Abstract
Background: Hyaluronan (HA) is a glycosaminoglycan with viscoelastic properties necessary for vocal fold (VF) vibration and voice production. Changes in HAs molecular mass, possibly related to human papilloma virus, could affect formation/persistence of recurrent respiratory papillomatosis (RRP).
Aims/Objective: Describing mass and localization of HA and localization of HA receptor CD44 in VF and false vocal folds (FVF) in RRP.
Materials and Methods: Biopsies from VF and FVF from 24 RRP patients. Twelve were studied with histo-/immunohistochemistry for HA and CD44 in epithelium, stroma and RRP lesions. Twelve samples were analyzed for HA molecular mass distribution with gas-phase-electrophoretic-molecular-mobility-analyzer (GEMMA).
Results: Three of 23 stains (VF and FVF combined) showed faint HA staining in the epithelium; there was more extensive staining in the stroma. CD44 was present throughout all areas in FVF and VF, it did not concur with HA. GEMMA analysis revealed very high mass HA (vHMHA) with more varying amounts in VF.
Conclusions/Significance: HA was mainly distributed in the stroma. CD44 not binding to HA might explain the non-inflammatory response described in RRP. Possibly crosslinked vHMHA was seen in VF and FVF, with more variable amounts in VF samples. Counteracting HA crosslinking could become a treatment option in RRP.
Chinese abstract
背景:透明质酸(HA)是一种糖胺聚糖, 具有声带(VF)振动和发声所必需的粘弹性。HA 分子量的变化可能与人乳头瘤病毒相关, 还可能影响复发性呼吸道乳头状瘤病(RRP)的形成或持续。
目的:描述HA的量和定位, HA受体CD44在VF中的定位和假性声带(FVF)在RRP中的定位。
材料和方法:24名RRP患者的VF和FVF的活组织检查。用组织/免疫组织化学方法研究12个样品的上皮、基质和RRP病变中的HA和CD44。用气-相-电泳 - 分子-迁移率-分析仪(GEMMA)分析另12个样品的HA分子量分布。
结果:23个染色中的3个(VF和FVF组合)在上皮细胞中显示出微弱的HA染色;基质中有更强的染色。 CD44存在于FVF和VF的所有区域, 它与HA不同时存在。 GEMMA分析显示非常高量的HA(vHMHA), 它在VF中的量多变。
结论/意义:HA主要分布在基质中。 CD44不与HA接合可能解释所描述的RRP中的非炎症反应。在VF和FVF中观察到可能交合的vHMHA, 而在VF样品中具有更多的变量。抗HA交合可能成为RRP的治疗选择。
Introduction
The structurally well-defined vocal folds (VF) can be exposed to various negative effects and strenuous events such as vocal abuse, tobacco smoke, air pollution and infections.
Vocal fold are usually infected by common cold viruses. In rare cases, specific human papilloma virus (HPV) genotypes persistently infect the VF, which is a prerequisite for the development of recurrent respiratory papillomatosis (RRP). This condition is characterized by benign wart-like lesions in the upper airway affecting voice- and respiratory function, commonly throughout life [Citation1]. Currently, there are no curative treatment options for RRP. For symptom control, patients are treated with non-curative surgery. Two HPV subtypes are associated with RRP: HPV 6 and 11. These HPV subtypes exhibit a tropism for the VF, implying pronounced morbidity due to voice impairment and airway obstruction [Citation1]. Despite RRPs tropism for the VF, Forslund et al. [Citation2] were able to show the presence of HPV mRNA in adjacent, macroscopically healthy false vocal folds (FVF) in 50% of their patients. However, overall viral load was higher in the RRP lesions than in macroscopically healthy adjacent FVF.
Three well defined layers constitute the VF: the epithelium, the subepithelial space or lamina propria and the muscular layer. In harmonic voice production, optimal viscoelasticity of the extracellular matrix in the subepithelial layer is essential. Besides viral infections, voice production can be affected by different changes in the composition of the extracellular matrix in VF, which, for example, can be observed with aging of the VF [Citation3].
Hyaluronan (HA) is a high molecular weight glycosaminoglycan (GAG) and a major component of loose connective tissue. Due to its viscoelastic properties, it contributes to the normal glottic wave [Citation4]. There seems to be a gender-based difference in the concentration of HA, since females have higher HA concentration in their VF as compared with men [Citation4].
An exceptionally wide range of biological functions have been attributed to HA, including for example cell proliferation and mitosis, cell recognition, influence on morphogenesis and differentiation, locomotion, interaction with inflammatory cells, and wound healing.
The general ability of HA to co-regulate healing processes, inflammation, and cell proliferation [Citation5] could be of importance in RRP. High molecular mass HA (HMHA) (commonly defined as >1000 kDa) has been attributed anti-angiogenic, anti-inflammatory and immunosuppressive properties [Citation6], whereas low molecular mass HA (LMHA) has been suggested as a substance which activates dendritic cells and thereby elicits the opposite effects [Citation6]. HA accumulates in adenocarcinomas including breast cancer, lung cancer and ovarian cancer [Citation7], and increased levels of HA as well as hyaluronidases have been shown in the saliva of patients with squamous cell cancer of the head and neck [Citation8]. Higher levels of HA are associated with tumor progression [Citation7].
Several HA receptors have been identified, among which CD44 is the main one [Citation9]. CD44 is involved in many biological functions including angiogenesis, tumor invasion, and metastasis [Citation9].
In human VF, only a few reports on the occurrence and distribution of HA are available [Citation4,Citation10]. In these reports, materials and methods differ, the small sample sizes affect the reliability of the study outcome, and the findings have been contradictory. Thus, additional studies are needed.
A report comparing laryngeal cancer samples with normal laryngeal tissue from autopsies found a significant decrease in high-molecular HA and a simultaneous increase of low-molecular HA in laryngeal cancer [Citation11].
Studies on the HA concentration, molecular mass, and organization in RRP patients can provide insights into RRP pathogenesis, which could help to identify potential targets in medical treatment of this condition.
It is important to study HA in VF since it is abundant in the VF and essential for voice production [Citation4]. Altered molecular weight of HA (such as seen in cancer [Citation7]) alters biological function of the molecule which in return could affect the course of RRP and voice production. Current treatment strategies of RRP today mostly rely on surgical debulking. It is of great importance to study antiviral treatment of RRP but even other treatment options and possibilities to influence factors that could be important in disease formation, such as HA, need to be studied.
The purpose of this investigation was to examine if there is a difference in HA expression in VF and FVF in light of the fact that RRP lesions have tropism for the VF [Citation1] despite presence of HPV in both VF and FVF [Citation2].
Our specific aim was to determine HA molecular mass and localization, as well as expression of its receptor CD44 in the VF compared to FVF.
We hypothesize that low-molecular HA is present in RRP patients as it is present in malignant conditions. Varying amounts of low-molecular HA could be part of the explanation for the fact that some patients exhibit a more aggressive course of their disease than others.
Material and methods
Study population
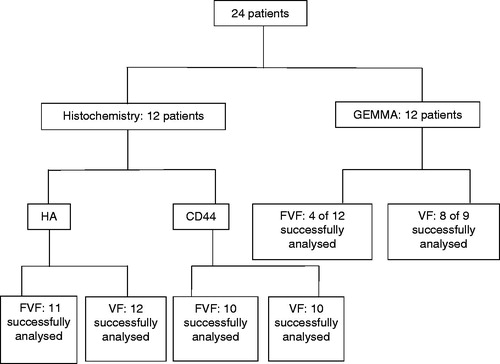
This is a descriptive, observational, case-based study on 24 RRP patients diagnosed at a tertiary hospital. Patients with established RRP and newly diagnosed patients were included. The Department of Otorhinolaryngology, University Hospital of Umeå, Sweden, provides clinical assessment and surgical treatment for RRP patients from a population of ∼900,000 inhabitants. The sample size was determined by access of RRP patients during the study time between September 2015 and November 2016. Written informed consent was obtained and none of the patients admitted during the inclusion period refrained from participating in the study. Twenty-four patients with RRP that were surgically treated at the Department of Otorhinolaryngology, University Hospital of Umeå, Sweden between September 2015 and November 2016 were included in the study. No power calculation was performed. At sampling, the 24 patients had a mean age of 40 (range 2–74), with 18 males (mean age 43 years) and 6 females (mean age 29 years). The cut-off point for juvenile onset RRP versus adult onset RRP was defined as onset at 18 years or younger. None of the eligible subjects smoked, and none of the juvenile RRP patients were premature at birth. Patients were included regardless of occurrence of allergy, gastroesophageal reflux disease (GERD), or asthma. The detailed outline of the patient characteristics is provided in .
Table 1. Sample characteristics of RRP patients, biopsy site: 1: false vocal folds, 2: vocal folds.
Surgery
The indications for surgery were the following: subjective severity of symptoms (hoarseness and mild airway obstruction) in patients with known RRP, and biopsy acquisition for objective histopathological verification of RRP in newly diagnosed patients. Surgery of the RRP lesions was performed under general anesthesia. One sample, sized 1–2 mm, of macroscopically healthy mucosa was taken from the FVF, and one sample of equal size was taken from the macroscopically RRP affected VF. Thereafter the RRP lesions were removed using an AcuPulse Lumenis CO2 laser (MikronMed). All therapeutic interventions were performed by one phoniatrician/otolaryngologist specialized and responsible for the RRP treatment at our hospital.
In three cases, the VF were not macroscopically affected. For these cases, biopsies were only taken from the FVF, since it was considered to be not ethically motivated to potentially traumatize macroscopically healthy VF in a lifelong surgically demanding treatment condition.
Randomization to GEMMA/histochemistry
Twelve of the 24 patients were randomly selected to be analyzed for HA molecular mass distribution with a gas-phase electrophoretic molecular mobility analyzer (GEMMA), whereas the remaining 12 were studied with histochemistry staining for HA and CD44. In the GEMMA group, biopsies from the VF were available in nine cases since three samples were not acquired due to the fact that the VF were macroscopically unaffected (patient flowchart, ).
Fixation, embedding and staining
Biopsies were immediately frozen and stored at –80 °C. Before embedding in paraffin, samples were fixed in 4% buffered formaldehyde for 48 h in room temperature. Paraffin sections were cut (4 µm) and then mounted on Superfrost Plus Slides (ThermoFisher Scientific Inc., MA) and dried overnight at 37 °C.
Immunohistochemistry for CD44
The slides were stained for CD44 expression using an automated Ventana Benchmark staining machine (Ventana Medical Systems). Citrate buffer at pH 8.0 for 8 min at 94 °C and EDTA buffer for 4 min at 100 °C were applied for antigen retrieval.
Thereafter, the primary antibody CD44 monoclonal IgG2a, diluted 1/100 (MA5-13890, ThermoFisher Scientific) was applied, followed by incubation for 32 min. The slides were then counterstained in a Ventana Benchmark staining machine with hematoxylin and bluing reagent (lithium carbonate).
Secondary antibodies part of AEC Detection Kit (760-020) for Ventana BenchMark XT (Ventana Medical Systems, Inc, Roche), were used for detection.
Human skin was used as positive control. Staining without primary antibody was used as negative control.
Histochemistry for HA
Slides were deparaffinized in xylene, rehydrated in ethanol, washed in phosphate-buffered solution (PBS) and incubated with a solution of 3% H2O2 in methanol for 5 min. Thereafter, slides were washed in distilled water and in PBS and incubated with bovine serum albumin (10 mg/mL) for 30 min to block non-specific binding sites. Control slides were pre-incubated with streptomyces hyaluronidase 50 units/ml (Sigma, St. Louis, MO), for 4 h at 37° C, which specifically degrades HA, serving as a control to guarantee specificity of the method. After washing in PBS, slides were incubated with a HA binding protein probe (HABP) (1:40 dilution) at 4 °C overnight. After cleansing slides in PBS 2 × 10 min, they were incubated with Vectastain-Elite Avidin-Biotin complex reagent (Vector Laboratories, Burlingame, CA) for 40 min, then washed 3 × 10 min in PBS and incubated for 5 min in a solution of 3,3′-diaminobenzidine (Vector Laboratories). Following a wash in tap water, the sections were counterstained with Mayer’s hematoxylin.
Histochemistry evaluation
Three of the authors independently evaluated the histological slides using the same scanned microscopic images, thereafter consensus was reached for each sample grade. The authors were blinded during evaluation. In the HA slides, staining intensity was scored in the epithelium, stroma and papilloma tissue using a modified grading scale by Opheim et al. [Citation10] designed to semiquantify the HA staining intensities with grading 0: no staining, 1: faint staining and 2: intense staining. In the CD44 slides, two parameters were evaluated for epithelium, stroma and RRP lesions respectively: The first was presence of CD44 (0: no stain, 1: visible staining) and the second congruence with HA staining (plus (+): congruence, minus (−): no congruence).
Isolation and purification of HA for GEMMA
To isolate HA from tissue samples, a slightly modified protocol from Tolg et al. [Citation12] was adopted. Tissue samples, wet weight 23–41.7 mg, were dried using a vacuum rotary evaporator. When the tissues were completely dry, they were homogenized by manual grinding.
Proteins in the homogenized tissues were digested with proteinase K (Sigma-Aldrich, MO). The digestion was carried out at 55 °C overnight.
HA was extracted by liquid–liquid extraction by adding chloroform. The aqueous phases were dialyzed against 0.1 M NaCl using an Amicon Ultra 3K concentration units (Millipore, MA). HA was then precipitated overnight in ethanol (EtOH) 99%.
Nucleic acids were digested by benzonase (Sigma-Aldrich, MO), followed by 5 h of incubation at 37 °C. The samples were dialyzed against 0.1 M NaCl using Amicon Ultra 3K concentration units followed by HA precipitation using EtOH.
Chondroitin was digested by dissolving the samples in buffer chondroitinase ABC (Sigma-Aldrich, MO) for exactly 10 min at 37 °C. The samples were then dialyzed against 0.1 M NaCl using Amicon Ultra 3K concentration units followed by HA precipitation using EtOH.
Sulfated GAGs and remaining non-HA contaminants were removed using anion exchange chromatography. Each sample was loaded on a prewashed anion exchange mini spin column (Thermo Scientific, MA) and centrifuged to wash out unwanted molecules based on NaCl binding. To remove salt prior to GEMMA analysis, the eluted HA fractions were dialyzed thoroughly against 20 mM of ammonium acetate at pH 8.0 in prewashed Amicon Ultra 3K concentration units (Millipore, MA).
Determination of HA molecular mass distribution with GEMMA analysis
All HA molecular mass analyses were performed using a nanoelectrospray gas-phase electrophoretic molecular mobility analyzer (GEMMA) (TSI Corp., MN). The samples were pushed through a capillary and sprayed into an electric field at the capillary tip where the liquid sample was charged and converted to aerosols using a 3480 electro spray generator. The aerosols were separated using a 3080 electrostatic classifier. HA particles were detected by a 3025A ultrafine condensation particle counter operated at high flow mode.
Each sample of purified HA was scanned three times in the GEMMA—the final size distribution spectrum is a sum of the three scans. The raw molecular counts from the GEMMA spectrum were calibrated according to previously described method [Citation13].
The molecule diameter analyzed in the GEMMA was converted to molecular mass by analyzing HA standards ranging from 30 to 2500 kDa (Hyalose L.L.C., OC).
The relation between the peak area in the GEMMA spectrum to the HA concentration enables an estimation of the relative concentration of different molecular mass of HA. The peak areas under the curve (AUC) were normalized to the dry weight of the sample. The 30.6 kDa HA standard (Hyalose L.L.C., OC), was used to estimate LMHA.
HA of different mass behaves differently in the gas phase of the GEMMA analysis. The relative amount of HA with a mass less than 70 kDa cannot be compared to HA of a mass greater than 70 kDa [Citation13].
In this study, LMHA was defined as a mass up to 50 kDa. Very high mass HA (vHMHA) was defined as more than approximately 10 MDa ().
Figure 2. Mass distribution analysis of hyaluronan (HA) using GEMMA (gas-phase electrophoretic molecular mobility analyzer). LMHA: Low mass Hyaluronan. HMHA: high mass hyaluronan. Two major peaks of HA were detected ranging from about 50 kDa to larger than HA standards (>10 MDa). There was a trend of relatively more of the very high mass HA (vHMHA) in the vocal folds compared to false vocal folds. HA from skin biopsy analyses at our laboratory did not show vHMHA.
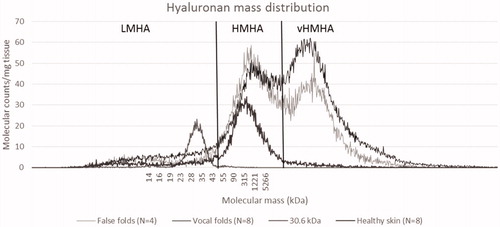
In this study, we based the above-mentioned ranges of HMHA and vHMHA on the two observed HA peaks. LMHA was defined as mass below HMHA.
HA extracted from tissue was degraded with hyaluronidase from Streptomyces hyalurolyticus (Sigma-Aldrich, MO) and reanalyzed to test for extraction specificity.
HA purified from skin biopsies from eight healthy individuals were analyzed for comparison to HA from patients’ VF.
Statistics
All statistical analyses were performed with the IBM SPSS Statistics analysis software (version 23) (NY). Differences between two groups regarding GEMMA analysis were compared using Independent Samples Mann–Whitney U test. Levene’s test for equality of variances was used to estimate differences in variance between groups.
Differences between groups in immunohistochemistry were calculated using Wilcoxon’s signed rank test. Statistical significance was set at p < .05.
Ethical statement
Ethical permission was granted by the Ethical Committee of the Regional Ethical Review Board in Umeå (2015-323-32M (2012-379-31M), 2015-11-11) courtesy to BioBank North, 472-13-08, 2013-03-26.
Results
Hyaluronan distribution:
HA staining succeeded in 11 FVF specimens and in all 12 VF specimens.
HA was present in the epithelium from the FVF in 1 of 11 stains (faint intensity) and for the VF samples in 2 of 12 (faint intensity).
In the stroma of the FVF, HA was stained in 10 of 11 samples, and in the VF in 10 of 12. HA staining intensity ranged from faint to intense in both FVF and VF, with a mean of 1.09 and 1.25 (range 0–2. 0: no staining, 1: faint staining and 2: intense staining), respectively. Comparing these values with Wilcoxon signed rank test revealed no statistical difference (p = .739).
In the macroscopically healthy FVF, RRP lesions were present in 5 of 11 cases, one of which exhibited a faint staining for HA. In the VF with macroscopic RRP lesions, only 7 of 12 were microscopically visible. These patients had earlier been diagnosed with RRP. In two of the microscopically verified RRP samples, faint HA staining was present. Results are shown in .
Table 2. Scoring of HA and CD44 throughout the epithelium, stroma and RRP lesion in vocal folds and false vocal folds.
CD44 distribution
CD44 staining succeeded in 10 FVF specimens and in 10 VF specimens.
CD44 was present throughout the epithelium, stroma, and RRP lesion, in both FVF and VF. However, CD44 staining did not coincide with HA staining in all but one case (RRP lesion in VF). Results shown in .
HA mass distribution
Eight of nine VF samples, and 4 of 12 FVF samples, contained enough HA to be analyzed with GEMMA. The area under the curve in the GEMMA analysis represents the amount of HA separated by size.
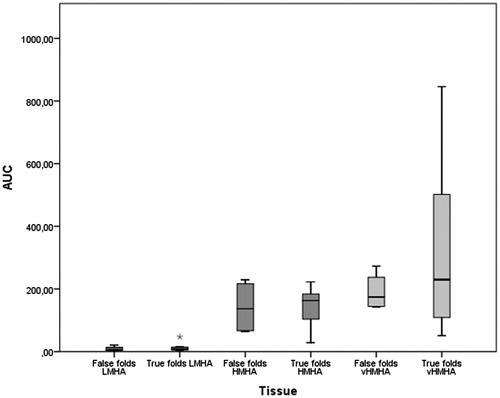
Only a small amount of low mass HA (LMHA) was detected, and there was no significant difference between VF and FVF with regard to LMHA (). The two major peaks of HA in the high mass region ranged from about 50 kDa to very high mass HA, larger than HA standards used (2.5 MDa) ().
Amounts of high mass HA (HMHA) were similar in VF and FVF. The amount of very high mass HA (vHMHA) varied more in the VF and was in some individuals larger as compared with the FVF (p = .046). However, the means for the two groups did not differ (p = .808) (). HA from skin biopsies from healthy individuals showed no presence of vHMHA ().
Figure 3. Area under curve (AUC) from GEMMA (gas-phase electrophoretic molecular mobility analyzer) mass distribution analysis. AUC equals relative amount of hyaluronan in tissue. Low mass hyaluronan (LMHA) and high mass hyaluronan (HMHA) showed little variation between true and false vocal folds. High variation was observed of very high mass hyaluronan (vHMHA) in true vocal folds compared to false vocal folds. Levene’s test for equality of variances, vHMHA p = .046
Discussion
This report constitutes the first evaluation of HA molecular mass and expression of HA with its receptor CD44 in VF and FVF in patients with RRP. It is evident that RRP results from persistent HPV infection, and it is widely recognized that RRP lesions have a tropism for the VF [Citation1]. The majority of RRP patients show long-term airway and voice problems related to disease effects on the laryngeal inlet. The main finding, noted in the epithelium and stroma of both the false and true VF, was the variable staining of HYA and CD44 and the fact that they did not coincide.
HA mass distribution analysis using GEMMA size distribution analysis revealed HA concentrated in the high mass region in VF tissue. Two peaks could be detected: a peak representing normal or native HA, and a second peak that consisted of very high mass HA (vHMHA). Larger than normal HA has previously been reported in the naked mole rat [Citation14] where it was named vHMW-HA. We adopted this term, with a little adjustment (vHMW-HA-very high molecular weight HA was changed to vHMHA-very high molecular mass HA) since we measured molecular mass in Dalton with the GEMMA size distribution analysis.
The accumulation of molecular complexes with similar mass in a distinct peak could indicate that a specific mechanism forms the detected HA peak in FVF and VF, since random entanglement or crosslinking would have led to a more even distribution of molecular complexes. Possible mechanisms forming the specific molecular complexes include entanglement of HA molecules, HA crosslinking with other HA molecules, and HA crosslinking with small amounts of molecules not removed in the purification of HA.
The amount of vHMHA in the VF was more varied and, in some individuals, larger than in FVF. An increased concentration of vHMHA is favorable for viscosity [Citation15] of the VF and voice. It could be speculated that the higher variation in amount of vHMHA could be related to the degree of viral load in VF [Citation2]. These results indicate that a high amount of vHMHA might be a compensatory mechanism in response to the increasing viral load in VF, since vHMHA facilitates vibration and voice production. Another possible explanation for the higher variation in amount of vHMHA could be that HPVs tropism for the VF and severity of the disease is connected to vHMHA since it has been suggested to have immunosuppressive properties [Citation6], beneficial for local HPV accumulation.
Viruses induce cellular aerobic glycolysis in the process of optimizing their own replication. Increased cellular glucose metabolism has been shown to increase HA synthesis [Citation16] and could be the source of the observed differences in HA mass distribution and concentration in VF and FVF.
HA complex forming has been reported in hepatitis virus infection in patients with chronic liver diseases [Citation17]. HA has been found covalently bound to the heavy chain of inter-α-inhibitor, a complex associated to inflammation in tissues causing an expanded HA molecular network. The binding of HA and the heavy chain is reported to be mediated by tumor necrosis factor (TNF)-stimulated gene 6 (TSG-6) [Citation18]. TSG-6 expression is induced by TNF-alpha. A main question is if RRP induces HA complex with the heavy chain in VF. If this is the case, one might be able to use anti TNF-alpha treatment to reduce the forming of complexes which could affect RRP disease activity and recurrence. Consequently, TNF-alpha inhibitors might serve as a medical treatment option in RRP in the future.
The finding of microscopic RRP lesions in 5 of 11 macroscopically normal-appearing FVF is in line with findings presented by Forslund et al. [Citation2]. They demonstrated presence of HPV 6 in macroscopically normal appearing FVF, however, with suppressed copies/cell as compared to the macroscopically diseased true VF.
Immunohistochemistry showed that staining demonstrating HA and CD44 did not coincide in the same tissue. HA was mainly distributed in the stroma whereas CD44 was equally distributed in the epithelium and the stroma. Fragmented LMHA has been reported to induce inflammatory cytokine release through Toll-like receptor binding [Citation5]. Almost no LMHA could be detected in the VF in the present study. The finding of incongruent receptor staining and absence of LMHA could imply that there is no intracellular signaling of HA through CD44 and TLR pathways. Further studies are needed to verify this theory.
The staining intensity of HA in the stroma of the VF was lower than expected. This finding could indicate an abnormally high turnover of HA. HA turnover is reported to occur by receptor mediated endocytosis [Citation19]. In this complicated catabolic process, large extracellular HA molecules are internalized in fragments, and these may act as endogenous signals of injury [Citation5].
Limitations
A limitation of the current study is the small sample sizes and lack of normal tissue from individuals without HPV infection. Although all accessible RRP patients were included during the study period, the incidence and treatment frequency rate were the limiting factors in the regional uptake area, and thus the limiting factor for a larger sample size for the study.
It would have been desirable to analyze healthy VF controls in order to confirm that presence of vHMHA is limited to tissue affected by HPV. However, considering patient discomfort when sampling from a healthy voice source and potentially harming voice production in healthy individuals, it was not deemed ethically justifiable. However, analysis of skin biopsies from healthy controls revealed a total absence of vHMHA.
Further studies using GEMMA analysis are warranted to detect HA mass distribution in healthy VF in order to properly interpret the higher variability of vHMHA amount in the VF compared to FVF in RRP patients. Functional experiments in order to quantify CD44 expression and ligand binding assays evaluating the binding of HA to its receptor CD44 need to be done in order to prove our theory of HA not binding to CD44.
The statistical power of the study should be regarded as low. Therefore, the results demand replication before they are transferred into clinical considerations.
Conclusion
HA was mainly distributed in the stroma of VF and FVF, and CD44 did not coincide with it. This could imply that CD44 does not bind to HA, which in return might explain the non-inflammatory response seen in RRP [Citation20]. vHMHA was found in both VF and FVF. However, there is relatively more vHMHA and a greater variation in amount in the VF compared to FVF, indicating that vHMHA could mirror a higher disease activity in the VF.
Although the pathogenic significance is unclear, this study warrants for further investigation of HA in RRP of the larynx and especially in regard to inflammatory crosslinking of HA that could be induced by HPV. The possibility to counteract such binding with medical treatment, for example with anti-TNF-alpha therapy, makes it interesting in the search for possible non-surgical treatments. Such medical treatment options could reduce the number of surgical treatment sessions along with risks related to anesthesia and local scarring of the voice source.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Derkay CS, Wiatrak B. Recurrent respiratory papillomatosis: a review. Laryngoscope. 2008;118:1236–1247.
- Forslund O, Schwartz S, Olofsson K, et al. Viral load and mRNA expression of HPV type 6 among cases with recurrent respiratory papillomatosis. Laryngoscope. 2016;126:122–127.
- Ohno T, Hirano S. Treatment of aging vocal folds: novel approaches. Curr Opin Otolaryngol Head Neck Surg. 2014;22:472–476.
- Lebl MD, Martins JR, Nader HB, et al. Concentration and distribution of hyaluronic acid in human vocal folds. Laryngoscope. 2007;117:595–599.
- Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–461.
- Stern R. Hyaluronan metabolism: a major paradox in cancer biology. Pathol Biol (Paris). 2005;53:372–382.
- Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539.
- Franzmann EJ, Schroeder GL, Goodwin WJ, et al. Expression of tumor markers hyaluronic acid and hyaluronidase (HYAL1) in head and neck tumors. Int J Cancer. 2003;106:438–445.
- Senbanjo LT, Chellaiah MA. CD44: a multifunctional cell surface adhesion receptor is a regulator of progression and metastasis of cancer cells. Front Cell Dev Biol. 2017;5:18.
- Opheim LR, Hellman U, Engstrom-Laurent A, et al. Hyaluronan in human vocal folds in smokers and nonsmokers-a histochemical study. J Voice. 2016 ;30:255–262.
- Skandalis SS, Stylianou M, Vynios DH, et al. The structural and compositional changes of glycosaminoglycans are closely associated with tissue type in human laryngeal cancer. Biochimie. 2007;89:1573–1580.
- Tolg C, Hamilton SR, Zalinska E, et al. A RHAMM mimetic peptide blocks hyaluronan signaling and reduces inflammation and fibrogenesis in excisional skin wounds. Am J Pathol. 2012;181:1250–1270.
- Malm L, Hellman U, Larsson G. Size determination of hyaluronan using a gas-phase electrophoretic mobility molecular analysis. Glycobiology. 2012;22:7–11.
- Tian X, Azpurua J, Hine C, et al. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature 2013 ;499:346–349.
- Cowman MK, Schmidt TA, Raghavan P, et al. Viscoelastic properties of hyaluronan in physiological conditions. F1000Res. 2015;4:622.
- Yevdokimova NY. Elevated level of ambient glucose stimulates the synthesis of high-molecular-weight hyaluronic acid by human mesangial cells. The involvement of transforming growth factor beta1 and its activation by thrombospondin-1. Acta Biochim Pol. 2006;53:383–393.
- Shen L, Zhuo L, Okumura A, et al. The SHAP-hyaluronan complex in serum from patients with chronic liver diseases caused by hepatitis virus infection. Hepatol Res. 2006;34:178–186.
- Milner CM, Day AJ. TSG-6: a multifunctional protein associated with inflammation. J Cell Sci. 2003;116:1863–1873.
- Harris EN, Kyosseva SV, Weigel JA, et al. Expression, processing, and glycosaminoglycan binding activity of the recombinant human 315-kDa hyaluronic acid receptor for endocytosis (HARE). J Biol Chem. 2007;282:2785–2797.
- DeVoti J, Hatam L, Lucs A, et al. Decreased Langerhans cell responses to IL-36gamma: altered innate immunity in patients with recurrent respiratory papillomatosis. Mol Med. 2014;20:28–37280.