77(8):630–635.
- Jokovic A, Locker D, Stephens M, et al. Measuring parental perceptions of child oral health-related quality of life. J Public Health Dent. 2003;63(2):67–72.
- Locker D, Jokovic A, Stephens M, et al. Family impact of child oral and oro-facial conditions. Community Dent Oral Epidemiol. 2002;30(6):438–448.
- Eythorsdottir DY, Frederiksen P, Larsen SC, et al. Associations between objective measures of physical activity, sleep and stress levels among preschool children. BMC Pediatr. 2020;20(1):258.
- Chaput JP, Dutil C, Sampasa-Kanyinga H. Sleeping hours: what is the ideal number and how does age impact this? Nat Sci Sleep. 2018;10:421–430.
- Zhou N, Zhu H, Chen Y, et al. Dental caries and associated factors in 3 to 5-year-old children in Zhejiang Province, China: an epidemiological survey. BMC Oral Health. 2019;19(1):9.
- Moure-Leite FR, Ramos-Jorge J, Ramos-Jorge ML, et al. Impact of dental pain on daily living of five-year-old Brazilian preschool children: prevalence and associated factors. Eur Arch Paediatr Dent. 2011;12(6):293–297.
- Santos PS, Martins-Júnior PA, Paiva SM, et al. Prevalence of self-reported dental pain and associated factors among eight- to ten-year-old Brazilian schoolchildren. PLOS One. 2019;14(4):e0214990.
- Chen H, Tanaka S, Arai K, et al. Insufficient sleep and incidence of dental caries in deciduous teeth among children in Japan: a population-based cohort study. J Pediatr. 2018;198:279–286.e5.
- Thomson WM, Malden PE. Assessing change in the family impact of caries in young children after treatment under general anaesthesia. Acta Odontol Scand. 2011;69(5):257–262.
- Collado V, Pichot H, Delfosse C, et al. Impact of early childhood caries and its treatment under general anesthesia on orofacial function and quality of life: a prospective comparative study. Med Oral. 2017;22:0–0.
- Abanto J, Tsakos G, Paiva SM, et al. Impact of dental caries and trauma on quality of life among 5- to 6-year-old children: perceptions of parents and children. Community Dent Oral Epidemiol. 2014;42(5):385–394.
- Chaffee BW, Rodrigues PH, Kramer PF, et al. Oral health-related quality-of-life scores differ by socioeconomic status and caries experience. Community Dent Oral Epidemiol. 2017;45(3):216–224.
- Nobrega AVD, Moura L, Andrade NS, et al. Impact of dental caries on the quality of life of preschoolers measured by PedsQL questionnaire. Cien Saude Colet. 2019;24:4031–4042.
- Lee GH, McGrath C, Yiu CK, et al. A comparison of a generic and oral health-specific measure in assessing the impact of early childhood caries on quality of life. Community Dent Oral Epidemiol. 2010;38(4):333–339.
- Plumridge G, Redwood S, Greenfield S, et al. Involving interpreters in research studies. J Health Serv Res Policy. 2012;17(3):190–192.
- Ludovichetti FS, Zuccon A, Lucchi P, et al. Mothers’ awareness of the correlation between their own and their children’s oral health. IJERPH. 2022;19(22):14967.
- Christensen LB, Twetman S, Sundby A. Oral health in children and adolescents with different socio-cultural and socio-economic backgrounds. Acta Odontol Scand. 2010;68(1):34–42.
- Jacobsson B, Koch G, Magnusson T, et al. Oral health in young individuals with foreign and Swedish backgrounds–a ten-year perspective. Eur Arch Paediatr Dent. 2011;12(3):151–158.
- Stecksén-Blicks C, Hasslöf P, Kieri C, et al. Caries and background factors in Swedish 4-year-old children with special reference to immigrant status. Acta Odontol Scand. 2014;72(8):852–858.
