Abstract
Background We have previously shown that proliferation in primary cultures of human osteoblast-like cells is lower after exposure to synovial fluid from patients with aseptic prosthesis loosening than after exposure to synovial fluid from patients with osteoarthrosis.
Materials and methods Insulin-like growth factors (IGF) I and II and IGF binding proteins (IGFBP) 3–6, were measured with radioimmunoassy in synovial fluid and in serum from patients with aseptic prosthesis loosening or osteoarthrosis. Proliferation in osteoblast-like MG-63 cells was studied with the CyQUANT assay.
Results IGF-I and IGFBP-4 concentrations were lower whereas the concentration of IGFBP-6 was higher in synovial fluids from patients with prosthesis loosening than in synovial fluid from patients with osteoarthrosis. IGF-I concentrations in serum from patients with prosthesis loosening were also lower than in the osteoarthrosis group, and were even below the normal range in most cases (72%). Synovial fluid from patients with aseptic loosening had a weaker stimulatory effect on MG63 osteoblast-like cell proliferation than synovial fluid from patients with osteoarthrosis, but there was no difference between the two groups when a human IGF-I antibody was added.
Interpretation Low levels of IGF-I in synovial fluid possibly result from low serum levelsand may be a mechanism leading to aseptic prosthesis loosening.
We have previously shown that synovial fluid from patients undergoing revision surgery after total hip arthroplasty due to aseptic prosthesis loosening has an inhibitory effect on normal human osteoblast-like cell proliferation, and that synovial fluid from patients with osteoarthrosis has a stimulatory effect (Andersson et al. Citation2000). These observations imply that synovial fluid may play a role in the pathogenesis of aseptic loosening. Other studies on cytokines in synovial fluid (Sabokbar and Rushton Citation1995) and periprosthetic pseudomembranes (Kim et al. Citation1993, Chiba and Rubash Citation1994, Atkins et al. Citation1997, Matsui et al. Citation1997) also suggest that the characteristics of the synovial fluid may be important for aseptic loosening.
Insulin-like growth factors (IGF) I and II are important for formation and growth of bone, and are produced in many tissues, including bone itself (Bautista et al. Citation1991, Mohan and Baylink Citation1991). The biological effect of IGFs is regulated by 6 IGF binding proteins (IGFBP) (Shimasaki and Ling Citation1991, Hwa et al. Citation1999). The most prevalent IGFBPs in human bone are IGFBP-3, 4, 5 and 6 (Malpe et al. Citation1997). IGFBP-3 generally has a stimulatory effect on bone cells (Schmid et al. Citation1996), while IGFBP-4 usually inhibits the stimulatory effect of IGFs (Schlitz et al. Citation1993, Mohan et al. Citation1995b). IGFBP-5 mainly enhances the effects of IGF on both bone in animal models and normal human osteoblasts (Schmid et al. Citation1995). However, in a study published by Kiefer et al. (Citation1993) IGFBP-5 reduced IGF-induced DNA synthesis in osteoblast-like cells. The effects of IGFBP-6 on bone are not as well characterized, but it is generally regarded to have mainly inhibitory effects (Srinivasan et al. Citation1996, Yan et al. Citation2001). Thus, the regulatory effects of IGFBPs on IGF activity are complex and pleiotropic. Since the levels of IGFs and IGFBPs are altered in synovial fluid from patients with osteoarthrosis (Schneiderman et al. Citation1995, Kanety et al. Citation1996, Matsumoto et al. Citation1996), IGFs and IGFBPs in synovial fluid may be important for periarticular bone turnover in joint disease.
We determined the levels of IGF-I and -II and IGFBP 3–6 in synovial fluid and IGF-I in serum from patients with aseptic prosthesis loosening undergoing revision surgery. Furthermore, we investigated the specific role of IGF-I in synovial fluid-induced osteoblast proliferation.
Material and methods
Synovial fluid and serum samples
Synovial fluid samples were obtained from 14 patients who underwent revision total hip arthroplasty due to aseptic prosthesis loosening and who had primary surgery for osteoarthrosis (). All the cases had radiographic periprosthetic bone loss varying from grade 1 to 3 according to the Endo Clinic classification (Engelbrecht and Heinert Citation1987). These samples were compared with synovial fluid from 12 patients who underwent total hip arthroplasty because of primary advanced osteoarthrosis. Synovial fluid samples were aspirated intraoperatively and before incision of the joint capsule. All samples were centrifuged and aliquoted before storage at –70°C. Serum samples were obtained from 18 patients undergoing revision surgery and 35 patients with osteoarthrosis (). These patients had the same inclusion criteria as was the case with those who donated synovial fluid. The serum samples were collected prior to surgery, centrifuged and immediately stored at –70°C. Before analysis, the samples were dissolved in acetic acid, and subjected to acid gel filtration using the Biospin protocol as previously published to remove IGFBP artifacts (Mohan and Baylink Citation1995). The ethical committee of Karolinska University Hospital approved the study.
Table 1. Clinical data regarding patients with osteoarthrosis of the hip and patients undergoing revision total hip arthroplasty, from whom serum and synovial fluid was obtained. There was no significant age difference between the two groups. Numbers are mean (SEM)
Radioimmunoassay for IGFs and IGFBPs
Before radioimmunoassay, the IGF pool was neutralized by addition of 0.05 mL of 1.2 M Tris base. IGF-I was measured using recombinant human IGF-I (a gift from Ciba-Geigy, Basel, Switzerland) as tracer and standard, and rabbit polyclonal antiserum (obtained from the National Hormone and Pituitary Program, Baltimore, MD) as described earlier (Mohan et al. Citation1990). IGF-II was determined using human IGF-II (Bachem Chemicals, Torrance, CA) as standard and tracer, and a monoclonal antibody against IGF-II (Amana International, Troy, VA) (Mohan et al. Citation1990). IGFBP-3 was measured using rabbit polyclonal antibody against human IGFBP-3 (a gift from Dr. V. Mukku, Gentech, SouthSan Francisco, CA) and recombinant human IGFBP-3 (a gift from Dr. A. Sommer, Celtrix Corp., Palo Alto, CA) as tracer and standard as described earlier (Mohan and Baylink Citation1995, Kanety et al. Citation1996). IGFBP-4 was determined using guinea pig antibodies and recombinant human IGFBP-4 (expressed in E. coli) as standard and tracer as previously described (Mohan and Baylink Citation1995, Honda et al. Citation1996). IGFBP-5 was measured using guinea pig antibody against recombinant human IGFBP-5 together with recombinant human IGFBP-5 (a gift from Drs. C. Dony and K. Lang, Boehringer Mannheim, Mannheim, Germany) as standard and tracer (Mohan and Baylink Citation1995, Mohan et al. Citation1995a). IGFBP-6 was determined using guinea pig antibodies and recombinant human IGFBP-6 as standard and tracer (Yan et al. Citation2001). 125I for radiolabeling was purchased from the International Chemical and Nuclear Corporation (Irvine, CA).
Cell culture and proliferation assay
MG63 human osteosarcoma cells were grown in Dulbecco's Modified Eagle's Medium (DMEM) containing penicillin (100 U/mL), streptomycin (100 μg/mL), fungizone (0.25 μg/mL) and 10% fetal bovine serum (Gibco, Grand Island, NY). The cells were incubated at 37°C in 5% CO2 with saturated humidity. After reaching subconfluency, the cells were detached with trypsin-EDTA (Gibco) and plated at a density of 1000 cells/well in 96-well plates in serum-free DMEM supplemented with 0.1% bovine serum albumin (Gibco). The cells were then cultivated for 24 h before addition of synovial fluids etc. After another 24 h of incubation, the cell medium was removed and the plates were stored at −70°C. Cell proliferation was measured with the CyQUANT cell proliferation assay kit (Molecular Probes, Eugene, OR).
To evaluate the role of IGF-I in synovial fluid-induced cell proliferation, MG63 cells were incubated for 24 hours with 10% pooled synovial fluid from the osteoarthrosis group or 10% pooled synovial fluid from the revision surgery group, or with 10 ng/mL recombinant human IGF I. The experiments were performed in the presence or absence of an antibody against human IGF-I at a concentration of 50 μg/mL (Sigma-Aldrich, Stockholm, Sweden).
Statistics
The data are presented as mean (SEM). Student's t-test was used to determine statistical significance. P-values less than 0.05 were considered to be significant. We used Sigma-Stat software (Jandel Corp., Sausalito, CA) for all calculations.
Results
Patient characteristics ()
There was no significant difference in age or body mass index (data not shown) between patients with osteoarthrosis and patients with prosthetic loosening. No bacterial growth was found in the synovial fluid samples from the patients with prosthetic loosening.
IGF-I, -II and IGFBP 3–6 levels in synovial fluid and serum ()
IGF-I and IGFBP-4 concentrations were significantly lower, whereas IGFBP-6 was higher in synovial fluid from patients undergoing revision surgery than in synovial fluid from patients with osteoarthrosis. The serum levels of IGF-I followed the same pattern, with a significantly lower IGF-I level in serum from patients undergoing revision surgery than in serum from patients with osteoarthrosis. In addition, most patients in the revision group had serum IGF-I levels below the normal range. There were no differences in IGF-II, IGFBP-3 and 5 levels in synovial fluid between the two patient groups.
Table 2. Concentrations of IGFs and IGFBPs (ng/mL) in synovial fluid from patients with osteoarthrosis of the hip and from patients undergoing revision total hip arthroplasty. Numbers are mean (SEM)
Table 3. Serum levels of IGF-I in patients with osteoarthrosis of the hip and in patients undergoing revision total hip arthroplasty. Numbers are mean (SEM)
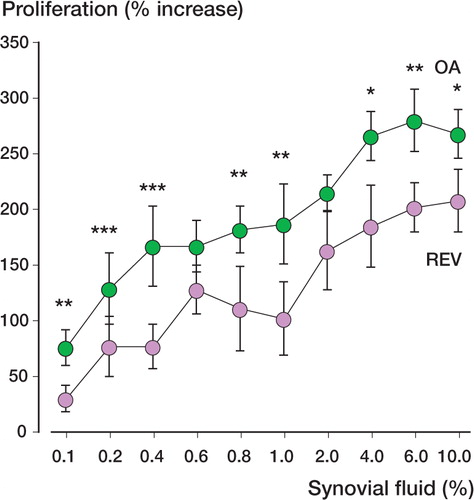
Synovial fluid-induced cell proliferation ()
Proliferation was significantly higher in MG63 cells incubated with 0.1–10% synovial fluid from patients with osteoarthrosis than in cells treated with the same concentrations of synovial fluid from patients undergoing revision surgery. The experiment shown represents 1 of 8 performed with synovial fluid from different patients, with similar results.
Figure 1. Proliferation in osteoblast-like MG63 cells exposed to 2 typical synovial fluid samples for 24 hours (8 cell culture wells), one from a patient with aseptic prosthesis loosening undergoing revision surgery (REV) and one from a patient with osteoarthrosis (OA). P-values for comparison between the two patient groups were between < 0.001 and 0.03 (* = p < 0.05, ** = p < 0.01, *** = p < 0.001), except for 0.6% and 2.0% synovial fluid (p = 0.2 and p = 0.1, respectively). Bars indicate SEM.
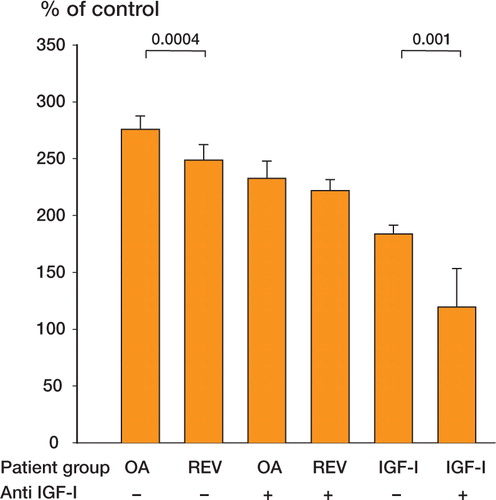
Role of IGF-I in cell proliferation induced by synovial fluid ()
The stimulatory effect of 10% pooled synovial fluid from 7 patients with osteoarthrosis on MG63 proliferation was significantly higher than that of 10% pooled synovial fluid from 7 patients with aseptic prosthesis loosening undergoing revision surgery. When human IGF-I antibody was added, there was no significant difference between the two groups. Human recombinant IGF-I at 10 ng/mL also had a significant stimulatory effect on MG63 proliferation compared to unstimulated cells and this effect was also inhibited by human IGF-I antibody. The experiment shown represents 1 of 2, both of which gave similar results.
Figure 2. Changes in cell proliferation in MG63 cells (8 cell culture wells) incubated for 24 hours with 10% pooled synovial fluid from 7 patients with osteoarthrosis (OA), or from 7 patients with aseptic prosthesis loosening undergoing revision surgery (REV). The experiments were conducted in the presence or absence of 50 μg/mL human IGF-I antibody. Without the antibody there was a difference between the two patient groups, but there was no difference between the two groups in the presence of the antibody (p = 0.1). Proliferation was also inhibited by human IGF-I antibody in cells incubated with 10 ng/mL IGF-I. Bars indicate SEM.
Discussion
Synovial fluid from patients with aseptic prosthesis loosening had a significantly lower content of IGF-I and IGFBP-4, but a higher level of IGFBP-6 than synovial fluid from patients with osteoarthrosis. Synovial fluid from patients with prosthesis loosening had a weaker proliferation-inducing effect on MG63 osteoblast-like cells than synovial fluid from patients with osteoarthrosis. Since there was no significant difference in proliferation when IGF-I antiserum was added, we suggest that the lower levels of IGF-I in the prosthesis loosening group account for the weaker effect. Both IGFBP-4 and IGFBP-6 mainly inhibit the effect of IGF-I. The lower IGFBP-4 content in synovial fluid from subjects with prosthesis loosening may therefore be compensated by a correspondingly higher concentration of IGFBP-6, which implies that the relative ratio of inhibitory to stimulatory IGFBP may not be different between the two patient groups. In addition to IGF-I, other cytokines and growth factors may also be important for synovial fluid-induced osteoblast proliferation (Sabokbar and Rushton Citation1995), since proliferation increased also in the presence of IGF-I antiserum. The comparison in this study between synovial fluid from loose prostheses and true synovial fluid from joints with osteoarthrosis is an intriguing issue. The former type of joint does not have the same type of biologically active surface as the later. Thus, it would be interesting to also study synovial fluid from prostheses without loosening, but the acquisition of such samples is controversial.
We did not study the exact in vivo role of IGF-I in synovial fluid on bone turnover. However, synovial fluid should reach the periprosthetic bone through discontinuities in the pseudomembrane around a loose prosthesis. We therefore speculate that a relative lack of IGF-I in synovial fluid could be important for the net loss of bone matrix around prostheses with aseptic loosening. Since IGF-I appears to have a key role for the difference of effect of synovial fluid on osteoblasts, serum IGF-I was also measured. Somewhat unexpectedly, the serum IGF-I levels were not only significantly lower in patients with aseptic loosening than in the osteoarthrosis group, but they were also below the normal range in most cases. The low IGF-I serum levels in patients with prosthesis loosening were probably not due to malnutrition, because there was no difference in body mass index between the two patient groups. These observations and the similarity between the levels of IGF-I in serum and synovial fluid in numerical terms indicate that the subnormal serum levels of IGF-I in these patients may induce decreased bone formation around the prosthesis via the synovial fluid. This possible mechanism, together with other events leading to increased bone resorption, could explain some of the pathophysiology in aseptic prosthesis loosening. IGF-I has been suggested to play a role in several pathological conditions (Juul Citation2003), including for example growth hormone disturbances, osteoporosis and hepatic disorders. In comparison to such disease, aseptic loosening may appear to have more “focal” than systemic characteristics. Our finding of low serum IGF-I in this condition suggests the opposite. Whether it is a low systemic IGF-I level that induces aseptic loosening, or the opposite, is unknown.
The key finding of this study is that the IGF-I concentration is lower in synovial fluid and serum from patients with aseptic prosthesis loosening than in subjects with osteoarthrosis. The lower stimulatory effect of synovial fluid on osteoblast proliferation in the aseptic loosening group seems to be dependent on IGF-I. Prospective studies of serum IGF-I should be performed to determine whether patients who subsequently develop prosthesis loosening are in a catabolic condition, with low serum IGF-I levels already at primary surgery, and are thus susceptible to future loosening of the prosthesis.
We are grateful to Mr. Daniel Brucht and Mrs. Barbro Granberg for excellent technical assistance. This work was supported by grants from the Sven Norén Foundation, the Foundation of Karolinska Institutet and the Swedish Society of Medicine.
- Andersson M K, Anissian L, Stark A, Bucht E, Fellander-Tsai L, Tsai J A. Synovial fluid from loose hip arthroplasties inhibits human osteoblasts. Clin Orthop 2000; 378: 148–54
- Atkins R M, Langkamer V G, Perry M J, Elson C J, Collins C M. Bone-membrane interface in aseptic loosening of total joint arthroplasties. J Arthroplasty 1997; 12: 461–4
- Bautista C M, Baylink D J, Mohan S. Isolation of a novel insulin-like growth factor (IGF) binding protein from human bone: a potential candidate for fixing IGF-II in human bone. Biochem Biophys Res Commun 1991; 176: 756–63
- Chiba J, Rubash H E. Letter to the editor. Clin Orthop 1994; 309: 278
- Engelbrecht E, Heinert K. Klassifikation und Behandlung-asrichtlinien von Knochensubstanzverlusten bei Revision-soperationen am Huftgelenk. Hrsg Endoklinik Hamburg. Springer-Verlag, Berlin 1987; 190–201
- Honda Y, Landale E C, Strong D D, Baylink D J, Mohan S. Recombinant synthesis of insulin-like growth factor-binding protein-4 (IGFBP-4): Development, validation, and application of a radioimmunoassay for IGFBP-4 in human serum and other biological fluids. J Clin Endocrinol Metab 1996; 81: 1389–96
- Hwa V, Oh Y, Rosenfeld R G. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev 1999; 20: 761–87
- Juul A. Serum levels of insulin-like growth factors I and its binding proteins in health and disease. Growth Horm IGF Res 2003; 13: 113–70
- Kanety H, Shimon I, Ehrenfeld M, Israeli A, Pariente C, Karasik A. Insulin-like growth factor I and its binding proteins 3 and 4 are increased in human inflammatory synovial fluid. J Rheumatol 1996; 23: 815–8
- Kiefer M C, Schmid C, Waldvogel M, Schlapfer I, Futo E, Masiarz F R, Barr P J, Zapf J. Recombinant human insulin-like growth factor binding proteins 4, 5, and 6: biological and physiochemical characterization. Growth Regul 1993; 3: 56–9
- Kim K J, Rubash H E, Wilson S C, D'Antontio J A, McClain E J. A histologic and biochemical comparison of interface tissues in cementless and cemented hip prostheses. Clin Orth 1993; 287: 142–52
- Malpe R, Baylink D J, Linkhart T A, Wergedal J E, Mohan S. Insulin-like growth factor (IGF)-I, -II, IGF binding proteins (IGFBP)-3, -4, and -5 levels in the conditioned media of normal human bone cells are skeletal site-dependent. J Bone Miner Res 1997; 12: 423–30
- Matsui H, Shimizu M, Tsuji H. Cartilage and subchondral bone interaction in osteoarthrosis of human knee joint: a histological and histomorphometric study. Microsc Res Tech 1997; 37: 333–42
- Matsumoto T, Gargosky S E, Iwasaki K, Rosenfeld R G. Identification and characterization of insulin-like growth factors (IGFs), IGF-binding proteins (IGFBPs), and IGFBP proteases in human synovial fluid. J Clin Endocrinol Metab 1996; 81: 150–5
- Mohan S, Baylink D J. Growth factors. Clin Orthop 1991; 263: 30–8
- Mohan S, Baylink D J. Development of a simple valid method for the complete removal of insulin-like growth factor (IGF)-binding proteins from IGFs in human serum and other biological fluids: comparison with acid-ethanol treatment and C18 Sep-Pak separation. J Clin Endocrinol Metab 1995; 80: 637–47
- Mohan S, Bautista C M, Herring S J, Linkhart T A, Baylink D J. Development of valid methods to measure insulin-like growth factors-I and -II in bone cell-conditioned medium. Endocrinology 1990; 126: 2534–42
- Mohan S, Libanati C, Dony C, Lang K, Srinavasan N, Baylink D J. Development, validation, and application of a radioimmunoassay for insulin-like growth factor binding protein-5 in human serum and other biological fluids. J Clin Endocrinol Metab 1995a; 80: 2638–45
- Mohan S, Nakao Y, Honda Y, Landale E, Leser U Dony C, Lang K, Baylink D J. Studies on the mechanisms by which insulin-like growth factor (IGF) binding protein-4 (IGFBP-4) and IGFBP-5 modulate IGF actions in bone cells. J Biol Chem 1995b; 270: 20424–31
- Sabokbar A, Rushton N. Role of inflammatory mediators and adhesion molecules in the pathogenesis of aseptic loosening in total hip arthroplasties. J Arthroplasty 1995; 10: 810–6
- Schiltz P M, Mohan S, Baylink D J. Insulin-like growth factor binding protein-4 inhibits both basal and IGF-mediated chick pelvic cartilage growth in vitro. J Bone Miner Res 1993; 8: 391–6
- Schmid C, Schlapfer I, Keller A, Waldvogel M, Froesch E R, Zapf J. Effects of insulin-like growth factor (IGF) binding proteins (BPs) -3 and -6 on DNA synthesis of rat osteoblasts: further evidence for a role of auto-/paracrine IGF I but not IGF II in stimulating osteoblast growth. Biochem Biophys Res Commun 1995; 212: 242–8
- Schmid C, Schlapfer I, Gosteli-Peter M A, Froesch E R, Zapf J. Effects and fate of human IGF-binding protein-5 in rat osteoblast cultures. Am J Physiol 1996; 271: 1029–35
- Schneiderman R, Rosenberg N, Hiss J, Lee P, Liu F, Hinz R L, Maroudas A. Concentration and size distribution of insulin-like growth factor-I in human normal and osteoarthritic synovial fluid and cartilage. Arch Biochem Biophys 1995; 324: 173–88
- Shimasaki S, Ling N. Identification and molecular characterization of insulin-like growth factor binding proteins (IGFBP-1, -2, -3, -4, -5 and -6). Prog Growth Factor Res 1991; 3: 243–66
- Srinivasan N, Edwall D, Linkhart T A, Baylink D J, Mohan S. Insulin-like growth factor-binding protein-6 produced by human PC-3 prostate cancer cells: isolation, characterization and its biological action. J Endocrinol 1996; 149: 297–303
- Yan T, Wegedal, Zhou Y, Mohan S, Baylink D J, Strong D D. Inhibition of human osteoblast marker gene expression by retinoids is mediated in part by insuline-like growth factor binding protein-6. Growth Horm IGF Res 2001; 11: 368–77
