Abstract
Background Therapeutic ultrasound is commonly used for treatment of injuries to tendons, ligaments and joint capsules. Opinions still differ, however, as to the beneficial effects of ultrasound treatment of the above-men-tioned conditions.
Methods We studied the effect of various ultrasound intensities on the healing of tenotomized, sutured and immobilized Achilles tendons of adult rabbits. Different intensities of pulsating ultrasound (0, 50, 100, 200, 500, 750, 1 000 and 2 000 mW/cm2, frequency 3 MHz) were given over the healing tendons for 5 min daily, using a gel as the coupling agent between the ultrasound probe and the skin. Eleven days after the tenotomy, the healing tendons were analyzed in a materials testing machine.
Results The extensibility of the healing tendons was greater after sonication at an intensity of 2 000 mW/cm2than after 50 mW/cm2. We found no significant effect on the load at rupture and normalized load at rupture of the ultrasound-treated healing tendons compared with mock-sonicated healing tendons. A gradual decline was observed, however, in the stiffness and collagen content of the healing tendons with increasing intensity of the ultrasound treatment.
Interpretation The pulsating ultrasound treatment applied did not improve the mechanical properties of healing Achilles tendons at day 11 after the operation. On the other hand, a slight decline in stiffness was observed with increasing intensity of ultrasound treatment.
In spite of the widespread use of ultrasound for treatment of injuries to tendons, ligaments and joint capsules (Cambier and Vanderstraeten Citation1997), opinions still differ concerning the effect of ultrasound treatment on soft tissue injuries, and as to which ultrasound intensity and time schedule should be chosen. In animal experiments, Jackson et al. (Citation1991) have shown a stimulatory effect of ultrasound on the healing of partial tendon tenotomy in rats. The intensity of the ultrasound treatment was 1500 mW/cm2and it was applied for 4 min daily. The breaking strength of the healing tendon was increased at 5, 9, 15 and 21 days after the injury. Enwemeka et al. (Citation1990) found increased breaking strength at day 10 in healing rabbit tendons which had been tenotomized, sutured and immobilized. The ultrasound treatment was 500 mW/cm2given 5 min daily for 10 days. On the other hand, Roberts et al. (Citation1982) found that ultrasound treatment at 800 mW/cm2impeded the healing of tenotomized, sutured and immobilized flexor tendons of rabbits.
To shed light on these contradictory findings, we applied pulsating ultrasound at a frequency of 3 MHz and at various intensities on the Achilles tendon of rabbits after tenotomy and healing for 11 days. We then tested the mechanical properties of the tendon with the scar tissue in a materials testing machine after removal of the sutures.
Animals and methods
We used 72 fully developed female New Zealand white rabbits. The rabbits had a body weight between 2.9 and 4.6 kg. The animals were fed high-fiber rabbit chow and water ad libitum and were housed one rabbit per standard rabbit cage at 21-23°C. The study was approved by the Danish Animal Experiment Inspectorate.
Surgical procedure
Each rabbit was anesthetized with a mixture of Midazolam (Roche, Basel, Schwitzerland) (2 mg/ kg) and Hypnorm (Janssen, Buckinghamshire, UK) (0.1 mg/kg). Preoperatively, Midazolam 2 mg/kg was given intramuscularly at the start of the operation. Hypnorm 0.1 mg/kg was given intramuscularly with an additional injection of Hypnorm (0.1 mg/kg) every 20 min.
The skin over and around the right Achilles tendon was shaved and the tendon was approached by a medial skin incision, and cut transversely midway between its calcaneal insertion and the musculotendinous junction. We used a razor blade to make a straight incision at a right angle through the tendon. The severed ends of the tendon were sutured with two loops of 4.0 non-absorbable suture (Dermalon 4–0) and the skin incision was sutured with the same suture. The incision was covered with a sterile bandage and the limb was immobilized in a lightweight fiberglass cast with the ankle fully plantar flexed and the knee in a flexion of 90°. A 10-cm2window was made in the cast over the tenotomy site to permit sonication of the tendon without cast removal. All surgical procedures were performed under aseptic conditions.
Ultrasound treatment
The animals were allocated randomly to the following treatment groups based on intensity of ultrasound: 0 (controls), 50, 100, 200, 500, 750, 1 000 and 2 000 mW. The ultrasound treatment was started on the day after surgery and was applied for a period of 5 min daily, consecutively for 10 days. Before ultrasound treatment, the bandage was removed. The skin was covered with a standard coupling gel (Sakura Tissue-tek compound, Zoekerwoude, Holland) in order to achieve contact between the ultrasound applicator and the skin. The ultrasound applicator (Enraf-Nonius, Delft, Holland) was set to pulsating ultrasound from 0 mW to 2 000 mW with a frequency of 3 MHz. The ultrasound applicator was held on the lateral side of the Achilles tendon. A total of 10 consecutive treatments were given, after which the tendon was dissected free and tested on the eleventh postoperative day. The immobilization splint of each rabbit was removed and each animal was weighed and killed with an overdose of pentobarbital. 30 mm of the Achilles tendon containing the scar tissue was dissected free and the sutures were removed without damaging the scar tissue.
Mechanical analysis
The Achilles tendon specimens were fixed between two clamps with a jaw space of 15 mm and the scar halfway between the clamps. The clamps were mounted in a materials testing machine with the tendon specimen immersed in buffered Ringer′s solution to avoid drying during the testing procedure. The clamps were moved apart at a constant speed of 50 mm/min until rupture of the scar tissue. Rupture of tenotomies healed for 11 days always took place in the scar tissue, never at the clamps. The load values were measured by means of a load cell and the deformation values were measured by an extensometer coupled to the measuring bridges of the materials testing machine (Alwetron; Lorentzen and Wettre, Stockholm, Sweden), the signals being recorded as continuous x-y curves. After mechanical testing, the tendinous tissue between the clamps with the torn scar tissue was analyzed for collagen content by hydroxyproline assessment according to Woessner (Citation1976). The amount of collagen per mm tendon length (= unit collagen = UC) was used as a measure of the cross-sectional area of the tendon (Oxlund et al. Citation1981). Normalized load values were load values divided by the collagen content per mm tendon length (= load/UC = Newton/UC). Strain was expressed as deformation (ΔL) of the tendon specimen divided by the start length (L0) of the specimen (i.e. ΔL/L0). The load-deformation curves were scanned into a computer, and the following parameters were calculated using SigmaScan software (Jandel Scientific Software, St. Raphael, CA, USA): strain at rupture i.e. extensibility, load at rupture, stiffness (slope of the linear part of the load-strain curve), normalized load at rupture and normalized stiffness.
Statistics
The distribution of the load values at rupture of the healing tendons did not follow a Gaussian distribution. The mechanical data were therefore analyzed by non-parametric statistics. Median values with 95% confidence limits were calculated. For analysis of differences between groups, the Krus-kal-Wallis test was applied; in case of significant differences, the Mann-Whitney U-test was used. In order to analyze the correlation between ultrasound intensity and the mechanical parameters, the Spearman rank correlation test was applied. P < 0.05 (two-tailed) was considered statistically significant (Sokal and Rohlf Citation1981).
Results (table)
7 rabbits were discarded from the study: 3 rabbits with hematoma and 4 rabbits with infection around the healing Achilles tendon. The discarded rabbits seemed to be equally distributed between the groups. The extensibility of healing tendons was increased after sonication with an intensity of 2 000 mW/cm2compared to the group sonicated with 50 mW/cm2(p < 0.01). No significant differences were found between the values of load at rupture or normalized load at rupture. The stiffness (i.e. slope of the load-strain curves, and normalized stiffness) decreased significantly, which could be correlated to the gradual increase in ultrasound intensities (Spearman′s R, p = 0.002 and p = 0.020, respectively, ). Likewise, there was an inverse correlation between the collagen content of the Achilles tendons and the intensity of ultrasound treatment (Spearman′s R, p = 0.022, ).
Figure 1 The normalized stiffness of rabbit Achilles tendon tenotomies was studied after healing for 11 days. Pulsating ultrasound at a frequency of 3 MHz and various intensities was applied daily during the healing period. The stiffness was normalized to the amount of collagen per mm tendon length, which was used as a measure of the tendon cross-sectional area. N = Newtons, UC = unit collagen (amount of collagen per mm tendon length). Horizontal bars show median values of normalized stiffness. A gradual decline in normalized stiffness was found with increasing ultrasound intensities. Spearman′s R = -0.325, p < 0.020.
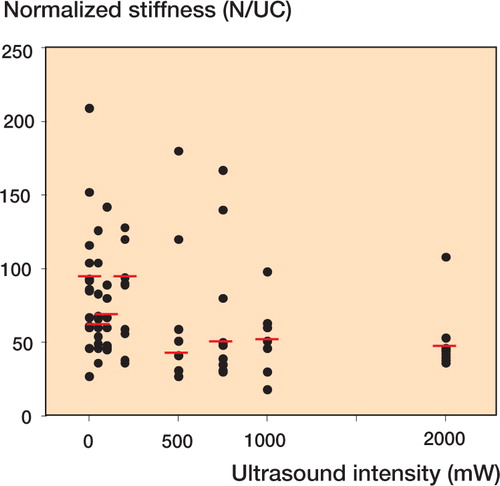
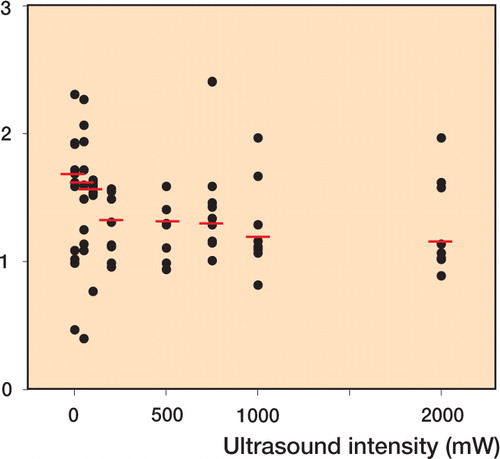
Figure 2 Collagen content per mm tendon length of rabbit Achilles tendon tenotomies was used as a measure of tendon cross-sectional area. Horizontal bars show median values of collagen content. A gradual decline in collagen content was found with decreasing ultrasound intensity. Spearman′s R = -0.320, p < 0.022.
Mechanical properties of healing rabbit Achilles tendons at day 11, after being given pulsed ultrasound (0-2 000 mW, 3 MHz) for 5 min daily. Median values with 95% confidence intervals in parenthesis. N = Newtons. UC = unit collagen (mg collagen/mm tendon)
Discussion
Therapeutic ultrasound is used by physiotherapists in the treatment of a plethora of conditions and is reputed to reduce edema, relieve pain and modify scar formation; but does ultrasound treatment enhance tissue repair processes? The results of experiments on this issue are contradictory. In our study, a range of pulsatile ultrasound intensities, 50–2 000 mW/cm2, at a frequency of 3 MHz was given for 5 min daily, and no significant effect on rupture load and normalized rupture load of the healing tenotomized Achilles tendons was observed at day 11 after the operation. A gradual decline in stiffness and collagen content was, however, observed with increasing ultrasound intensities.
Our results contrast with the findings of Enwemeka (Citation1989a), who, at day 10, found a significantly increased breaking strength and increased energy absorption in rabbit tendons which had been tenotomized, sutured and immobilized. The ultrasound treatment in that investigation was continuous waves at a spatial averaged intensity of 1 000 mW/ cm2, with a frequency of 1 MHz, given for 5 min daily. The cross-sectional area of the healing tendons given ultrasound was found to be higher than that of the mock-sonicated healing tendon specimens. The tensile stress of these specimens was not reported. In another paper, Enwemeka (Citation1989b) also studied the process of inflammation and fibrillogenesis, and the cellular and subcellular events by light and electron microscopy, in tenotomized Achilles tendons in rabbits. Electron micrographs revealed an initial period of inflammation lasting for at least five days, a subsequent period of fibroplasias and fibrillogenesis, and a third period of progressive alignment and organization of the collagen fibrils into bundles that were oriented in the longitudinal axis of the tendon. Also, Enwemeka et al. (Citation1990) showed that lower-intensity ultrasound could influence the healing of tenotomized Achilles tendons in a beneficial way. They gave healing tendons continuous ultrasound waves at a space-averaged intensity of 500 mW/cm2at 1 MHz for 5 min daily over 10 days, which resulted in increased breaking strength and energy absorption capacity of the tendons. In this study of Enwemeka et al. (Citation1990), the ultrasound treatment did not increase the cross-sectional area of the healing tendons relative to that of the mock-sonicated tendons, and the tensile stress values were increased compared to the mock-sonicated healing tendons. Frieder et al. (Citation1988) and Jackson et al. (Citation1991) have also shown a stimulating effect of continuous ultrasound on the healing of partial tendon tenotomy in rats. The intensity of the ultrasound treatment was 1 500 mW/cm2, with a frequency of 1 MHz, applied for 4 min daily, and the tendons were examined at 2, 5, 9, 15 and 21 days after injury for measurement of tendon breaking strength, and at 3 and 5 days post-injury for analysis of collagen synthesis (Jackson et al. Citation1991). The breaking strengths of the ultra-sound-treated tendons were significantly greater than the strengths of the untreated tendons 5, 9, 15 and 21 days post-injury. The cross-sectional area of the tendon specimens was not measured in this study, and therefore, tensile stress values were not reported. Collagen synthesis was elevated in the treated tendons compared with the untreated tendons 5 days after injury. In a recent publication (DaCunha et al. Citation2001), healing tenotomized Achilles tendons of rats were treated with pulsating or continuous ultrasound at an intensity of 500 mW/ cm2, frequency 1 MHz, 5 min daily for 14 days. The pulsating mode resulted in the best organization and aggregation of collagen fiber bundles.
In contrast to the above results, Roberts et al. (Citation1982) found that pulsating ultrasound, intensity 800 mW/cm2, frequency 1.1 MHz, markedly impeded the healing of tenotomized, sutured and immobilized tendons of rabbits. Using mature White Leghorn hens, Stevenson et al. (Citation1986) found that ultrasound treatment with an intensity of 750 mW/cm2had no effect on the breaking strength of repaired profound tendons.
The choice of transducer depends on the depth of the target to be treated; deeper targets require lower frequencies because of the frequency dependence of ultrasonic attenuation. The output may be continuous or pulsed. Pulsed exposures are often chosen when thermal effects are to be kept to a minimum (ter Haar Citation1999). Most ultrasound machines are set at a frequency of 1 or 3 MHz. Low-frequency ultrasound waves have greater depth of penetration, but are less focused. Ultrasound at a frequency of 1 MHz is absorbed primarily by tissues at a depth of 3-5 cm and is therefore recommended for deeper injuries. A frequency of 3 MHz is recommended for more superficial lesions at a depth of 1-2 cm. Consequently, 3 MHz was chosen in the present study.
Traditionally, biophysical effects of ultrasound have been differentiated into thermal and non-ther-mal effects. Thermal effects of ultrasound upon tissues may include increased blood flow, reduction in muscle spasms, increased extensibility of collagen fibres, and a pro-inflammatory response. Excessive thermal effects-seen with higher ultrasound intensities in particular-may damage the tissue. It has been suggested that the non-thermal effects of ultrasound, including cavitation and acoustic microstreaming, are more important in the treatment of soft tissue lesions than are the thermal effects. Cavitation is the formation of tiny gas bubbles in the tissues as a result of ultrasound vibration. Microstreaming is localized liquid flow in the fluid around the vibrating bubbles, and microstreaming is secondary to cavitation. It is still being debated whether cavitation takes place in vivo at the intensities used for therapeutic ultrasound (Baker et al. Citation2001), the hypothesis being that microstreaming alters membrane permeability, resulting in mast cell degranulation, growth factor releases, uptake of calcium ions, increases in protein synthesis, fibroblast proliferation and angiogenesis. These phenomena have been demonstrated to result from ultrasound treatment in vitro, but it is still debatable whether they take place in vivo as a result of therapeutic ultrasound. On the other hand, damage to fibroblasts treated with therapeutic ultrasound has been reported (DeDeyne and Kirsch-Volders Citation1995, Ramirez et al. Citation1997). Although many labo-ratory-based research studies have demonstrated a number of physiological effects of ultrasound upon living tissues, there is remarkably little evidence for beneficial effects in the treatment of soft tissue injuries (Baker et al. Citation2001, Robertson and Baker Citation2001).
Low-intensity ultrasound has the potential to influence bone signaling, bone growth and structure through the strong sensitivity of bone tissue to physical stimuli. Double-blind, prospective, pla-cebo-controlled clinical trials have demonstrated that pulsed, low-intensity, high-frequency (30 mW/ cm2, 1.5 MHz) ultrasound treatment for 20 min daily could reduce the healing time of fractures in the tibial diaphysis and distal radius, both radiographically and clinically (Heckman et al. Citation1994, Kristiansen et al. Citation1997). Likewise, scaphoid fractures may benefit from treatment with low-inten-sity, high-frequency ultrasound (Mayr et al. Citation2000).
The ultrasound regimen used in our study did not improve the mechanical properties of healing rabbit tendons. On the contrary, there was a slight decrease in stiffness with the highest ultrasound intensities.
The authors wish to thank Nordjyllands Amts Forskningsfond, Det Obelske Familiefond, Nordjyske Exogen A/S and Kebo-Care. We are also grateful to E. K. Mikkelsen for skilled technical assistance, and the experimental animal care unit at Aalborg Hospital is also gratefully acknowledged.
No competing interests declared.
- Baker K G, Robertson V J, Duck F A. A review of therapeutic ultrasound: biophysical effects. Phys Ther 2001; 81(7)1351–8
- Cambier D C, Vanderstraeten G G. Failure of therapeutic ultrasound in healing burn injuries. Burns 1997; 23(3)248–9
- DaCunha A, Parizotto N A, Vidal B C. The effect of therapeutic ultrasound on repair of the Achilles tendon of the rat. Ultrasound Med Biol 2001; 27(12)1691–6
- De Deyne P G, Kirsch-Volders M. In vitro effects of therapeutic ultrasound on the nucleus of human fibroblasts. Phys Ther 1995; 75(7)629–34
- Enwemeka C S. The effects of therapeutic ultrasound on tendon healing. Am J Phys Med Rehab 1989a; 68(6)283–7
- Enwemeka C S. Inflammation, cellularity, and fibrillogenesis in regenerating tendon, implications for tendon rehabilitation. Phys Ther 1989b; 69(10)816–25
- Enwemeka C S, Rodriguez O, Mendoza S. The biomechanical effects of low-intensity ultrasound on healing tendons. Ultrasound Med Biol 1990; 16(8)801–7
- Frieder S, Weisberg J, Fleming B, Stanek A. A pilot study: The effect of ultrasound following partial rupture of Achilles tendons in male rats. J Orthop Sports Phys Therapy 1988; 10(2)39–46
- Heckman J D, Ryaby J P, McCabe J, Frey J J, Kilcoyne R F. Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound. J Bone Joint Surg (Am) 1994; 76(1)26–34
- Jackson B A, Schwane J A, Starcher B C. Effect of ultrasound therapy on the repair of Achilles tendon injuries in rats. Med Sci Sports Exerc 1991; 23(2)171–6
- Kristiansen T K, Ryaby J P, McCabe J R N, Frey J J, Roe L R. Accelerated healing of distal radial fractures with the use of specific, low-intensity ultrasound. A multicenter, prospective, randomized, doubleblind, placebo-controlled study. J Bone Joint Surg (Am) 1997; 79(7)961–73
- Mayr E, Rudzki M M, Rudzki M, Borchardt B, Hausser H, Ruter A. Does low intensity, pulsed ultrasound speed healing of scaphoid fractures?. Handchir Mikrochir Plast Chir 2000; 32(2)115–22
- Oxlund H, Manthorpe R, Viidik A. The biomechanical properties of connective tissue in rabbits as influenced by short-term glucocorticoid treatment. J Biomech 1981; 14(3)129–33
- Ramirez A, Schwane J A, McFarland C, Starcher B. The effect of ultrasound on collagen synthesis and fibroblast proliferation in vitro. Med Sci Sports Exerc 1997; 29(3)326–32
- Roberts M, Rutherford J H, Harris D. The effect of ultrasound on flexor tendon repairs in the rabbit. Hand 1982; 14(1)17–20
- Robertson V J, Baker K G. A review of therapeutic ultrasound: effectiveness studies. Phys Ther 2001; 81(7)1339–50
- Sokal R R, Rohlf F J. Biometry. W H Freeman, San Francisco 1981
- Stevenson J H, Pang C Y, Lindsay W K, Zuker R M. Functional, mechanical, and biochemical assessement of ultrasound therapy on tendon healing in the chicken toe. Plast Reconstr Surg 1986; 77(6)965–72
- ter Haar G. Therapeutic ultrasound. Eur J Ultrasound 1999; 9: 3–9
- Woessner J F. Determination of hydroxyproline in connective tissues. The methodology of connective tissue research, D A Hall. Joynson-Bruvvers, Oxford 1976; 227–33