Abstract
Background
Inflammatory bowel disease, which includes ulcerative colitis (UC), is an inflammatory disorder with potential impact on periodontal disease, but evidence to date for this association is limited. The primary aim of this study was to investigate the prevalence of periodontitis according to the 2017-classification in a cohort of subjects with UC. The secondary aim was to assess a potential correlation of periodontal status with previous UC disease parameters and to assess oral health-related quality-of-life.
Method
A cohort from a community hospital in Norway with confirmed extensive UC was comprehensively examined. Periodontal parameters, OHIP-14 and demographic variables were collected. Previous UC data including colon activity index (CAI), Mayo score and years of UC diagnosis was used to explore a potential correlation with periodontal status.
Results
A total of 50 out of 63 invited patients participated. According to the 2017-classification, 74% of the patients presented periodontitis. No correlation was found between periodontitis (stage, grade, bleeding on probing or probing pocket depth ≥6mm) and CAI, Mayo score, or years with UC diagnosis.
Conclusions
Within the limitations of this study, the prevalence of periodontitis among patients with mild UC for more than 12 years was in line with that reported from a Norwegian general population. No correlation between periodontitis and UC disease indices or years with UC diagnosis was observed. The study suggests that the susceptibility to periodontitis may be limited in patients with well treated or mild UD who regularly attend the dental office, despite a considerable UC disease duration.
Introduction
The two main forms of inflammatory bowel disease (IBD) are Crohn’s disease (CD) and ulcerative colitis (UC). They are chronic, relapsing, and remitting diseases of multifactorial aetiology which involve the gastrointestinal tract [Citation1]. CD is characterized by transmural inflammation and may occur at any site of the entire gastro-intestinal tract. In UC, the inflammatory process is restricted to the colon and invariably involves the rectum and may extend proximally in a continuous fashion [Citation2]. In Europe, incidence rates for UC range from 0.9 to 24.0 per 100 000 person-years [Citation3]. The prevalence of UC varies from 2.4 to 294 cases per 100 000 persons. In a Norwegian population cohort study, the prevalence of UC was found to be 0.51% [Citation4]. In a recent study from Norway the incidence of UC was found to be 24.7 and 28.4 per 100 000 persons-years in the years 2010 and 2017 [Citation5].
UC is currently not curable, and treatment is aimed at symptomatic relief, reduction of inflammation during exacerbations, maintenance of remission, and increasing quality of life [Citation6]. Surgical treatment is indicated in patients who fail drug treatment or develop severe complications. The use of surgery in the treatment of UC has recently declined over time in Europe [Citation7].
The peak age for diagnosis of UC is 30–40 years [Citation8] and the disease occurs more frequently in men: 53.8–60% [Citation5,Citation8,Citation9]. The first signs are often increased frequency of bowel movements and/or haematochezia. Extraintestinal manifestations like arthritis, uveitis, erythema nodusum, and aphtous ulcers are common, and many patients report that IBD negatively influences their health-related quality of life (HRQoL) and ability to work [Citation10–12].
IBD and periodontitis share some similar immunopathogenic responses in addition to being non-curable and chronic diseases. UC and periodontitis are characterized by a hypersensitivity immune response to commensal gut bacteria and dental plaque bacteria, respectively, which may disrupt local homeostasis in susceptible individuals [Citation13]. Periodontitis is described as the result of an imbalance of the oral microbiota in the dento-gingival complex, which results in a host response leading to inflammation and destruction of the periodontium in susceptible individuals. Previous studies have shown greater attachment loss and higher prevalence and severity of periodontitis in adults with IBD than in controls [Citation13]. The risk of having periodontitis with the IBD diagnosis varies across studies with odds ratios ranging from 3.95 to 7.0 [Citation13–15]. It has also been found that one sixth of IBD individuals are also diagnosed with a rheumatoid diagnosis, and that there is higher risk for increased probing depths and attachment loss in individuals with rheumatoid arthritis [Citation16,Citation17]. According to current knowledge there is a significant association between inflammatory bowel disease and periodontitis [Citation18,Citation19], although the overall quality of evidence is considered weak to moderate [Citation20]. Since there are marked differences between CD and UC [Citation21], information on oral health may be lost when combined as IBD.
No formal validated definition of mild, moderate or severe UC exists to date. Several severity scores have been developed using variables which include clinical symptoms, laboratory studies and endoscopic assessment. Also, the terms disease activity and severity have often been used interchangeably in the literature. Disease activity refers to cross-sectional, single assessments of inflammation, whereas disease severity may include longitudinal and historical factors. One of the mostly used disease scores in clinical practice is the Mayo score, which includes stool frequency, rectal bleeding, findings of flexible sigmoidoscopy and the physician’s global assessment. The colitis activity index (CAI) is a validated symptom-based index score (score 0–19) which has a good correlation with more complicated disease activity indices [Citation22]. The score is based on variables including the frequency of bowel movements, presence of blood in the stool, urgency of defacation, general well-being and extracolonic features.
In the present study, the clinical periodontal status in a cohort diagnosed with ulcerative colitis was assessed. The primary aim of this cross-sectional study was to assess the prevalence of periodontitis in a Norwegian cohort with established and medically treated UC. The secondary aims were to assess a potential correlation between severity of periodontal disease and UC severity defined by Mayo scores (MAYO), clinical activity index (CAI), years with UC, and to assess OHRQoL.
Materials and methods
The present article was written following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [Citation23]. The study was performed at the dental clinic at Lovisenberg Diaconal Hospital (LDS) in Oslo, Norway, in collaboration with the Faculty of Dentistry at the University of Oslo. The cohort was recruited from a previous study that considered DNA markers for cancer in stool samples from patients with UC [Citation24]. Both degree of inflammation according to the Mayo endoscopic score (Mayo score) [Citation25] and colitis activity index (CAI)[Citation22] as a measure of disease activity, were registered by two dedicated gastroenterologists in the original study.
The CAI is a questionnaire used to measure symptoms of UC. The Mayo score is characterized by four classes of severity ranging from 0 to 3, with 0 indicating inactive disease with normal mucosa; 1 mild disease (erythema and mild friability); 2 moderate disease (marked erythema, friability, absent vascular pattern and erosions); and 3 severe disease (spontaneous bleeding and diffuse ulceration).
Due to general data protection regulations (GDPR), all patients from the original study initially had to be contacted by personnel directly involved in the multicentre study. The main investigator, hospital dentist and postgraduate student in periodontology (HOH) was kept unaware of the identity until they had accepted the invitation for an oral examination. The study protocol was approved by the Regional Committee for Medical and Health Research Ethics (REC Project NO. 2010/1093) with updates in 2019.
Patient population
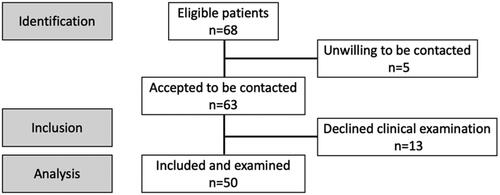
Patients were recruited from a population-based surveillance cohort of UC patients registered in the database of LDS from 1999–2013. The patients in the original study were invited if they had extensive UC documented by endoscopy at any time during the course of disease, and a disease duration of 8 years or more. For the present study, eligible patients had to be at least 18 years old. Pregnant or lactating women were excluded as well as persons unable to provide informed consent. This resulted in 68 potential study patients, of which 63 accepted to be contacted. Patients underwent an oral examination from June 2020–January 2021 following informed consent to participate. A questionnaire was used to record the age of the UC debut, self-reported smoking habits and the use of complementary medication.
Background information on oral-health-related behavior was collected, including self-reported medication, comorbidities, oral dryness (hyposalivation) according to the Challacombe Scale [Citation26], and decayed-missing-filled teeth (DMFT). A questionnaire on OHRQoL (Oral Health Impact Profile, OHIP-14) was used [Citation27]. All subjects were asked for information concerning disease activity, comorbidity and medication.
Clinical recordings
All periodontal recordings were done on six sites per tooth excluding third molars. Clinical data included oral hygiene (plaque registered dichotomously) [Citation28] with a manual probe (LM 52851 Perioprobe ErgoNorm, LM instruments, Parainen, Finland), periodontal probing depth (PPD), clinical attachment level (CAL), bleeding on probing (BoP) (10 s) [Citation29], horizontal furcation defect (degree I-III) with a Nabers probe [Citation30] and mobility (0–III) [Citation31]. Clinical attachment level (CAL) was defined as the probing depth and the distance from the gingival margin to the cementum enamel junction (CEJ). All recordings were done by an experienced dentist and postgraduate student in periodontology (HOH), trained in periodontal recordings.
Radiographic recordings
Fourteen intra-oral periapical radiographs and two vertical bitewings were obtained from all patients using conventional position holders (Eggensholder) [Citation32] and Digora® photostimulable phosphor plates. Periapical radiographs were taken at 63-kV with an exposure time of 0.08–0.12 s depending on anterior or posterior positioning. Intraoral standardized radiographs were taken with the long-squared cone using parallel technique. Bone loss (BL) was evaluated by measuring the linear distance between the CEJ to the level where a normal width of lamina dura could be detected. These measurements were done with a dedicated dental software (VisiQuick). The clinical assessment was performed during the same appointment as the radiographic recordings and the questionnaire. Both clinical and radiographic recordings and interpretations were done by the main investigator (HOH).
Periodontitis case definition
The definition of periodontitis according to the 2017 World Workshop on the Classification of Periodontal and Peri‐implant Diseases and Conditions was used [Citation33]. Periodontitis was defined when the distance between the CEJ and the alveolar crest (AC) exceeded 2 mm at ≥2 non-adjacent teeth on radiographic recordings. Alternatively, periodontitis was diagnosed by the clinical assessment with one of two criteria: either (1) interdental CAL detectable at ≥2 non‐adjacent teeth, or (2) buccal or oral CAL of ≥3 mm with pocketing >3 mm detectable at ≥2 teeth. Clinical attachment loss was defined as clinical attachment level exceeding 2 mm. In cases of <10% BoP, <4 mm PPD a periodontitis case was regarded as stable. In cases where BoP exceeded 10% with presence of PPD ≥4mm a case was defined as unstable.
Statistics
All statistical analysis was performed using SigmaPlot version 14.0 (Systat Software GmbH). A Spearman correlation test was performed to analyse correlation between periodontitis and the UC parameters of endoscopic inflammation (Mayo score), CAI and years with UC diagnosis. The demographic data are presented as descriptive statistics. An intra-class correlation coefficient (ICC) for intra-rater agreement of classification of periodontal stages was calculated by the examiner (HOH) based on 10 patients assessed twice. The ICC was 0.45 (95% CI −1.2, 0.86).
Results
A flow diagram of the recruitment is provided in . The demographic data and oral disease characteristics are presented in . The study sample consisted of 50 individuals (29 males), aged between 36 and 81 years (mean 51.9 years). Eight of the individuals reported on general health problems (e.g. high blood pressure, diabetes, rheumatic disease and cardiovascular disease) and were on various different medications in addition to UC medication.
Table 1. Patient demographics and disease characteristics. (UC = ulcerative colitis).
All but 11 subjects regularly visited a dentist or dental hygienist (78%), and all but 3 subjects reported that they never had received any form of “gum treatment”. The number of remaining teeth varied between 18–32 (mean 27,2). Three subjects presented 1 implant each, and 2 subjects had 2 implants each. None of the participants had lost teeth due to periodontitis.
Four individuals were current smokers (3–15 cig/day) and 19 were former smokers. Nine individuals reported daily use of moistened snuff.
Of the patients, 58% were treated with mesalazin, a type of 5-aminosalicylic acid (5-ASA). Other combinations of 5-ASA medication were also reported. None of the patients were currently treated with steroids or immunosuppressant drugs ().
Table 2. Medication used for ulcerative colitis.
Mucosal oral lesions were observed in 4 individuals displaying leukoplakia. The average DMFT score was 11.4 (range 25–1). Mean hyposalivation according to the Challacombe scale was 0.34. A total of 39 participants scored 0.
The distribution of periodontitis stages and grades is shown in and . Patients not receiving UC medication (n = 9) were represented in all stages of periodontitis identified in the population (I, II, III) as well as among the non-periodontitis cases. The periodontal disease status (stage/grade/PPD > 5mm/full-mouth bleeding score; FMBS) did not correlate to previous assessments of CAI, Mayo score, or with number of years with UC disease ().
Figure 2. Distribution of participants with periodontal health, gingivitis, and periodontitis. Definitions of stable periodontitis and periodontitis with some inflammation/unstable are modifications of the 2017 classification. BoP: Bleeding on probing; PPD: Probing pocket depth.
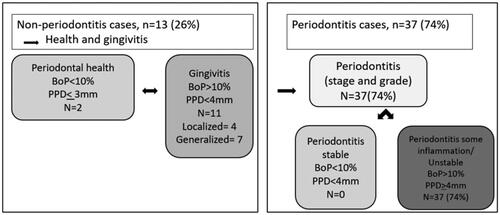
Table 3. Allocation to periodontal stage and grade in number of patients and percentage (%) of the total population based on clinical and radiographic examination.
Table 4. Correlation of previous data on intestinal inflammation (Mayo score & CAI), UC disease duration and periodontal variables performed with Spearman correlation.
The results from the OHIP-14 questionnaire revealed highest scores for the functional limitation domain. The OHIP-14 score according to domains is shown in .
Table 5. Mean score (standard deviation; SD), median (interquartile range; IQR) and range for the items of OHIP-14.
Discussion
In the present study, 74% of the subjects presented with periodontitis, of which 34% stage III, 22% stage II and 18% stage I. Only 26% of the population were diagnosed as non-periodontitis cases. Two patients were diagnosed as periodontally healthy and 11 patients with gingivitis. No correlations were found between previous scores of UC disease severity and symptoms (Mayo score and CAI), years with UC diagnosis and the severity of the periodontal disease (stage, grade, FMBS, PPD ≥ 6mm).
A recently published study highlighted a need to perform studies on periodontal disease according to the World Workshop on the Classification scheme for periodontal and peri‐implant diseases and conditions 2017 in subjects with IBD [Citation34,Citation35]. With respect to periodontitis prevalence and classification, the findings of the present study is in agreement with a recent study in Norway by Stødle et al. [Citation36]. In their sample population of nearly 5000 individuals from Trøndelag county [Citation37], the prevalence of periodontitis was reported to be 2.3%, 15%, 40% and 12% for stages IV, III, II, and I, respectively, and only 28% were not diagnosed with periodontitis. The present study recorded no case of stage IV but a higher prevalence of stage III. This difference may be explained by the small sample size of the present study but also by the diagnostic means used. In contrast to Stødle et al. the present study included a more comprehensive examination which included clinical assessments of furcation defects and CAL. This most likely led to the diagnosis of more stage III localized cases at the expense of stage II cases. When summarizing all periodontitis cases regardless of stage, the populations were similar (74% vs 72.4%). This is a higher prevalence compared to other recent studies from Norway [Citation38,Citation39], which may be explained by different demographics, inter-observational differences between examiners and different thresholds for defining periodontitis.
The lack of correlation between periodontal status and previous Mayo scores, CAI, or years with UC may be explained by the time lapse between the UC records obtained some years prior to the periodontal examination. The most plausible explanation, however, is likely the fact that most patients were medically well-maintained, reported mild symptoms of UC, and that the majority of the patients (78%) reported no symptoms of UC at the time of the periodontal examination. The mild form of UC in this population is further supported by the exclusion of patients with refractory colitis and colorectal cancer in the original cohort, which accordingly were not included in the present study. Therefore, severe UC cases were not part of this study. The fact that none of the patients were on biological agents, such as anti-TNF-α, reflects the mild and well-treated course of disease for many of the patients in this study population. Biological agents are traditionally reserved for effective medical treatment of moderate-to-severe UC. When UC symptoms are in remission, patients are often kept at 5-ASA (e.g. Mezalasin) to prevent mucosal dysplasia. As pointed out by Vavrica et al. there are very few studies that have analysed the effect of IBD medication or disease activity on the periodontal status [Citation13]. Due to a small study population with limited symptoms and medication, no such analysis was considered feasible in the present study.
A recent systematic review presented clear evidence for an association between periodontal disease and IBD, and in particular for UC [Citation34]. A study from Jordan reported a higher prevalence of periodontitis and proportion of clinical attachment level >4 mm in subjects with UC as compared to subjects with no IBD [Citation15]. A recent study from China reported UC as a risk indicator for periodontitis with an odds ratio of 4.7 (95% CI: 2.49–8.71). The FMBS was 53.9% in the present population was higher than corresponding numbers from an Italian case-control study (31%) [Citation19], where FMBS was reported as a significant predictor for IBD and UC.
These studies as many others on the association between periodontitis and UC, are cross-sectional case–control studies, with limited discrimination of UC disease activity or severity. Therefore, it is likely that such studies have included severe forms of UC, and not only mild cases, which may explain the reported associations in the literature. The study by Vavricka et al. [Citation13], included CAI in their UC population. In their study, of the UC population, 27% were on systemic steroids, 20% anti-TNF-a, and 20% on thiopurines, reflecting a population with more severe UC disease than in the present study. The mean CAI score of 3.7 in their population underscores this difference of populations, when compared to the mean CAI of 0.4 in the present study. Despite the higher CAI scores in Vavricka et al. [Citation13], no significant correlation with periodontitis was found, which was in agreement with the present study. The mean disease duration, however, was considerably longer in the present study and almost three times that of the population in the study by Vavricka and co-workers. Although UC is a disease with a relapsing and remitting clinical course over time, no correlation was found between disease duration and the periodontal variables. This may indicate that UC disease severity may be a stronger predictor for periodontitis than is disease duration.
Previous studies have reported on the symptom-relieving effect of tobacco use among UC patients [Citation40], and therefore information on smoking habits and the use of smokeless tobacco was analysed in this study [Citation41]. A recent study by Kang et al. [Citation42] reported that periodontitis and smoking increase the risk of UC. Since few patients reported on tobacco use in the current study, no conclusions could be drawn. Interestingly, three patients reported cannabis smoking as self-medication. This finding is supported by a newly published article which reported that approximately 10%–20% patients with IBD are active cannabis users [Citation41].
The OHIP-14 data demonstrated limited negative impact on QoL in this population. This is partly in contrast to studies reporting on the negative influence of UC on health-related quality of life [Citation43]. A recent study by Goldinova et al. [Citation44], reported a near-significant correlation of the OHIP-14 scores with a simple clinical colitis activity index (SCCAI) and IBD questionnaire (IBDQ-9). In a national cross-sectional Norwegian study from 2011 using the OHIP-14 [Citation45], the proportion of individuals who reported problems ranged from 11% to 56%, with pain as the most frequently reported item. The most frequently experienced problems were physical pain, such as aching in the mouth and discomfort eating food, which is in agreement with our study and other Scandinavian studies [Citation46,Citation47].
Several limitations and important confounders are noteworthy of the present study, and the small number of participants is the most obvious. However, the recruitment of a high number of participants with long-term UC diagnosis may be challenging. The use of Mayo score and CAI obtained at a single time point some years before the periodontal examination can be questioned. Although the study coincided with the first and second waves of the COVID-19 pandemic in Norway, most invitees (50/63) were willing to participate in the study (78%), which reflects a highly motivated and compliant cohort. Prior to the present study, this motivated cohort had also remained throughout the course of the original prospective surveillance study. This study was conducted in a community hospital in which patients may present with a less aggressive UC than seen in advanced units, as pointed out in the study by Klepp et al. [Citation24]. It is therefore likely that those included in the present study represent a selection of the most compliant patients from a population with limited severe UC cases, as refractory colitis cases were excluded in the original study. The population examined was successfully treated as suggested by the OHRQoL-data and the patient-reported data presented. A non-response analysis was not performed, and one may speculate that participants agreeing to a clinical periodontal examination were the healthiest with respect to both UC and periodontal disease. The most frequent reason for not participating was Covid-19-related.
The strength of the study is the comprehensive periodontal diagnostic means according to the new classification, consideration of patients’ OHRQoL with a long duration of UC, and the data on UC disease severity. In agreement with Lorenzo-Pouso et al. [Citation34], more evidence of a potential link between periodontitis and UC is needed.
Conclusion
Within the limitations of this study, the prevalence of periodontitis among patients with mild UC for more than 12 years was in line with that reported from a Norwegian general population. No correlation between periodontitis or periodontal inflammatory indices and UC disease indices or years with UC diagnosis was observed. The study suggests that the susceptibility to periodontitis may be limited in patients with well treated or mild UD who regularly attend the dental office, despite a considerable UC disease duration. These findings require confirmation in future studies with a larger population of UC patients.
Ethical approval and consent to participate
The study protocol was approved by the Regional Committee for Medical and Health Research Ethics (REC Project NO. 2010/1093) with amendments in 2019 and all methods are in accordance with the Declaration of Helsinki. All participants signed an informed consent on participation and for publication of the results.
Author contributions
HOH and AV designed the study. HOH collected the data. HOH and AV analyzed, and interpreted the data. HOH and AV drafted the manuscript. HOH, AV and PK reviewed the manuscript. HOH, AV and PK revised the manuscript. All authors have read and approved the final version of the manuscript.
Acknowledgment
The authors thank Anita Tollisen, Unger-Vetlesen Institute, Department of Internal Medicine, Lovisenberg Hospital, Lovisenberggt.17, 0456 Oslo, Norway.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analysed and questionnaires during the current study are available from the corresponding author on request.
Additional information
Funding
References
- Levine JS, Burakoff R. Inflammatory bowel disease: medical considerations. In: Greenberger NJ, Blumberg RS, Burakoff R, editors. CURRENT diagnosis & treatment: gastroenterology, hepatology, & endoscopy. 3rd ed. New York (NY): McGraw-Hill Education; 2016.
- Jin J. Inflammatory bowel disease. JAMA. 2014;311(19):2034.
- Burisch J, Munkholm P. The epidemiology of inflammatory bowel disease. Scand J Gastroenterol. 2015;50(8):942–951.
- Bengtson MB, Solberg C, Aamodt G, et al. Familial aggregation in crohn’s disease and ulcerative colitis in a norwegian population-based cohort followed for ten years. J Crohns Colitis. 2009;3(2):92–99.
- Lirhus SS, Høivik ML, Moum B, et al. Incidence and prevalence of inflammatory bowel disease in Norway and the impact of different case definitions: a nationwide registry study. Clin Epidemiol. 2021;13:287–294.
- Ho EY, Cominelli F, Katz J. Ulcerative colitis: what is the optimal treatment goal and how do we achieve it? Curr Treat Options Gastroenterol. 2015;13(1):130–142.
- Zhao M, Gönczi L, Lakatos PL, et al. The burden of inflammatory bowel disease in Europe in 2020. J Crohns Colitis. 2021;15(9):1573–1587.
- Cosnes J, Gower-Rousseau C, Seksik P, et al. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140(6):1785–1794.
- Alatab S, Sepanlou SG, Ikuta K, et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. 2020;5(1):17–30.
- Høivik ML, Moum B, Solberg IC, et al. Health-related quality of life in patients with ulcerative colitis after a 10-year disease course: results from the IBSEN study. Inflamm Bowel Dis. 2012;18(8):1540–1549.
- Høivik ML, Moum B, Solberg IC, et al. Work disability in inflammatory bowel disease patients 10 years after disease onset: results from the IBSEN study. Gut. 2013;62(3):368–375.
- Alvarado-Julio A, Chumacero-Palma K, Buenahora MR, et al. Oral manifestations associated with inflammatory bowel disease and early endoscopic findings in patients with spondyloarthritis. BMC Oral Health. 2022;22(1):477.
- Vavricka SR, Manser CN, Hediger S, et al. Periodontitis and gingivitis in inflammatory bowel disease: a case-control study. Inflamm Bowel Dis. 2013;19(13):2768–2777.
- Flemmig TF, Shanahan F, Miyasaki KT. Prevalence and severity of periodontal disease in patients with inflammatory bowel disease. J Clin Periodontol. 1991;18(9):690–697.
- Habashneh RA, Khader YS, Alhumouz MK, et al. The association between inflammatory bowel disease and periodontitis among jordanians: a case–control study. J Periodontal Res. 2012;47(3):293–298.
- Fuggle NR, Smith TO, Kaul A, et al. Hand to mouth: a systematic review and Meta-Analysis of the association between rheumatoid arthritis and periodontitis. Front Immunol. 2016;7:80.
- Ossum AM, Palm Ø, Cvancarova M, et al. Peripheral arthritis in patients with long-term inflammatory bowel disease. Results from 20 years of follow-up in the IBSEN study. Scand J Gastroenterol. 2018;53(10-11):1250–1256.
- Albandar JM, Susin C, Hughes FJ. Manifestations of systemic diseases and conditions that affect the periodontal attachment apparatus: case definitions and diagnostic considerations. J Clin Periodontol. 2018;45(S20): s 171–S189.
- Baima G, Muwalla M, Testa G, et al. Periodontitis prevalence and severity in inflammatory bowel disease. A Case-Control Study. J Periodontol. 2023;94:313–322.
- Hirschfeld J, Chapple ILC. Inflammatory bowl disease and periodontitis. In: Rangé H, Bouchard P, editors. Periodontitis and systemic diseases: clinical evidence and biological plausibility. Berlin: Quintessence; 2021. p. 112–131.
- Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3(7):521–533.
- Walmsley RS, Ayres RCS, Pounder RE, et al. A simple clinical colitis activity index. Gut. 1998;43(1):29–32.
- von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499.
- Klepp P, Tollisen A, Røseth A, et al. Real-life chromoendoscopy for dysplasia surveillance in ulcerative colitis. World J Gastroenterol. 2018;24(35):4069–4076.
- Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317(26):1625–1629.
- Osailan SM, Pramanik R, Shirlaw P, et al. Clinical assessment of oral dryness: development of a scoring system related to salivary flow and mucosal wetness. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(5):597–603.
- Slade GD. Derivation and validation of a short-form oral health impact profile. Community Dent Oral Epidemiol. 1997;25(4):284–290.
- O’Leary TJ, Drake RB, Naylor JE. The plaque control record. J Periodontol. 1972;43(1):38.
- Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975;25(4):229–235.
- Hamp SE, Nyman S, Lindhe J. Periodontal treatment of multirooted teeth. Results after 5 years. J Clin Periodontol. 1975;2(3):126–135.
- Nyman S, Lindhe J, Lundgren D. The role of occlusion for the stability of fixed bridges in patients with reduced periodontal tissue support. J Clin Periodontol. 1975;2(2):53–66.
- Eggen S. Standardiserad intraoral röntgenteknik. Sver Tandlakarforb Tidn. 1969;17:867–872.
- Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Periodontol. 2018;89(Suppl 1):s159–S172.
- Lorenzo-Pouso AI, Castelo-Baz P, Rodriguez-Zorrilla S, et al. Association between periodontal disease and inflammatory bowel disease: a systematic review and meta-analysis. Acta Odontol Scand. 2021;79(5):344–353.
- Papapanou PN, Sanz M, Buduneli N, et al. Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and Peri-Implant diseases and conditions. J Clin Periodontol. 2018;45(Suppl 20):S162–S170.
- Stødle IH, Verket A, Høvik H, et al. Prevalence of periodontitis based on the 2017 classification in a norwegian population: the HUNT study. J Clin Periodontol. 2021;48(9):1189–1199.
- Krokstad S, Langhammer A, Hveem K, et al. Cohort profile: the HUNT study, Norway. Int J Epidemiol. 2013;42(4):968–977.
- Holde GE, Oscarson N, Trovik TA, et al. Periodontitis prevalence and severity in adults: a Cross-Sectional study in norwegian circumpolar communities. J Periodontol. 2017;88(10):1012–1022.
- Bongo AS, Brustad M, Oscarson N, et al. Periodontal health in an indigenous sámi population in Northern Norway: a cross-sectional study. BMC Oral Health. 2020;20(1):104.
- Thomas T, Chandan JS, Li VSW, et al. Global smoking trends in inflammatory bowel disease: a systematic review of inception cohorts. PLoS One. 2019;14(9):e0221961.
- Rozich JJ, Holmer A, Singh S. Effect of lifestyle factors on outcomes in patients With inflammatory bowel diseases. Am J Gastroenterol. 2020;115(6):832–840.
- Kang EA, Chun J, Kim JH, et al. Periodontitis combined with smoking increases risk of the ulcerative colitis: a national cohort study. World J Gastroenterol. 2020;26(37):5661–5672.
- Huppertz-Hauss G, Høivik ML, Langholz E, et al. Health-related quality of life in inflammatory bowel disease in a european-wide population-based cohort 10 years after diagnosis. Inflamm Bowel Dis. 2015;21(2):337–344.
- Goldinova A, Tan CX, Bouma G, et al. Oral health and salivary function in ulcerative colitis patients. United European Gastroenterol J. 2020;8(9):1067–1075.
- Dahl KE, Wang NJ, Skau I, et al. Oral health-related quality of life and associated factors in norwegian adults. Acta Odontol Scand. 2011;69(4):208–214.
- Einarson S, Gerdin EW, Hugoson A. Oral health impact on quality of life in an adult swedish population. Acta Odontol Scand. 2009;67(2):85–93.
- Lahti S, Suominen-Taipale L, Hausen H. Oral health impacts among adults in Finland: competing effects of age, number of teeth, and removable dentures. Eur J Oral Sci. 2008;116(3):260–266.