Abstract
Transplantation of a vascularized limb or its components is defined as composite tissue allotransplantation, and is one of the newest areas in surgery. To date, 24 hands have been transplanted onto 18 recipients. The initial results have been promising, and hand transplantation may become an important procedure for functional restoration of upper limbs. However, the ethical aspects of using chronic immunosuppression for a condition which is not life threatening have been the subject of debate. In this article, we review the field of composite tissue allotransplantation.
The first human hand transplantation in the modern era of immunosuppression was performed in 1998 (Dubernard et al. Citation1999). Since then, 23 more have been performed worldwide (). Hand transplantations have aroused public interest and started debates in the hand surgery community (Siegler Citation1998, Foucher Citation1999, Lee and Mathes Citation1999, Lee Citation2002, Lundborg Citation1999, Herndon Citation2000, Altman Citation2001, Manske Citation2001, Breidenbach et al. Citation2002, Cooney and Hentz Citation2002, Jones Citation2002, Hettiaratchy et al. Citation2003, Lubbe Citation2003, Reigstad and Hetland Citation2003). The transplantations may either be described as a breakthrough in reconstructive surgery or as “technology over good sense” (Strickland Citation1999) due to the need for long-term immunosuppression for a non-life threatening condition.
Table 1. Hand transplantations that have been performed worldwide
Hand transplants are defined as composite tissue allografts. They differ from solid organ transplants, since they consist of several types of tissue such as bone, muscle, cartilage, tendon, skin, nerves and vessels, with different antigenicities (Lee et al. Citation1991, Llull Citation1998). All of these tissues have been transplanted individually in humans with success (Guimberteau et al. Citation1992, Mackinnon and Hudson Citation1992, Wendt et al. Citation1994, Hofmann et al. Citation1995, Citation1998, Jones et al. Citation1998, Mackinnon et al. Citation2001, Strome et al. Citation2001). Transplantation of a vascularized limb or its tissue components is one of the newest areas in surgery, and has been made possible by the advent of microvascular surgery combined with advances in our knowledge of transplantation immunology. The availability of composite tissue allografts would greatly expand the horizons of reconstructive surgery, since it would allow us to replace like with like while eliminating donor-site morbidity. The implications of such reconstructions are staggering. Total small-bowel transplants (Goulet et al. Citation1992) and partial abdominal wall transplants (Levi et al. Citation2003) are now a reality. In future, there may be partial body transplantation for reconstructions after excision of tumors, or for congenital or posttraumatic reconstructions.
Three major obstacles currently limit the future of composite tissue allotransplantation (CTA). The first obstacle is acute rejection, which is the most frequent complication of allotransplantation. Global experience in hand transplantation has shown that this phenomenon occurs without exception at least once within the first year of transplantation. Immunologically, it is caused by a T cell-mediated attack of the donor allograft by the recipient, resulting in early graft loss. The second obstacle is chronic rejection, which is a poorly characterized process that occurs late after allotransplantation. It is thought to be caused by both humoral and cell-mediated destruction of the allograft. Pathologically, there may be evidence of transplant vascular sclerosis. In visceral organ transplantation, chronic rejection is the major cause of late graft loss with current rates of 4-8% per year at most centres (Neuberger Citation1999). The third obstacle is the necessary chronic immunosuppression, which may lead to drug side effects such as opportunistic infections, malignancies or organ failure (First and Peddi Citation1998, Fishman and Rubin Citation1998, Mathew Citation1998, Brenner et al. Citation2002). These three issues have dominated the debate in the hand surgery community regarding the life-enhancing benefits and ethics of human hand transplantation. At present, clinical hand transplantation still remains experimental. In this article, we review the field of CTA.
Historical background
Historically, organ transplantation was preceded by composite tissue allotransplantation. The earliest accounts of limb transplantation are credited to the patron saints Cosmas and Damian in 348 A.D. (Da Varagine Citation1952, Danilevicius Citation1967, Kahan Citation1983). According to legend, they amputated the gangrenous leg of a sleeping man and replaced it with that of a recently deceased Ethiopian Moor. On awakening the man discovered that his ill-looking and painful leg had been amputated and that he had a new one, but it was the leg of a black man.
The Italian surgeon Gaspare Tagliacozzi is regarded as the Father of Plastic Surgery. In his book, “De curtorum chirurigia per insitionem” (Tagliacozzi Citation1597), he described a method for nasal reconstruction. Skin from the forearm of a slave was used to reconstruct the nose of a wealthy patient who had accidentally severed it during swordplay. In return, the slave was granted freedom. The flap was eventually rejected and Tagliacozzi concluded that though allografts were possible, there existed practical difficulties in “binding” two tissues from different people for a sufficient length of time.
At the beginning of the 20th century, advances in microvascular surgery served as the stimulus for early transplantation experiments. In 1908, Carrel described successful orthotopic hind-limb transplantation in dogs. In the same year, the first whole-joint transplantations were performed by Judet (Citation1908) and Lexer (Citation1908) in dogs. The whole joint transplants were non-vascularized and therefore did not require immunosuppression. Thereafter, infrequent reports of allotransplantation followed, but the immunological phenomenon of allograft rejection was first discovered by Sir Peter Brian Medawar in 1944.
The evolution of immunosuppressive drugs in transplantation
Historically, technical developments in microvascular surgery took the lead over advancements in our understanding of the immunological behaviour of allografts. After Medawar's demonstration that rejection was an immunological event, immunosuppression was used to manipulate the immune system. In the 1960s, the antimetabolites 6-mercaptopurine and its derivate azathioprine were introduced, along with agents such as antilymphocyte globulin (Murray et al. Citation1962, Citation1963, Starzl et al. Citation1963, Starzl Citation1964). However, initial graft survival and patient survival after organ transplantation were poor. It was in this era that the first hand transplantation in the world was performed in Ecuador in 1964 (Medical Tribune World-Wide Report Citation1964). A combination of systemic steroids and 6-mercaptopurine was used, which was replaced by azathioprine after 24 hours. Nevertheless, severe rejection set in and the hand had to be amputated after 3 weeks.
Experience gained in organ transplantation has provided insight into the immunological consequences of transplantation and the efficacy and toxicity of immunosuppressive drugs. The field of transplantation has evolved from transplanted kidneys (Merrill et al. Citation1956), bone marrow (Thomas et al. Citation1957), hearts (Barnard Citation1967), livers (Starzl et al. Citation1968), lungs (Derom et al. Citation1971), pancreas (Lillehei et al. Citation1976), multiple abdominal viscera (Starzl et al. Citation1989) and small bowel (Goulet et al. Citation1992), to CTA (). Even though the initial results of grafts and patient survival after organ transplantation in the 1960s were discouraging, advances in pharmacological immunosuppression during the past three decades have allowed significant improvements in graft survival. For example, the 5-year cadaveric kidney survival rate improved from approximately 16% to 66% between 1963 and 1995 (Moss et al. Citation2000). The introduction of cyclosporine A (CsA) in 1976 was the first major advance in transplantation since the introduction of prednisone and azathioprine made transplantation possible in the early 1950s and 1960s (Borel and Kis Citation1991). The calcineurin inhibitor tacrolimus (FK-506) (Goto et al. Citation1987) was approved in 1994. Tacrolimus led to dramatic improvements in solid organ transplantation (Fung et al. Citation1990, Todo et al. Citation1990, Armitage et al. Citation1991), allowing highly antigenic lymph node bearing grafts, such as the small bowel, to be transplanted (Todo et al. Citation1992). Recently, tacrolimus monotherapy has successfully allowed combined small bowel and partial abdominal wall transplantation in humans (Levi et al. Citation2003). The success of tacrolimus and CsA has made them key drugs in the modern era of transplantation (Gorantla et al. Citation2000). The purine synthesis inhibitor mycophenolate mofetil (MMF) (Allison and Eugui Citation1993) was approved in 1995, and the drug sirolimus (rapamycin) (Vézina et al. Citation1975) was introduced in 1999. Combining these drugs with a calcineurin inhibitor significantly reduced acute rejection and improved solid organ graft survival, with a reduction in adverse effects (Kahan et al. Citation1998, McAlister et al. Citation2000, Shapiro et al. Citation2000).
Table 2. The first composite tissue allotransplantations performed (excluding hand transplantations)
Until MMF became available for combination with calcineurin inhibitors in the mid-1990s, results of experimental small animal extremity transplantation were discouraging. In one study involving 114 rat hindlimb transplants, none of the limbs or animals had survived at 1 year when high doses of monotherapy with CsA were used (Min and Jones Citation1995). However, limb and animal survival improved to 89% and 100%, respectively, when MMF was combined with CsA (Benhaim et al. Citation1996). Others have subsequently reproduced these results in the same model using MMF combined with tacrolimus (Perez-Abadia et al. Citation2003).
Similar long-term transplant survival was achieved in a pig model of composite forelimb flaps (Üstüner et al. Citation2000) containing most tissue types except for the joint and with the possible exception of lymph nodes. The immunosuppression in this study was a combination of MMF and prednisone with either CsA (Üstüner et al. Citation1998) or tacrolimus (Jones Jr et al. Citation1999). Thus, the success of this combination regime provided sufficient evidence, in both a small and a large animal model, that human extremity CTA was a realistic goal. These preclinical studies were critical to the advancement of trials involving human hand transplantation.
Chronology of clinical CTA including hand transplantations
Several composite tissue allograft components including nerves (Mackinnon and Hudson Citation1992, Mackinnon et al. Citation2001), tendons (Guimberteau et al. Citation1992, Guimberteau Citation2001), skin (Wendt et al. Citation1994), bone and joints (Hofmann et al. Citation1995, Citation1998, Hofmann and Kirschner Citation2001), vessels (Carpenter and Tomaszewski Citation1997), and muscle (Jones et al. Citation1998) had been transplanted with success clinically before the first hand transplant.
Whereas clinical nerve allografting has been performed since the late 19th century, the first reported success was by Mackinnon and Hudson (Citation1992). In the period between 1988 and 1998, Mackinnon and coworkers transplanted nerve allografts on 7 patients with massive peripheral nerve deflcits, using modern immunosuppressants (Mackinnon et al. Citation2001). Motor and sensory function returned in 6 patients, whereas the graft was rejected in 1 patient. Guimberteau and coworkers (Citation1992) published the first report on clinical tendon allografting. This included transplantation of the digital flexor tendon system based on the ulnar artery. Substantial improvement in finger range of motion was reported without evidence of rejection. The first clinically successful vascularized femoral diaphysis allotransplant was performed in 1994 (Hofmann et al. Citation1995) using an immunosuppression regimen similar to that used in solid organ transplantation. In 1996, the same group performed the first allogeneic vascularized knee joint transplantation (Hofmann et al. Citation1998). Vein allografts as an alternative bypass conduit for patients who lack adequate autogenous veins, were reported by Carpenter and Tomaszewski (Citation1997), but poor patency rates were obtained. The first muscle allograft transplantation, reported by Jones et al. (Citation1998), was performed on a renal transplant patient to cover a large defect in the scalp. No immunosuppression was used in addition to that used for the renal transplant. The larynx, a compound structure of epithelium, cartilage, muscle and connective tissue, was first transplanted in 1998 in a human-with rein-nervations and a good functional outcome (Strome et al. Citation2001).
The clinical feasibility of transplanting limbs and the direction in which clinically oriented limb transplantation research should be heading was discussed at the first conference on CTA, which was held in Washington D.C. in 1991. It was predicted that CTA would become clinically feasible in the 2-4 years that followed (Black and Hewitt Citation1991). However, no such trials were undertaken during that time. 6 years later, in November 1997, the First International Symposium on Composite Tissue Allotransplantation was convened at Jewish Hospital in Louisville, Kentucky. At this meeting, the scientific, clinical and ethical barriers standing in the way of performing the first human hand transplant in the modern era of immunosuppression were discussed. International experts in the field predicted at the meeting that limb allotransplantation was not far from becoming a clinical reality. The final conclusion was to proceed with the work (Barker et al. Citation1998).
Since the first consensus conference, 24 hand transplantations in 18 patients have been performed worldwide (). The first hand transplant in the modern era of immunosuppression was performed in Lyon, France in September 1998 (Dubernard et al. Citation1999). The next one was performed in Louisville in January 1999 (Jones Jr. et al. Citation2000). Two more transplants were performed thereafter-in Guangzhou, China in September 1999 (Pei et al. Citation2001) and in Guangxi, China in January 2000 (data from www.handregistry.com). The world's first bilateral hand transplant was done in Lyon in January 2000 (Vallet et al. Citation2001), and a second bilateral hand transplant followed in Innsbruck, Austria in March 2000 (Margreiter et al. Citation2002). A unilateral transplant between homozygous twins was done in Malaysia in May 2000 (Pathmanathan Citation2000).
The Second International Symposium on Composite Tissue Allotransplantation was held in Louisville in May 2000 to evaluate the status of all composite tissue allografts performed thus far ( and ) (Barker et al. Citation2000, Strauch Citation2000). The first American hand transplant recipient and the first larynx transplant recipient (Jones Jr. et al. Citation2000, Strome et al. Citation2001) were presented at the conference and were evaluated by clinicians and researchers. The final consensus of the meeting was cautiously optimistic, but confirmed without question that these CTAs warranted further clinical trials (Strauch Citation2000). After the conference, a third bilateral hand transplant was performed in Guangzhou in September 2000 (Pei et al. Citation2001). A unilateral hand transplant was done in Milan, Italy in October 2000, followed by a second unilateral transplant in October 2001 (Lanzettà Citation2003). A fourth bilateral hand transplantation was performed in Harbin, China in January 2001 (data from www.handregistry.com). A second American recipient (unilateral) was transplanted in February 2001 (Breidenbach III et al. Citation2002).
The Third International Symposium on Composite Tissue Allotransplantation was held in Lyon in November 2001, to discuss global experience of hand transplantation. After the meeting, one unilateral transplant was performed in Brussels, Belgium in June 2002 and another unilateral transplant in Milan in November 2002. Thereafter, a fifth bilateral hand transplantation was performed in Innsbruck in February 2003 and a sixth bilateral transplant followed in Lyon in May 2003 (data from www.handregistry.com). The International Symposium on Composite Tissue Allotransplantation is now held annually. The Fourth Symposium was held in Varenna, Italy in 2002, and the Fifth Symposium was convened in Brussels in 2003. This year, the proceedings of the International Symposium will be conducted as part of the XXth International Congress of the Transplantation Society in Vienna, Austria.
Procedural aspects of hand transplantation
Hand transplantation is a complex procedure, but may not be as challenging as a hand replantation. This is because a replantation usually occurs in the setting of tissue loss with or without mutilation and contamination of limb components. The hand transplantation can last from 8-12 hours. A typical heart transplant takes 6-8 hours and a liver transplant, 8-12 hours.
Currently, there are no accepted ideal criteria for recipient selection in hand transplantation. The criteria used today include a patient between the age of 18 and 50 years (Lanzettà Citation2001) with unilateral or bilateral amputation(s) between mid-forearm and wrist, and who understands the advantages and risks involved in this experimental surgical technique. The patient has to demonstrate non-acceptance of prosthetic alternatives to transplantation, which usually requires 6-12 months after the amputation for full evaluation. Ideally, the patient should be healthy, without any uncontrolled systemic illness or pre-existing medical condition. Hand transplantation in a solid organ transplant recipient has therefore been argued by some to be a contraindication for the procedure. Recipient management includes clinical, radiographic, neurophysiological, electromyographic, hematological, pathological and psychological evaluation. Psychological screening is essential to assess patient compliance with rigorous pre- and posttransplant rehabilitation programs and drug regimens lasting for the rest of their lives.
Donor selection is similar to that in solid organ transplants. Donors are matched with recipients for gender, skin tone, bone size, race, age, viral status and blood type. Considerations in donor management include histocompatibility testing for tissue matching, and ABO blood group crossmatching (Granger et al. Citation2002). A history of previous malignancy, viral hepatitis (B and C), or HIV are absolute contraindications for use as donors.
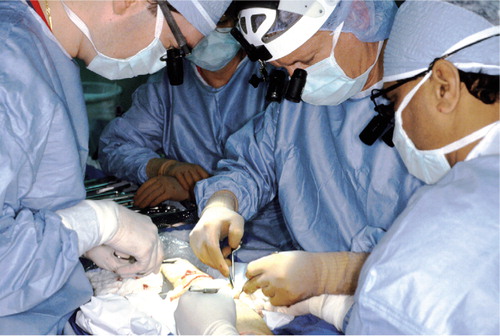
Experimental studies have shown that 2 hours of warm ischemia may predispose to rejection more severely than allotransplantation after 4 or 15 hours of ex vivo preservation (Qayumi et al. Citation1998). To minimize the warm ischemia time as much as possible, the US protocol is therefore to dissect the limb after all solid organs are dissected, and harvest the limb before the solid organs are harvested. The harvested limb is then cooled by topical application of sterile saline and arterial infusion of preservation solution at 4°C. The limb is then wrapped in moist sponges and placed in a sterile bag surrounded by ice and transported to the hospital. Since also extension of the ex vivo preservation time may predispose to more severe rejection (Qayumi et al. Citation1998), the surgical procedure requires two surgical teams to minimize the cold ischemia time. One team identifies and tags the anatomical structures such as muscles, tendons, vascular bundles, nerves, and bones of the donor limb, whereas the other team simultaneously prepares the amputation stump of the recipient. The surgeon will progress with tissue repair () in the following order: bone fixation, arterial anastomosis, anastomosis of a few veins, tendon repair, nerve repair, and finally anatomosis of the remaining veins. The sequence may vary according to the preferences of the surgical team. Postoperatively, the patient is managed according to established guidelines for major replants.
Functional outcomes in hand transplant recipients
Although critics have argued that the long-term functional outcomes of hand transplantation are unknown (Hettiaratchy et al. Citation2003), initial results suggest a high degree of patient satisfaction with minimal morbidity and no mortality (Francois et al. Citation2000, Owen et al. Citation2001, Petit et al. Citation2003). The two exceptions to this encouraging record have been the first French patient (Petruzzo and Dubernard Citation2001) and the first Chinese patient (Guoxian et al. Citation2004). The French patient requested amputation of the transplanted hand at 2 years because of unsatisfactory functional recovery, several episodes of rejection, and inability to cope with the immunosuppressive protocol. The hand of the Chinese patient was amputated at 3 years, presumably after suffering from acute rejection. The experience with these patients has provided clues regarding the evolution of the rejection process associated with non-compliance and treatment withdrawal. They have also highlighted the importance of careful psychological screening in patient selection.
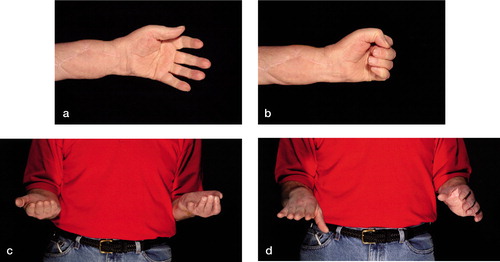
The motor function of the first 4 hand transplants (Francois et al. Citation2000) was evaluated by Carroll (Citation1965), using a test which assesses integrated upper extremity and global hand function on a scale from 0-99 points. Outcome is considered to be poor if the test result is less than 50 points, fair if it is between 51 and 74 points, good if it is between 75 and 84 points, and excellent if it is above 85 points. The functional capacity of the hand transplant 8-20 months postoperatively for the first Louisville patient was 52 points, and for the two Guangzhou patients, 65 and 75 points. The functioning of the hand transplant mirrors that achieved after forearm replantation, something that cannot be achieved using prostheses (Graham et al. Citation1998). Nerve regeneration evaluated by the Tinel test was considered to be remarkably rapid during the first few months for the 4 patients. This observation was attributed to the favorable effect of tacrolimus on axonal regeneration, previously observed in animals (Doolabh and Mackinnon Citation1999) and humans (Mackinnon et al. Citation2001). The patients did, however, only report perception of temperature, pain, and deep pressure, with diminished or complete loss of protective sensation. The first US patient is the recipient of the longest surviving hand transplant in the world. At the 5-year follow-up (unpublished data), his Carroll test score was 63 points, whereas it was 61 and 63 at the 3- and 4-year follow-up. He can perceive hot and cold at the fingertips, detect shapes with the hand and 256 cps of vibration at all fingertips. Tinel's sign has continued to progress well, and is a maximum of 4.5 cm distal to the PIP joint crease. Overall, the patient has continued to demonstrate improvements in intrinsic muscle strength, and total active motion ().
Figure 2. Functional results of the first US hand transplant recipient at the 5-year follow-up: a) extension, b) flexion, c) supination, and d) pronation.
As hand transplants may be described as “visible”, one may anticipate psychological problems postoperatively due to the “alien” nature of the hand. This may be the case particularly in the early postoperative phase, when the transplanted hand has not yet developed function. The hand transplant patients undergo different psychological phases after the operation. It may take up to 10 months to overcome the denial phase and accept the hand as one's own (Bachman and Burloux Citation2001). Integration or lack of integration of the transplanted part into the premotor cortex are not issues which are raised with organ grafts. However, a phenomenon of brain “plasticity” has been observed to occur after hand transplantation, whereby the cortical map of the homunculus is reorganized to accommodate the transplanted hand (Girau et al. Citation2001). Functional MRI studies have demonstrated neural integration of the transplanted hand and arm into the original cortical locus of the sensory-motor cortex 6 months after surgery (Girau et al. Citation2001).
Immunological responses in CTA
Unlike allografts such as kidney, liver and heart, which mainly consist of homogeneous parenchymal tissue, composite tissue allografts consist of multiple tissues - each with different degrees of antigenicity and rejected through different mechanisms (Lee et al. Citation1991, Llull Citation1998). For example, transplanted muscle appears to elicit mainly a cell-mediated immune response, whereas skin elicits both cellular and humoral responses (Duquesnoy Citation1998). In general, rejection of skin and bone marrow appears to be more severe and precedes rejection of muscle, bone, cartilage or tendon. It is clear that for success, an effective immunosuppressive protocol must be capable of preventing rejection of all of the components of the hand.
In transplantation, whether of a solid organ or hand, three main types of rejection may occur: hyperacute, acute, or chronic rejection (VanBuskirk et al. Citation1997). Clinically, regardless of the type of rejection, warning signs include fever, influenza-like symptoms, hypertension, edema or sudden weight gain, changes in heart rate, shortness of breath, and pain and tenderness over the graft site.
Hyperacute rejection. Occurring within minutes to days of transplantation, hyperacute rejection is due to preformed IgG antibodies in the recipient that react against class I MHC/HLA in the transplanted organ. Organ function is lost as a result of antibody deposition, complement activation, and vascular destruction. Kidney transplants are most susceptible to hyperacute rejection. However, hyperacute rejection can be prevented by detecting the antibody reaction with simple cross-matching prior to transplantation, and it is now rare.
Acute rejection. The most common form of rejection is acute rejection, and occurs most frequently in the first 3-6 months after transplantation (). After 6 months, the body adapts to the new organ, and acute rejection is less likely. Acute rejection is mediated by T cells which infiltrate the allograft, undergo clonal expansion, and cause tissue destruction (). Immunosuppressive drugs are most effective in preventing this type of rejection.
Figure 3. (a) Clinical signs of acute rejection of the skin in the second US hand transplant recipient. Note the maculopapular rash and edema in the donor allograft, in the proximal triangular area.
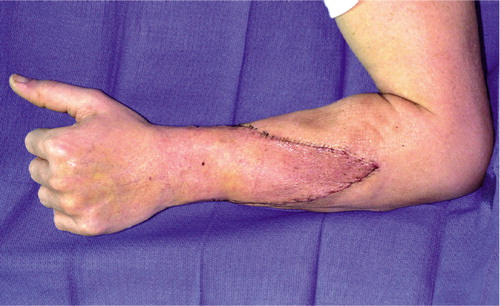
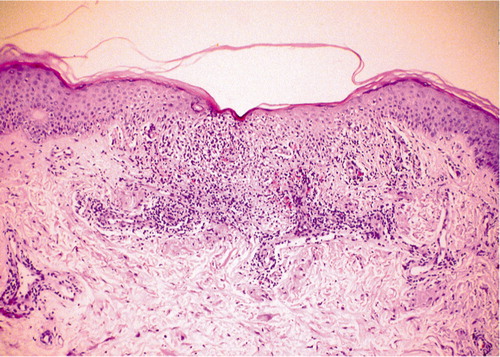
Figure 3. (b) Histological confirmation of moderate acute cellular rejection. Skin biopsy revealing perivascular and dermal lymphocyte infiltration, and mild degeneration of epidermis (hematoxylin and eosin, × 40).
Chronic rejection. Chronic rejection is the term used when allograft function slowly deteriorates and there is histological evidence of intimal hypertrophy and fibrosis. Although chronic rejection is most likely to occur in the late post-transplantation course, it may develop as early as 6-12 months after transplantation. Chronic rejection is the most prevalent cause of graft failure in the first 10 years after transplantation, and occurs in 25% of patients (Leendert et al. Citation2001). It may occur in all types of transplants, and it is the major cause of late graft loss with current rates of 4-8% at most centres (Neuberger Citation1999). The etiology of chronic rejection is unclear. There is, however, some evidence that chronic rejection may represent a low-grade acute rejection (Leendert et al. Citation2001). Preservation injury at the time of transplantation may also contribute to its occurrence. For all organs, the pathophysiology is similar: progressive intimal hypertrophy of the small to medium-sized arteries, which in turn leads to interstitial fibrosis, atrophy, and eventual failure of the organ transplant. Unfortunately, there is no standard treatment for chronic rejection. An analysis of the United States Renal Data System material has shown a median cadaveric kidney graft survival of 8.5 years for 1992- 1993, which is an improvement of 50% compared to data from 1986-1987 (Leendert et al. Citation2001).
Graft versus host disease (GvHD). In addition to rejection, transplantation of foreign tissue brings with it the risk of GvHD. GvHD is a potentially lethal disorder in which mature immunocompetent T cells present in the donor graft can initiate an immune response against the host's body. The primary effector cells for GvHD are chiefly cytotoxic CD8+ T cells and NK cells from the donor. Acute GvHD occurs within the first 3 months of a transplant, and consists of the triad of dermatitis, enteritis and hepatitis. Chronic GvHD develops after 3 months and consists of an autoimmune syndrome directed toward multiple organs. In both animals and humans, GvHD occurs after transplantation of solid organs such as liver, kidney, pancreas and small intestine. The greatest risk of GvHD, however, is after bone marrow transplantation. The incidence of acute GvHD in bone marrow transplantation ranges from less than 10% to more than 80%, depending on the degree of histoincompatibility, the number of T cells in the graft, the age of the patient, and prophylactic regimen, whereas the incidence of chronic GvHD ranges from 30 to 60% (Ferrara and Deeg Citation1991, Lee et al. Citation2003). Also, lymph nodes contain the highest numbers of T cells compared to other tissues. Thus, limb transplants that contain bone marrow (such as hand or arm transplants) and in some instances lymph nodes (epitrochlear nodes), can precipitate GvHD.
Potential risks of hand transplantation
The risks common to all transplants are principally those of the immunosuppression. One of the most important insights from the Louisville pig experiments was the finding that survival after CTA could be accomplished with the same levels of immunosuppression as used in human kidney transplantation (Üstüner et al. Citation2000). The triple immunosuppressive regimen used in hand transplantation is approximately the same as that currently used for kidney transplantation. The risks to which a hand transplant recipient would be exposed may therefore be anticipated from the literature based on extensive experience in kidney transplantation. Studies performed before the introduction of tacrolimus in 1994 would therefore lead to overestimation of the risk, since the previous immunosuppressive regimens carried a higher risk (Moss et al. Citation2000). The risks include overwhelming and sometimes fatal infections, direct organ toxicities, impaired wound healing, anemia, diabetes, GvHD, rejection, and malignancies (Ferrara and Deeg Citation1991, First and Peddi Citation1998, Fishman and Rubin Citation1998, Brenner et al. Citation2002, Lee et al. Citation2003). The predominant cancer types are skin and lip cancer (37%), lymphoma (16%), lung carcinoma (6%), and Kaposi's sarcoma (4%) (Penn Citation1999). A multicenter study of a kidney transplant population put the risk of non-melanoma skin cancer at 11% during the first 3 years (Mathew Citation1998), whereas the incidence of the more malignant lymphoma within the first year after transplantation is 0.22%-which is 20 times higher than the rate in the general population (Opelz and Henderson Citation1993). During subsequent years, the incidence is 0.04%. The incidence is 6 times higher in heart transplant recipients (Opelz and Henderson Citation1993).
In the first 4 hand transplant patients (Francois et al. Citation2000), the following complications were observed during the first 8-20 months postoperatively: insulin-dependent diabetes mellitus (Lyon), Cushing's syndrome (Guangzhou 1), cytomegalovirus colitis (Louisville), herpetic cutaneous infection (Lyon), and recurrent cutaneous mycosis (Louisville, Guangzhou 1). All of these complications were treated successfully with dose reduction of the immunosuppressants, insulin therapy, temporary intravenous and long-term oral ganciclovir, and topical treatment.
As noted earlier, 2 patients have had their hand amputated due to rejection, and more patients have noted signs of rejection, for which they have needed temporarily higher doses of immunosuppressants. However, unlike a recipient of any solid organ transplant who is in some degree of organ failure, the potential recipient of a hand transplant is likely to be healthy. This may significantly reduce the risk of complications after surgery. Furthermore, hand transplants have the advantage over most solid organ transplants that if severe complications arise, the allograft can easily be removed and immunosuppression stopped immediately.
The potential health risk of chronic immunosuppression has fuelled ethical debate. The goal of any intervention is to do more good than harm. It is, however, difficult to compare the benefits of hand transplantation to its potential detrimental effects. McCabe and coworkers (McCabe et al. Citation1998) introduced a decision analysis, and found that on average the benefit vs. risk ratio was unfavorable, whereas others have expressed the opposite opinion (Siegler Citation1998). Also, patients may proceed from a different frame of reference and have prioritization of values that is different from that of doctors when making decisions about surgery (Edgell et al. Citation2001). This illustrates how complex the judgement about whether one should or should not perform the hand transplantation is at present.
Predicted future developments in CTA and hand transplantation
Progress in hand transplantation depends on development of new strategies to achieve long-term allograft survival while minimizing risks and costs. Currently, several approaches are in the early stages of testing, which try to achieve this goal. These may be summarized as (1) the use of new immunosuppressive drugs, and (2) tolerance induction.
The first and most realistic possibility for the near future is the development of new immunosuppressive drugs (Gorantla et al. Citation2000). As mentioned earlier, current drug regimens are substantially better than those that were available only a decade ago. In the future, immunosuppressive drugs may be developed to specifically interfere with the transcription of genes necessary for T cell activation, to block signals of T and B cells, to suppress expression of cytokine receptors, to inhibit trafficking of immunocompetent cells and to decrease the capacity for immune cell adhesion.
The second and slightly more distant possibility is the induction of tolerance. Tolerance is defined as a reduced or absent immune response towards an allograft, allowing long-term survival without the need for continuous nonspecific immunosuppression. Tolerance induction protocols can be sub-categorized into those involving depletion of recipient T cells prior to transplantation, those using donor bone marrow cells to induce chimerism, and those using signal blockade to block alloreactive cell signaling.
T cell depletion can be achieved through the use of CD-3 immunotoxin (Thomas et al. Citation2000) or by the use of Campath 1H (Friend et al. Citation1991, Kirk et al. Citation2003). The first uses of Campath 1H in human CTA have successfully prevented rejection using a steroid-free tacrolimus monotherapy protocol (Levi et al. Citation2003). It remains to be seen whether Campath 1H can induce tolerance in humans with total elimination of the need for immunosuppression. Bone marrow mixed chimerism can induce tolerance, as shown in animal and human models (Prabhune et al. Citation2000, Citation2003). Signal blockade can be achieved by using co-stimulatory blocking agents (Vincenti Citation2003). These strategies are promising, but are all at preliminary stages of research and clinical testing. The past 30 years have witnessed many advancements in pharmacotherapeutics that have been implemented clinically. These have been greater than the advances in the field of transplantation tolerance. This is ample proof that the path to tolerance will strongly depend on the knowledge gained from the use of immunosuppression in allotransplantation.
Hand and composite tissue allotransplantation registry
The increasing interest in hand and composite tissue allotransplantation has resulted in the founding of an international society for hand and composite tissue allotransplantation. Information websites such as www.handtransplant.com, and www.handregistry.com (the International Registry of Hand and Composite Tissue Transplantation) have also been established. The registry serves as an up-to-date collection of scientific data, contributed by surgical units performing hand transplantations around the world. With the advent of newer drugs, sharing of state-of-the-art knowledge, comparison of innovative therapies and statistical analysis of transplant data can help in modification of drug regimens, which can in turn improve allograft outcome.
Conclusion
In the modern era of immunosuppression, 24 hands have been transplanted onto 18 recipients to date. The initial period of this closely followed trial of transplantations on kidney-level immunosuppression has proved successful. However, the outcomes of this experimental procedure are still being determined. The ethical aspects of using chronic immunosuppression for a condition which is not life-threatening have also been debated in the surgical communities. Consequently, the future of hand transplants and other composite tissue allografts lies in the development of less toxic immunosuppressive drugs and/or safer methods of tolerance induction. Experimental trial of hand transplantations must be carried out under strict and ethical research guidelines, should be open to both professional and lay scrutiny, and should be subject to periodic reporting in scientific symposia.
No competing interests declared.
The authors are grateful for the support from Professor Olav Reikerås, Department of Orthopedic Surgery, National Hospital, Oslo.
- Allison A C, Eugui E M. Immunosuppressive and other effects of mycophenolic acid and an ester prodrug, mycophenolate mofetil. Immunol Rev 1993; 136: 5–28
- Altman L K. A short, speckled history of a transplanted hand. NY Times (Print). 2001; Febr 27: F1, F2
- Armitage J M, Kormos R L, Griffith B P, Hardesty R L, Fricker F J, Stuart R S, Marrone G C, Todo S, Fung J, Starzl T E. A clinical trial of FK 506 as primary and rescue immunosuppression in cardiac transplantation. Transpl Proc 1991; 23(1)1149–52
- Bachman D, Burloux G. The psychiatrist and the hand transplant. Composite Tissue Allografts, J-M Dubernard. John Libbey Eurotext, Paris 2001; 85–91
- Barker J H, Jones J W, Jr, Breidenbach W C, III. Closing remarks. Proceedings of the International Symposium on Composite Tissue Allotransplantation, Louisville, Kentucky, 1998. 30: 2787, Transplant Proc (Special Issue)
- Barker J H, Breidenbach W C, III, Hewitt C W. Second International Symposium on Composite Tissue Allotransplantation. 2000. 20: 359, Introduction. Microsurgery (Special Issue), (8)
- Barnard C N. A human cardiac transplant: An interim report of a successful operation performed at Groote Schuur Hospital, Cape Town. S A Med J 1967, 37: 1271–4
- BBC News. Tongue transplant patient doing well. July 22, 2003, http://news.bbc.co.uk/1/hi/health/3086509.stm
- Benhaim P, Anthony J P, Ferreira L, Borsanyi J-P, Mathes S J. Use of combination of low-dose cyclosporine and RS61443 in a rat hindlimb model of composite tissue allotransplantation. Transplantation 1996; 61(4)527–32
- Birchall M. Tongue transplantation. Lancet 2004; 363(9422)1663
- Black K S, Hewitt C W. Report: Composite tissue transplantation workshop. Department of Veterans Affairs, Rehabilitation Research and Development Service, Washington, D.C 1991
- Borel J F, Kis Z L. The discovery and development of cyclosporine (Sandimmune). Transplant Proc 1991; 23(2)1867–74
- Breidenbach W C, III, Tobin II G R, Gorantla V S, Gonzales R N, Granger D K. A position statement in support of hand transplantation. J Hand Surg (Am) 2002; 27(5)760–70
- Brenner M J, Tung T H, Jensen J N, Mackinnon S E. The spectrum of complications of immunosuppression: is the time right for hand transplantation?. J Bone Joint Surg (Am) 2002; 84(10)1861–70
- Carpenter J P, Tomaszewski J E. Immunosuppression for human saphenous vein allograft bypass surgery: A prospective randomized trial. J Vasc Surg 1997; 26(1)32–42
- Carrel A. Results of the transplantation of blood vessels, organs and limbs. JAMA 1908; 51: 1662–7
- Carroll D. A quantitative test of upper extremity function. J Chronic Dis 1965; 18: 479–91
- Cooney W, Hentz V R. Position statement: Council of the American Society for Surgery of the Hand. Hand trans-plantation–primum non nocere. J Hand Surg (Am) 2002; 27(1)165–8
- Danilevicius Z. SS Cosmas and Damian. The patron saints of medicine in art. 1967; 201(13)1021–5
- Da Varagine J. Leggenda Aurea. Libreria Editrice Fiorentina, FlorenceItaly 1952; 648–52
- Derom F, Barbier F, Ringoir S, Versieck J, Rolly G, Berzsenyi G, Vermeire P, Vrints L. Ten-month survival after lung homotransplantation in man. Thoracic Cardiovasc Surg 1971; 61(6)835–46
- Doolabh V B, Mackinnon S E. FK506 accelerates functional recovery following nerve grafting in a rat model. 1999; 103(7)1928–36
- Dubernard J-M, Owen E, Herzberg G, Lanzettà M, Martin X, Kapila H, Dawahra M, Hakim N S. Human hand allograft: report on first 6 months. Lancet 1999; 353(9161)1315–20
- Duquesnoy R J. Is histocompatibility testing needed for composite tissue transplantation?. Transpl Proc 1998; 30: 2724–8
- Edgell S E, McCabe S J, Breidenbach W C, III, Neace W P, LaJoie A S, Abell T D. Different reference frames can lead to different hand transplantation decisions by patients and physicians. J Hand Surg (Am) 2001; 26(2)196–200
- Ferrara J LM, Deeg H J. Graft-versus-host disease. N Engl J Med 1991; 324(10)667–74
- First M R, Peddi V R. Malignancies complicating organ transplantation. Transpl Proc 1998; 30: 2768–70
- Fishman J A, Rubin R H. Infection in organ-transplant recipients. N Engl J Med 1998; 338(24)1741–51
- Foucher G. Prospects for hand transplantation. Lancet 1999; 353: 1286–7
- Francois C G, Breidenbach W C, III, Maldonado C, Kakoulidis T P, Hodges A, Dubernard J-M, Owen E, Pei G, Ren X, Barker J H. Hand transplantation: comparisons and observations of the first four clinical cases. Microsurgery 2000; 20: 360–71
- Friend P J, Waldmann H, Hale G, Cobbold S, Rebello P, Thiru S, Jamieson N V, Johnston P S, Calne R Y. Reversal of allograft rejection using the monoclonal antibody, Campath-1G. Transplant Proc 1991; 23(4)2253–4
- Fung J J, Todo S, Jain A, McCauley J, Alessiani M, Scotti C, Starzl T E. Conversion from cyclosporine to FK 506 in liver allograft recipients with cyclosporine-related complications. Transpl Proc (Suppl 1) 1990; 22(1)6–12
- Giraux P, Sirigu A, Schneider F, Dubernard J M. Cortical reorganization in motor cortex after graft of both hands. Nat Neurosci 2001; 4(7)691–2
- Gorantla V S, Barker J H, Jones J W, Jr, Prabhune K, Maldonado C, Granger D K. Immunosuppressive agents in transplantation: mechanisms of action and current anti-rejection strategies. Microsurgery 2000; 20(8)420–9
- Goto T, Kino T, Hatanaka H, Nishiyama M, Okuhara M, Kohsaka M, Aoki H, Imanaka H. Discovery of FK-506, a novel immunosuppressant isolated from Streptomyces Tsukubaensis. Transplant Proc (Suppl 6) 1987; 19(5)4–8
- Goulet O, Revillon Y, Brousse N, Jan D, Canion D, Ram-baud C, Cerf-Bensussan N, Buisson C, Hubert P, de Potter S, Mougenot J-F, Fischer A, Ricour C. Successful small bowel transplantation in an infant. Transplantation 1992; 53(4)940–3
- Graham B, Adkins P, Tsai T-M, Firrell J, Breidenbach W C, III. Major replantation versus revision amputation and prosthetic fitting in the upper extremity: a late functional outcomes study. J Hand Surg (Am) 1998; 23(5)783–91
- Granger D K, Breidenbach W C, III, Pidwell D J, Jones J W, Baxter-Lowe L A, Kaufmann C L. Lack of donor hyporesponsiveness and donor chimerism after clinical transplantation of the hand. Transplantation 2002; 74(11)1624–30
- Guimberteau J-C. Ten years follow-up of two cases of vascularized digital flexor system allotransplants. Composite tissue allografts, J-M Dubernard. John Libbey Eurotext, Paris 2001; 41–2
- Guimberteau J-C, Baudet J, Panconi B, Boileau R, Potaux L. Human allotransplant of a digital flexion system vascularized on the ulnar pedicle: a preliminary report and 1-year follow-up of two cases. Plast Reconstr Surg 1992; 89: 1135–47
- Guoxian P, Liqiang G, Lixin Y. Long-term follow-up of hand allografts. Abstract. J Reconstr Microsurg 2004; 20(1)111
- Herndon J H. Composite-tissue transplantation - A new frontier. N Engl J Med 2000; 343(7)503–5
- Hettiaratchy S, Butler P E, Lee W PA. Lessons from hand transplantations. Lancet 2001; 357(9255)494–5
- Hettiaratchy S, Randolph M A, Lee W PA. Long-term consideration of hand transplantation. Transplantation 2003; 75(9)1605
- Hofmann G O, Kirschner M H. Allogeneic vascularized bone and joint transplantation. First five years experience. Composite tissue allografts, J-M Dubernard. John Libbey Eurotext, Paris 2001; 43–7
- Hofmann O G, Kirschner M H, Bühren V, Land W. Allogenic vascularized transplantation of a human femoral diaphysis under cyclosporin A immunosuppression. Transpl Int 1995; 8(5)418–9
- Hofmann G O, Kirschner M H, Wagner F D, Brauns L, Gonschorek O, Bühren V. Allogeneic vascularized grafting of human knee joints under postoperative immunosuppression of the recipient. World J Surg 1998; 22: 818–23
- Jones J W, Jr, Üstüner E T, Zdichavsky M, Edelstein J, Ren X, Maldonado C, Ray M, Jevans A W, Breidenbach W C, III, Gruber S A, Barker J H, The Louisville Hand Transplant Team. Long-term survival of an extremity composite tissue allograft with FK506-mycophenolate mofetil therapy. Surgery 1999; 126(2)384–8
- Jones J W, Jr, Gruber S A, Barker J H, Breidenbach W C, III. Successful hand transplantation. One-year follow-up. N Engl J Med 2000; 343(7)468–73
- Jones N F. Concerns about human hand transplantation in the 21st century. Debate. J Hand Surg (Am) 2002; 27(5)771–87
- Jones T R, Humphrey P A, Brennan D C. Transplantation of vascularized allogeneic skeletal muscle for scalp reconstruction in renal transplant patient. Transplant Proc 1998; 30(6)2746–53
- Judet H. Essai sur la greffe des tissus articulaires. C R Acad Sci 1908; 146: 193–6
- Kahan B D. Cosmas and Damian revisited. Transplant Proc (Suppl 1) 1983; 15(4)2211–6
- Kahan B D, Podbielski J, Napoli K L, Katz S M, Meier-Kri-esche H-U, Van Buren C T. Immunosuppressive effects and safety of a sirolimus/cyclosporine combination regimen for renal transplantation (Clinical Transplantation). Transplantation 1998; 66(8)1040–6
- Kirk A D, Hale D A, Mannon R B, Kleiner D E, Hoffmann S C, Kampen R L, Cendales L K, Tadaki D K, Harlan D M, Swanson S J. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (Campath-1H) (Clinical Transplantation). Transplantation 2003; 76(1)120–9
- Lanzettà M. Indications for hand allografts. Composite tissue allografts, J-M Dubernard. John Libbey Eurotext, Paris 2001; 83
- Lanzettà M. Functional results of the italian hand transplantation program. J Hand Surg (Br) (Suppl 1) 2003; 28: 74
- Lee S J, Vogelsang G, Flowers M ED. Chronic graft-versus-host disease. BB&MT 2003; 9: 215–33
- Lee W PA. The debate over hand transplantation. J Hand Surg (Am) 2002; 27(5)757–9
- Lee W PA, Mathes D W. Hand transplantation: pertinent data and future outlook. J Hand Surg (Am) 1999; 24(5)906–13
- Lee W PA, Yaremchuk M J, Pan Y-C, Randolph M A, Tan C M, Weiland A J. Relative antigenicity of components of a vascularized limb allograft. Plast Reconstr Surg 1991; 87(3)401–11
- Leendert C P, de Fijter J W, Sijpkens Y WJ. Chronic renal transplant rejection. Kidney Transplantation. Principles and practice, P J Morris. W.B. Saunders Company, Philadelphia 2001; 408–18
- Levi D M, Tzakis A G, Kato T, Madariaga J, Mittal N K, Nery J, Nishida S, Ruiz P. Transplantation of the abdominal wall. Lancet 2003; 361(9376)2173–6
- Lexer E. Substitution of whole or half joints from freshly amputated extremities by free plastic operation. Surg Gyn Obst 1908; 6: 601–7
- Lillehei R C, Ruix J O, Aquino C, Goetz F. Transplantation of the pancreas. Acta Endocrinol Suppl (Copenh) 1976; 205: 303–20
- Llull R. An open proposal for clinical composite tissue allotransplantation. Transpl Proc 1998; 30(6)2692–6
- Lubbe A S. Successful hand transplantation or too early to tell?. Transplantation 2003; 75(11)1916–7
- Lundborg G. Hand transplantation. Editorial. Scand J Plast Reconstr Hand Surg 1999; 33: 369–71
- Mackinnon S E, Hudson A R. Clinical application of peripheral nerve transplantation. Plast Reconstr Surg 1992; 90(4)695–9
- Mackinnon S E, Doolabh V B, Novak C B, Trulock E P. Clinical outcome following nerve allograft transplantation. Plast Reconstr Surg 2001; 107(6)1419–29
- Manske P R. Hand transplantation. Editorial. J Hand Surg (Am) 2001; 26(2)193–5
- Margreiter R, Brandacher G, Ninkovic M, Steurer W, Kreczy A, Schneeberger S. A double-hand transplant can be worth the effort!. Transplantation 2002; 74(1)85–90
- Mathew T H, for the Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group. A blinded, long-term, randomized multicenter study of mycophenolate mofetil in cadaveric renal transplantation: results at three years (Clinical Transplantation). Transplantation 1998; 65(11)1450–4
- McAlister V C, Gao Z, Peltekian K, Domingues J, Mahalati K, MacDonald A S. Sirolimus-tacrolimus combination immunosuppression. Lancet 2000; 355(9201)376–7
- McCabe S J, Rodocker G, Julliard K, Breidenbach W C, III, Marcel C, Shirbacheh M V, Barker J. Using decision analysis to aid in the introduction of upper extremity transplantation. Transpl Proc 1998; 30: 2783–6
- Medawar P B. The behaviour and fate of skin autografts and skin homografts in rabbits. J Anat 1944; 78: 176–203
- Medical Tribune-World Wide report. Transplant is successful with a cadaver forearm. Med Trib Med News 1964; 5(20)1, 38
- Merrill J P, Murray J E, Harrison J H, Guild W R. Successful homotransplantation of the human kidney between identical twins. JAMA 1956; 160(4)277–82
- Min Z, Jones N F. Limb transplantation in rats: immunosuppression with FK-506. J Hand Surg (Am) 1995; 20(1)77–87
- Moss A, Najarian J S, Sutherland D ER, Payne W D, Gruessner R WG, Humar A, Kandaswamy R, Gillingham K J, Dunn D L, Matas A J. 5,000 kidney transplants - A single-center experience. Clinical Transplants, J M Cecka, P I Terasaki. UCLA Immunogenetics Center, LA, California 2000; 159–71
- Murray J E, Merrill J P, Dammin G J, Dealy J B, Alexandre G W, Harrison J H. Kidney transplantation in modified recipients. Ann Surg 1962; 156: 337–55
- Murray J E, Merrill J P, Harrison J H, Wilson R E, Dammin G J. Prolonged survival of human-kidney homografts by immunosuppressive drug therapy. N Engl J Med 1963; 268(24)1315–23
- Neuberger J. Incidence, timing, and risk factors for acute and chronic rejection. Liver Transpl Surg (Suppl 1) 1999; 5(4)30–6
- Opelz G, Henderson R, for the Collaborative Transplant Study. Incidence of non-Hodgkin lymphoma in kidney and heart transplant recipients. Lancet 1993; 342(88867)1514–6
- Owen E, Dubernard J-M, Lanzettà M, Hakim N, Kapila H, Martin X, Noli R. What we have learnt from human hand allograft transplantation. Composite tissue allografts, J-M Dubernard. John Libbey Eurotext, Paris 2001; 5–6
- Pathmanathan V. Hand transplantation between homozygous twins. June, 2000, Personal communication with Breidenbach III W C
- Pei G, Zhu L, Gu L. The experience of three cases of human hand allografts in China. Composite Tissue Allografts, J-M Dubernard. John Libbey Eurotext, Paris 2001; 69–70
- Penn I. Posttransplant malignancies. Transpl Proc 1999; 31: 1260–2
- Perez-Abadia G, Laurentin-Perez L, Gorantla V S, Francois C G, Vossen M, Brouha P CR, Orhun H I, Anderson G L, Maldonado C, Pidwell D J, Breidenbach W C, III, Barker J H. Low-dose immunosuppression in a rat hindlimb transplantation model. Transp Int 2003; 16(12)835–42
- Petit F, Minns A B, Dubernard J-M, Hettiaratchy S, Lee W PA. Composite tissue allotransplantation and reconstructive surgery. First clinical applications. Ann Surg 2003; 237(1)19–25
- Petruzzo P, Dubernard J-M. Hand transplantation: Lyon experience. Composite tissue allografts, J-M Dubernard. John Libbey Eurotext, Paris 2001; 63–7
- Prabhune K A, Gorantla V S, Maldonado C, Perez-Abadia G, Barker J H, Ildstad S T. Mixed allogeneic chimerism and tolerance to composite tissue allografts. Microsurgery 2000; 20(8)441–7
- Prabhune K A, Gorantla V S, Perez-Abadia G, Francois C G, Vossen M, Laurentin-Perez L A, Breidenbach W C, III, Wang G G, Anderson G L, Pidwell D J, Barker J H, Maldonado C. Composite tissue allotransplantation in chimeric hosts part II. A clinically relevant protocol to induce tolerance in a rat model [Experimental transplantation]. Transplantation 2003; 76(11)1548–55
- Pubmed reference. Recipient of first tongue transplant recovering. CDS Rev 2003; 96(7)44
- Qayumi A K, Nikbakht-Sangari M N, Godin D V, English J C, Horley K J, Keown P A, Lim S P, Ansley D M, Koehle M S. The relationship of ischemia-reperfusion injury of transplanted lung and the up-regulation of major histocompatibility complex II on host peripheral lymphocytes. J Thorac Cardiovasc Surg. J Thorac Cardiovasc Surg 1998; 115(5)978–89
- Reigstad A, Hetland K R. Allotransplantasjon av hånd. Tidsskr Nor Lægeforen 2003; 7: 946
- Shapiro A MJ, Lakey J RT, Ryan E A, Korbutt G S, Toth E, Warnock G L, Kneteman N M, Rajotte R V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000; 343(4)230–8
- Siegler M. Ethical issues in innovative surgery: should we attempt a cadaveric hand transplantation in a human subject?. Transplant Proc 1998; 30: 2779–82
- Starzl T E. Anti-rejection therapy. Experience in renal transplantation, T E Starzl. W.B. Saunders Company, Philadelphia 1964; 130–41
- Starzl T E, Marchioro T L, Waddell W R. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg Gyn Obst 1963; 117(4)385–95
- Starzl T E, Groth C G, Brettschneider L, Penn I, Fulginiti V A, Moon J B, Blanchard H, Martin A J, Porter K A. Orthotopic homotransplantation of the human liver. Ann Surg 1968; 168(3)392–413
- Starzl T E, Todo S, Tzakis A, Podesta L, Mieles L, Demetris A, Teperman L, Selby R, Stevenson W, Stieber A, Gordon R, Iwatsuki S. Abdominal organ cluster transplantation for the treatment of upper abdominal malignancies. Ann Surg 1989;; 210(3)374–86
- Strauch B (2000) Foreword. Proceedings of the Second International Symposium on Composite Tissue Allotransplantation. 2000. Louisville, Kentucky, 20: 357, Microsurgery (Special Issue)
- Strickland J W. Hand transplant: technology over good sense. The Indiana Hand Center Newsletter 1999; 3: 2–4
- Strome M, Stein J, Esclamado R, Hicks D, Lorenz R R, Braun W, Yetman R, Eliachar I, Mayes J. Laryngeal transplantation and 40-month follow-up. N Engl J Med 2001; 344(22)1676–9
- Tagliacozzi G. De cutorum chirurgia per insitonem. University of Milan Library, VeniceItaly 1597
- Thomas E D, Lochte H L, Lu W C, Ferrebee J W. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med 1957; 257(11)491–6
- Thomas F, Ray P, Thomas J M. Immunological tolerance as an adjunct to allogeneic tissue grafting. Microsurgery 2000; 20(8)435–40
- Todo S, Fung J J, Demetris A J, Jain A, Venkataramanan R, Starzl T E. Early trials with FK 506 as primary treatment in liver transplantation. Transpl Proc (Suppl 1) 1990; 22(1)13–6
- Todo S, Tzakis A G, Abu-Elmagd K, Reyes J, Nakamura K, Casavilla A, Selby R, Nour B M, Wright H, Fung J J, Demetris A J, Van Thiel D H, Starzl T E. Intestinal transplantation in composite visceral grafts or alone. Ann Surg 1992; 216(3)223–34
- Üstüner E T, Zdichavsky M, Ren X, Edelstein J, Maldonado C, Ray M, Jevans A W, Breidenbach W C, III, Gruber S A, Barker J H, Jones J W. Long-term composite tissue allograft survival in a porcine model with cyclosporine/ mycophenolate mofetil therapy. Transplantation 1998; 66(12)1581–7
- Üstüner E T, Majzoub R K, Ren X, Edelstein J, Maldonado C, Perez-Abadia G, Breidenbach W C, III, Barker J H. Swine composite tissue allotransplant model for preclinical hand transplant studies. Microsurgery 2000; 20: 400–6
- Vallet B, Parmentier H, Lagouy V, Dziesmiazkiewiez N. Bilateral hand transplant: functional results after 18 months. Composite tissue allografts, J-M Dubernard. John Libbey Eurotext, Paris 2001; 75–6
- VanBuskirk A M, Pidwell D J, Adams P W, Orosz C G. Transplantation immunology. JAMA 1997; 278(22)1993–9
- Vézina C, Kudelski A, Sehgal S N. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 1975; 28(10)721–6
- Vincenti F. New monoclonal antibodies in renal transplantation. Minerva Urol Nefrol 2003; 55(1)57–66
- Wendt J R, Ulich T R, Ruzics E P, Hostetler J R. Indefinite survival of human skin allografts in patients with long-term immunosuppression. Ann Plast Surg 1994; 32: 411–7