Abstract
Background Bone morphogenetic proteins (BMPs), which are capable of stimulating the production of new bone, must be sterilized before preclinical and clinical use to reduce the risk of infections and associated complications. In this study, we investigated the effects of gamma sterilization on the osteoinductivity of native reindeer BMP extract in the Balb/C mouse thigh muscle pouch model.
Methods 5 mg of native reindeer BMP extract and 5 mg of bovine serum albumin were administered separately either in gelatine capsules or mixed with gelatine as injections. The dose of gamma irradiation was 4.1 MRad. Unsterile capsules and injections served as controls. New bone formation was evaluated based on the incorporation of Ca45and also radiographically 3 weeks after implantation.
Results Albumin-containing implants and injections did not induce new bone formation, as monitored in radiographs. Gamma sterilization did not reduce the osteoinductivity of native BMP extract in capsules, but a significant decrease in osteoinductivity-measured as area (50%) and Ca45incorporation of new bone (27%)-was seen after injection. Gamma sterilization had no effect on the optical density of new bone induced by native BMP extract administered in capsules or by injection.
Interpretation We conclude that, as gamma irradiation did not reduce the osteoinductivity of reindeer BMP extract in gelatine capsules, this method appears to be suitable for sterilization of BMPs to be given in capsule form. Native reindeer BMP extract was more sensitive to irradiation in soluble collagen (gelatine) than BMP in gelatine capsules. This finding must be given serious consideration regarding treatment of patients, but the remaining activity may be sufficient for the induction of bone formation in preclinical and clinical situations.
Bone morphogenetic proteins (BMPs) have been shown to stimulate the production of bone when combined with an appropriate carrier material (Tuominen et al. Citation2000, Kujala et al. Citation2002, Citation2004, Wozney Citation2002, Pekkarinen et al. Citation2003). In clinical situations, it is necessary to sterilize all medical implants and parenteral drug delivery systems prior to surgical placement or injection, to reduce the risk of infections and associated complications. Gamma irradiation and ethylene oxide gas sterilization have been used, but the optimal method for the sterilization of BMPs has been the subject of debate (Munting et al. Citation1988, Wientroub and Reddi Citation1988, Wientroub et al. Citation1990, Aspenberg et al. Citation1990, Aspenberg and Lindqvist Citation1998, Sigholm et al. Citation1992, Ijiri et al. Citation1994, Zhang et al. Citation1997, Andriano et al. Citation2000, Ripamonti et al. Citation2000, Pekkarinen et al. Citation2004). The doses of ethylene oxide that are sufficient for sterilization have a detrimental effect on the osteoinductivity of BMPs (Munting et al. Citation1988, Aspenberg et al. Citation1990, Aspenberg and Lindqvist Citation1998, Pekkarinen et al. Citation2004), whereas gamma irradiation appears to be less harmful in this respect. Most of the reports considering the effects of gamma sterilization on the osteoinductivity of these growth factors have been perfomed on demineralized bone matrix and recombinant BMPs (Munting et al. Citation1988, Wientroub and Reddi Citation1988, Wientroub et al. Citation1990, Zhang et al. Citation1997 Andriano et al. Citation2000, Ripamonti et al. Citation2000). There has only been one paper considering the effects of gamma sterilization on native purified BMP (Ijiri et al. Citation1994).
We investigated the effects of gamma sterilization on reindeer BMP extract administered either as a separate substance in gelatine capsules or mixed with gelatine as injections in the mouse thigh muscle pouch model.
Materials and methods
Preparation of native reindeer (Rangifer tarandus tarandus) BMP extract
Native reindeer BMP extract was prepared from reindeer diaphyseal bone. Cortical bones from each animal were chilled immediately after death. The epiphyseal ends, bone marrow and periosteum were removed mechanically, and after freezing in liquid nitrogen, the cleaned cortical bones were ground to a particle size of 1.0 mm3. The pulverized bone was demineralized in 0.6 M HCl and extracted in 4 M guanidine hydrochloride (GuHCl) at 4°C. The GuHCl-extracted solution was filtered with a tangential flow system and concentrated. The concentrated solution was dialyzed against deionized water, and the water-insoluble material was collected. After re-dissolution in 4 M GuHCl solution, the water-insoluble material was dialyzed against 0.25 M citrate buffer, pH 3.1. The citrate-buffer-insoluble material was washed with deionized water and lyophilized. (Jortikka et al. Citation1993)
Reconstitution of test materials and sterilization
Implants. 5 mg of BMP extract was introduced into each gelatine capsule (no. 1). The control implants contained 5 mg bovine serum albumin (BSA).
Injection. 75 mg BMP extract and 150 mg gelatine (type A from porcine skin, Bloom 300, Sigma, St. Louis, MO) were mixed with 0.9% saline to obtain 1.5 mL homogenized emulsion. 100 μL of this emulsion was used per injection, and each injection thus contained 5 mg BMP extract. The control injections contained 5 mg BSA. The preparation of implants and injections was done aseptically.
Gamma sterilization of the test materials was performed by a specialized company (Isotron Ltd., Swindon, UK). The dose was 4.10 MRad.
Groups
Gelatine capsule + native reindeer BMP (n = 15), non-sterilized BMP group.
Gelatine capsule + native reindeer BMP, irradiated (n = 15), sterilized BMP group.
Gelatine + native reindeer BMP injection (n = 15), non-sterilized BMP/injection group.
Gelatine + native reindeer BMP injection, irradiated (n = 15), sterilized BMP/injection group.
Gelatine capsule + albumin (n = 6), non-steril-ized BSA group.
Gelatine capsule + albumin, irradiated (n = 6), sterilized BSA group.
Gelatine + albumin injection (n = 6), non-steril-ized BSA/injection group.
Gelatine + albumin, irradiated (n = 6), sterilized BSA/injection group.
Implantation and injection techniques
We used male Balb/C mice aged 10–12 weeks, and the administration of capsules and injections was done under neuroleptic analgesia (Hypnorm Janssen, Belgium; Dormicum Roche, Switzerland).
Implantation.Capsules were introduced under sterile conditions into the thigh muscle pouches in the bilateral hind legs. After the implantation, the muscle was closed with 5-0 resorbable sutures, and the skin with 3-0 resorbable sutures.
Injections. 100 μL emulsion was injected under sterile conditions into both thigh muscles using a 1-mL syringe and 20-G needle.
All animals were killed in a chamber with carbon dioxide 21 days later, and the hind legs were harvested (Reddi Citation1981, Jortikka et al. Citation1993). The study protocol was accepted by our institutional Ethics Committee.
Radiographic evaluation of area and density of new bone
After harvest, standard lateral position radiographs (100 mA, 20 kV, 0.08 s/exp; Mamex de Maq, Soredex, Orion, Finland) were taken of all hind legs. The radiographic images were transferred into a computer by using an optical scanner (HP Laserjet/ Desk Scan). New bone formation was evaluated as the area (in mm2) of calcified tissue visible in the radiographs, defined by using Scion Image Beta 4.02 software (Scion Corp., Maryland). The mean optical density (mm Al) of the defined area was measured with the same equipment. Calibration of the equipment for the measurement of optical density was performed using an aluminium wedge.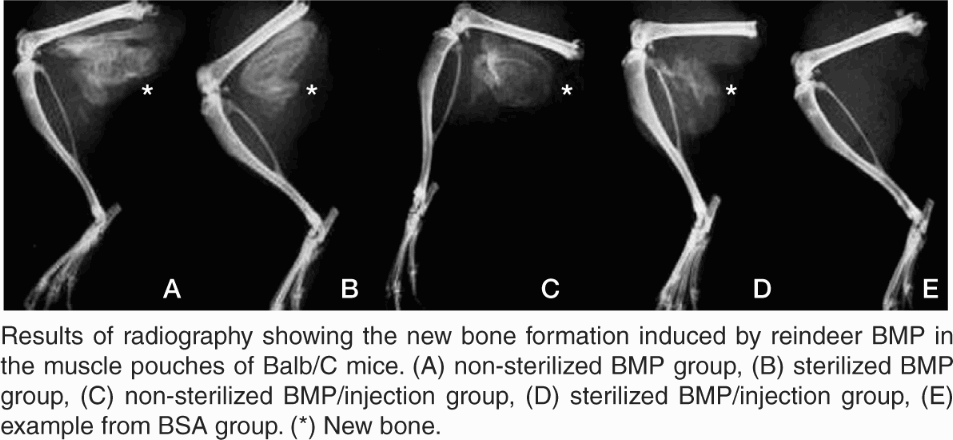
Ca45 activity
24 h before killing, all mice received an intraperitoneal injection of diluted carrier-free Ca45solution (Amersham, UK; 40 μCi/kg of body weight). The muscle tissue of each harvested hind leg, including the implant and the newly formed bone, was taken en bloc for a specimen immediately after the radiography. A piece of intact foreleg muscle was used as reference (10 samples). All specimens were weighed and digested in a mixture of 0.2 mL 70% perchloric acid and 0.4 mL 33% peroxide at 70°C for 3 h. 0.6 mL of the digested solution was pipetted into a diffuse scintillation vial, and 5 mL scintillation cocktail (OptiPhase Hi-safe 3; Wallac, Finland) was added. The samples were counted in a liquid scintillation counter (Wallac 1410, Pharmacia, Finland) with an internal spectrum library. Ca45incorporation into tissue was expressed as DPM/mg tissue.
Statistics
We performed statistical analysis using the SPSS statistical package version 9.0 (SPSS Inc., Illinois). The non-parametric Kruskall-Wallis test was used to evaluate the statistical differences between the groups and the Mann-Whitney test was used for pairwise comparison. Values of p ≤ 0.05 were considered statistically significant.
Results
The injections and implantations were well tolerated by the mice, and no complications occurred during or after the procedures.
Area of new bone formation evaluated radiographically
There was no new radiographically detectable bone formation in the control groups, whereas there was an abundance of new bone in the groups that received BMP extract (, Figure).
. Area and optical density of new bone formation measured from radiographs in the different groups. Mean (SD)
There was no difference in the new bone area between the non-sterilized BMP group and the sterilized BMP group (p = 0.4). A decrease in new bone area could be seen in the sterilized BMP/ injection group compared to the non-sterilized BMP/injection group (p < 0.001) ().
Optical density of new bone formation evaluated radiographically
Because the control groups showed no new bone formation radiologically, their optical density could not be measured. There were no differences in optical density between the non-sterilized and sterilized BMP groups (p = 0.3) or between the non-sterilized and sterilized BMP/injection groups (p = 0.2) ().
Ca45 incorporation
The mean Ca45incorporation was manifold in all groups containing BMP extract compared with the corresponding control groups (p < 0.001) (). There was no difference in Ca45incorporation between the non-sterilized BMP group and the sterilized BMP group (p = 0.7). A decrease in Ca45incorporation could be seen in the sterilized BMP/injection group compared to the non-steril-ized BMP/injection group (p = 0.05).
. Ca45incorporation in different groups. Mean (SD)
Discussion
An important problem in the clinical application of BMPs and their carriers is sterilization. Ethylene oxide gas is used in many protocols, but it reduces the osteoinductive activity of BMPs, and the formation of free radicals during ethylene oxide sterilization is a further cause for concern (Munting Citation1988, Aspenberg et al. Citation1990, Aspenberg and Lindqvist Citation1998, Ijiri et al. Citation1994, Zhang et al. Citation1997, Pekkarinen et al. Citation2004). Many reports have suggested that gamma sterilization is less harmful in these respects, and it would thus be a better alternative for the sterilization of BMPs (Wientroub and Reddi Citation1988, Wientroub et al. Citation1990, Andriano et al. Citation2000, Ripamonti et al. Citation2000).
Wientroub et al. (Citation1988, Citation1990) used allogenic demineralized bone matrix with endogenous native BMPs and reported that samples could tolerate up to 5–7 MRad of gamma irradiation as long as appropriate temperatures (4oC or less) were maintained. Andriano et al. (Citation2000) showed that gamma irradiation at doses of 1.5–2.5 MRad did not reduce the activity of a combination of polymeric and bovine-derived BMPs, and thus appeared to be a promising method for sterilization of BMPs. These authors even reported that irradiation of the polymer matrix actually improved the extent of new bone formation, but this trend was not supported by statistical analysis (Andriano et al. Citation2000). Ripamonti et al. (Citation2000) investigated the effects of gamma irradiaton at doses of 2.5–3.0 MRad on the osteoinductivity of human OP-1, and observed that gamma-irradiated human OP-1 combined with irradiated xenogeneic bovine collagenous matrix carrier is effective in regenerating and maintaining the architecture of induced bone. Our results are, by and large, in accordance with these studies. We have shown that gamma irradiation at a dose of 4.1 MRad does not reduce the osteoinductivity of BMP extract in gelatine capsules.
There have also been reports with results that conflict with ours. Zhang et al. (Citation1997) and Munting et al. (Citation1988) reported that gamma irradiation at a dose of 2.5 Mrad reduced the osteoinductive capacity of demineralized bone matrix (DBM) by about 40–50%. Ijiri et al. (Citation1994) reported that exposure of bovine-derived BMP with type-I collagen carrier to 2.5 MRad of gamma irradiation reduced the activity of BMP to 4.4% of that of the controls. Moreover, these authors suggested that irradiation of bovine type-I collagen carrier alone with 2.5 MRads dramatically reduced the osteoinductivity of non-irradiated bovine-derived BMP released from this carrier. Here, we observed a reduction in the osteoinductivity of BMP extract when it was exposed to 4.1 MRad of gamma irradiation in an injectable mixture with soluble gelatine.
It has been shown that irradiation changes the consistency of collagen. Buring (Citation1970) reported that gamma irradiation in excess of 2.0 MRad increased the solubility of collagen and destroyed the fibrillar network of the bone matrix. It is possible that these changes have harmful effects on BMPs, or that collagen potentiates these deterious effects of irradiation on BMPs by some unknown mechanism.
Currently, the standard dose recommended by the Food and Drug Administration (Rockville, MD) is 2.5 MRad. However, even when we used a larger dose (4.1 MRad) here, BMP maintained its osteoinductivity well. Because the safety requirements allow the use of even lower doses than the one used here, gamma irradiation can be recommended for the sterilization of BMP material for clinical purposes.
We conclude that gamma irradiation does not reduce the osteoinductivity of native reindeer BMP extract in gelatine capsules and is a suitable sterilization method for BMPs administered in this way. Reindeer BMP extract in soluble collagen (gelatine) for injections seemed to be more sensitive to irradiation than BMP in gelatine capsules, and this difference must be given serious consideration. The remaining activity may, however, be sufficient for the induction of bone formation in preclinical and clinical situations.
The authors wish to express their thanks to Mrs. Marja Juustila for her kind assistance in preparation of the implants and the nucleic techniques.
No competing interests declared.
- Andriano K P, Chandrashekar B, McEnery K, Dunn R L, Moyer K, Balliu C M, Holland K M, Garrett S, Huffer W E. Preliminary in vivo studies on the osteogenic potential of bone morphogenetic proteins delivered from an absorbable puttylike polymer matrix. J Biomed Mater Res 2000; 53: 36–43
- Aspenberg P, Lindqvist S B. Ethene oxide and bone induction. Controversy remains. Acta Orthop Scand 1998; 69: 173–6
- Aspenberg P, Johnsson E, Thorgren K G. Dose-dependent reduction of bone inductive properties by ethylene oxide. J Bone Joint Surg (Br) 1990; 72: 1036–7
- Buring K. Ionizing radiation for sterilization of bone. Sterilization and preservation of biological tissues for transplantation. International Atomic Energy Agency, Vienna 1970; 71–8, In:
- Ijiri S, Yamamuro T, Nakamura T, Kotani S, Notoya K. Effect of sterilization on bone morphogenetic protein. J Orthop Res 1994; 12: 628–36
- Jortikka L, Marttinen A, Lindholm T S. Purification of monocomponent bovine morphogenetic protein in a water-soluble form. Ann Chir Gyn 1993; 82: 25–30
- Kujala S, Raatikainen T, Ryhänen J, Kaarela O, Jalovaara P. Composite implant of native bovine bone morphogenetic protein (BMP) and biocoral in the treatment of scaphoid nonunions. A preliminary study. Scand J Surg 2002; 9: 186–90
- Kujala S, Raatikainen T, Ryhänen J, Kaarela O, Jalovaara P. Composite implant of native bovine bone morphogenetic protein (BMP), collagen carrier and biocoral in the treatment of resistant ulnar nonunions: report of five preliminary cases. Arch Orthop Trauma Surg 2004; 1: 26–30
- Munting E, Wilmart J -F, Wijne A, Hennebert P, Delloye C. Effect of sterilization on osteoinduction. Comparison of five methods in demineralized rat bone. Acta Orthop Scand 1988; 59: 34–8
- Pekkarinen T, Lindholm T S, Hietala O, Jalovaara P. New bone formation induced by injection of native reindeer BMP. Scand J Surg 2003; 92: 227–30
- Pekkarinen T, Lindholm T S, Hietala O, Jalovaara P. Influence of ethylene oxide sterilization on the activity of native reindeer bone morphogenetic protein. Int Orthop 2004; 28: 97–101
- Reddi A H. Cell biology and biochemistry on endochondral bone development. Coll Relat Res 1981; 1: 209–26
- Ripamonti U, Van Den Heever B, Crooks J, Tucker M M, Sampath T K, Rueger D C, Reddi A H. Long-term evaluation of bone formation by osteogenic protein-1 in the baboon and relative efficacy of bone-derived bone morphogenetic proteins delivered by irradiated xenogeneic collagenous matrix. J Bone Miner Res 2000; 15: 1798–809
- Sigholm G, Gendler E, McKellop H, Marshall G J, Moore T M, Sarmiento A. Graft perforations favor osteoinduction. Studies of rabbit cortical grafts sterilized with ethylene oxide. Acta Orthop Scand 1992; 63: 177–82
- Tuominen T, Jämsä T, Tuukkanen J, Nieminen P, Lindholm T C, Lindholm T S, Jalovaara P. Native bovine bone morphogenetic protein improves the potential of biocoral to heal segmental canine ulnar defects. Int Orthop 2000; 24: 289–94
- Wientroub S, Reddi A H. Influence of irradiation on the ostoinductive potential of demineralised bone matrix. Calcif Tissue Int 1988; 42: 255–60
- Wientroub S, Weiss J F, Catravas G N, Reddi A H. Influence of whole body radiation on local shielding on matrix-induced endochondral bone differentiation. Calcif Tissue Int 1990; 46: 38–45
- Wozney J M. Overview of bone morphogenetic proteins. Spine 2002; 27(16S)2–8
- Zhang Q, Cornu O, Delloye C. Ethylene oxide does not extinguish the osteoinductive capacity of demineralized bone. A reappraisal in rats. Acta Orthop Scand 1997; 68: 104–8