?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background: Hearing preservation is thought to be achievable following atraumatic surgery with thin cochlear implant electrodes; therefore, the surgical approach and implant electrode design are crucial considerations.
Objective: To assess the feasibility of hearing preservation with long electrodes for patients meeting the criteria for conventional cochlear implantation.
Methods: One hundred and two patients (132 ears) who underwent cochlear implant surgery were analyzed. Inclusion criteria included measurable residual hearing in the low frequency before implantation and not meeting the criteria for electric acoustic stimulation (EAS).
Results: Of the 18 patients with residual hearing in the low frequency enrolled, 17 subjects (94.4%) retained low frequency hearing. A younger age at surgery tended to contribute to better hearing preservation than that observed in older patients. There was no clear trend regarding the influence of insertion depth angle of the electrode on hearing preservation.
Conclusion: It is possible to achieve hearing preservation in the lower frequency by the use of longer electrodes. This study underscores the importance of atraumatic surgery, even for patients with only limited residual hearing, and longer electrodes should be adopted for EAS.
Chinese abstract
背景:听力保存被认为可以通过使用薄耳蜗植入电极进行无创伤手术来实现;因此, 手术方式和植入电极设计是至关重要的考虑因素。
目的:评估对于符合常规人工耳蜗植入标准的患者, 使用长电极来保存听力的可行性。
方法:对102例接受人工耳蜗植入手术的患者(132个耳)进行分析。入选标准包括植入前低频率可测残余听力, 以及不符合电声刺激(EAS)的标准。
结果:18例低频残余听力患者入选。总体而言, 17名受试者(94.4%)保留了低频听力。手术年龄较小的年龄往往比较大年龄更有助于提高听力保持率。电极的插入深度角对听力保持的影响不明显。
结论:通过使用更长的电极, 可以在较低频率下实现听力保持。该项研究强调, 应该认识到无创伤手术的重要性, 即使对于残余听力有限的患者也是如此。并且强调应采用更长的电极来进行电声刺激。
Introduction
The criteria for cochlear implant (CI) candidates vary by country or region, as well as in terms of hearing threshold or speech perception score. Formerly, CI was only applied for candidates with profound hearing loss in all frequencies. Today, however, the criteria for CI have changed dramatically on a national level. Hearing preservation (HP) has been achieved for patients with a functional level of residual hearing in the low frequency, and many studies have demonstrated that the addition of acoustic stimulation for residual hearing improves speech perception, especially in noisy conditions [Citation1,Citation2]. This combination of the electrical stimulation provided by CI with acoustic stimulation in the low frequency (electric acoustic stimulation [EAS]) has afforded great benefits to hearing loss patients, and also has made the expansion of the eligibility criteria possible.
Hearing preservation is thought to be achievable following atraumatic CI surgery with flexible and thin electrodes; therefore, the surgical approach and implant electrode design are crucial considerations. Several types or lengths of CI are available in order to minimize intra-cochlear damage. Shorter electrodes of approximately 20–24 mm in length are generally adopted as the standard procedure for EAS. One reason for this is the atraumacity of the electrode, with longer electrodes considered to have the potential to damage natural hearing on the basis of several studies which reported that a deep insertion angle with a longer electrode appears to be correlated with a poorer hearing preservation rate [Citation3]. On the other hand, several reports also raise the possibility of preservation of residual low frequency hearing even with the use of longer electrodes [Citation4,Citation5]. The advantage of using longer electrodes with deep insertion into the cochlea is that they can provide better speech perception on electrical stimulation, suggesting each electrode better approximates the tonotopic location of the contact through wider coverage of the native cochlea [Citation6]. Buchman et al. clearly demonstrated that deep insertion with a longer electrode (31 mm) offered better speech perception compared with that afforded by a shorter one (24 mm), although subjective assessment showed no differences between them [Citation7]. Taking into consideration the above factors, we believe that it is important to have a longer electrode that can provide more favorable electrical stimulation and assure coverage of the cochlear region when low frequency hearing is lost, despite the potential for the longer electrode to be detrimental to residual hearing. The aim of this study was to obtain preliminary hearing preservation results for patients meeting the criteria for conventional CI but not meeting those for EAS using a longer electrode.
Subjects and methods
All 102 patients (132 ears) who underwent implant surgery with conventional CI with a full-length electrode at the Department of Otorhinolaryngology, Shinshu University Hospital between January 2013 and January 2017 were analyzed. All patients met conventional CI criteria, with bilateral severe to profound hearing loss with an average threshold of above 90 dBHL from 500 to 4000 Hz, and poor speech perception under best-aided conditions. Inclusion criteria were as follows: (1) age at implantation above 6 years old for the reliable assessment of pure-tone audiometric results, (2) MED-EL FLEX28 or FLEXsoft electrode array implanted using a hearing preservation procedure as described below in the Methods section, (3) measurable residual hearing in the low frequency with a threshold less than 80 dBHL for the average of values at 125, 250, and 500 Hz before implantation, and (4) not meeting the criteria for electric acoustic stimulation (EAS). All subjects were evaluated for residual hearing over time after surgery.
Cochlear implant and surgical procedure
The MED-EL CONCERT CI implant system with a FLEX28 (28 mm in length) or FLEXsoft electrode array (31 mm in length) was implanted. The CI surgery was performed by postauricular incision and standard mastoidectomy. To insert the electrode array, the round window approach was applied to reduce insertion damage to the cochlea. The bony overhang of the round window was removed with a low-speed drill and the round window membrane was widely exposed. A small incision was made and the electrode was fully inserted carefully and slowly [Citation4]. We generally perform this procedure for all patients regardless of the presence or absence of residual hearing.
Audiological assessment
Hearing thresholds, assessed by pure tone audiometry (PTA), were measured pre- and from 1 to 6 months post-operatively. Post-operative HP rates were calculated using the equation developed by Skarzynski et al. and the HEARRING group [Citation8] as follows:
Preservation numerical scale (S)
The results obtained for the preservation numerical scale (%) were categorized as follows: complete HP, defined as greater than 75%; partial HP, 25–75%; minimal HP, 1–25%; and complete loss of hearing; 0%. It is important to note that the scale results were substantially lower than those in other reports, so that the levels of hearing preservation in this study also appear lower. This was due to the fact that the PTAmax, the maximum pure-tone average, was 100 dB in this study, while the default setting is 110 dB.
Postoperative radiological assessment
The final position of the implanted electrode array was assessed by X-ray images of the horizontal plane of the cochlear basal turn obtained in modified Stenver’s view. Measurement of the insertion depth angel (IDA) was performed in a blinded fashion by two experienced otolaryngologists. We measured IDA based on the method for the determination of insertion depth described by Trieger et al. [Citation9].
Results
Patient data, demographics and hearing preservation
Eighteen patients met the inclusion criteria for the hearing preservation analysis. shows the patient demographics and hearing preservation outcomes based on the numerical preservation scale. The median age at implantation was 44 years (range 6–70 years). All patients had progressive hearing loss with various onset ages. Patients included 9 males and 9 females, with 15 patients receiving implantation with a FLEX28 electrode and 3 patients receiving a FLEXsoft electrode on the right side. According to the HP classification, 9 of the 18 subjects had complete (50%), 2 had partial (11%), and 6 had minimal (33%) preservation, while 1 patient had no detectable (6%) hearing. Overall, substantial hearing levels were observed in more than half of the subjects, and 17 subjects (94.4%) retained low frequency hearing.
Table 1. Subject demographics and hearing preservation outcomes.
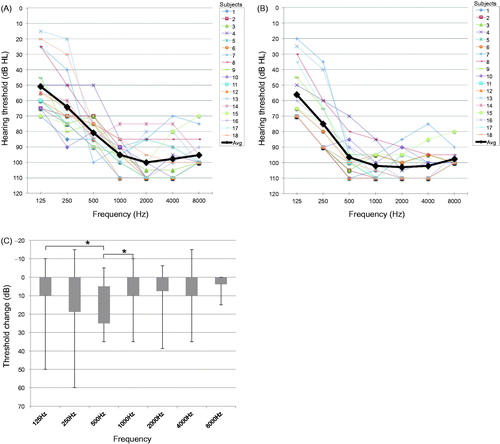
Hearing thresholds changes
shows the pre-operative and 6-month post-operative audiograms (A,B) and average threshold changes for each frequency (C). Six months after surgery, threshold changes from the pre-operative values were observed at all frequencies, with a large deterioration in hearing observed at 500 Hz. There were significant differences in threshold changes between 125 and 500 Hz, and between 500 and 1000 Hz (Tukey’s test, <0.05).
Figure 1. Individual and average (bold black line) hearing thresholds pre- (A) and at 6 months post-operatively (B). Threshold changes from 125 to 8000 Hz between the pre-operative and 6 months post-operative values (C). Statistically significant differences were calculated using Tukey’s test (*significant difference <0.05).
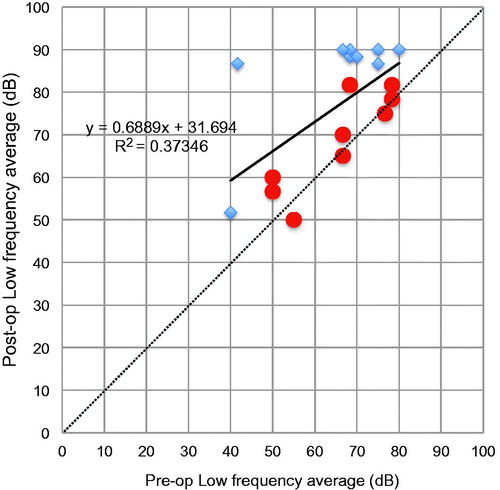
Correlation between hearing preservation and each factor
As shown in , the scatter plots for age at CI versus numerical HP scale at 6 months post-operatively show that there was a moderately positive relationship between age and hearing preservation. A younger age at surgery tended to contribute to better hearing preservation than that observed in older patients (). The results for insertion depth angle (IDA) were separated by the two types of electrode, with the average IDA being 613.3° (range: 550°–695°) for FLEX28 and 725.0° (range: 713°–735°) for FLEXsoft electrodes. There was no clear trend regarding the influence of electrode length on hearing preservation (). With regard to pre- and post-operative hearing thresholds in the low frequency, shows that even though the individuals had only limited residual hearing pre-operatively, their post-operative hearing levels were retained to a substantial degree. As a consequence, a lower pre-operative hearing level did not mean that the rate of hearing preservation would also be poor.
Figure 2. Scatter plots for the numerical hearing preservation scale [Citation8] versus age at implantation (A), and versus insertion depth angle of the electrode (B). There is a moderate relationship between the hearing preservation value and age at surgery, whereas no trend was observed regarding the influence of electrode length on hearing preservation.
![Figure 2. Scatter plots for the numerical hearing preservation scale [Citation8] versus age at implantation (A), and versus insertion depth angle of the electrode (B). There is a moderate relationship between the hearing preservation value and age at surgery, whereas no trend was observed regarding the influence of electrode length on hearing preservation.](/cms/asset/9096517c-5247-48e2-ad45-12d6ea6cdbf4/ioto_a_1508888_f0002_c.jpg)
Discussion
There are a number of patients who have a reasonable level of residual hearing in the low frequency, but still need CI in order to hear properly. EAS has made it possible for them to achieve better hearing; however, they continue to face a considerable issue in terms of the potential risk of deterioration in their residual hearing, which needs to be addressed. As a further consideration, there have been many studies on the use of longer electrodes for hearing preservation [Citation10–14]. In this study, we examined hearing preservation in the low frequency after implantation of electrodes of conventional length for patients who met conventional CI criteria. Among the 18 of 102 implantees (17.6%) with residual hearing before surgery, 17 (94.4%) retained low frequency hearing, with complete hearing preservation achieved in 9 patients (50%). The results for hearing preservation appeared to be correlated with age at CI surgery. Anagiotos et al. reported that the hearing preservation rate was better in younger than in older patients among their cohort study of 1- to 80-year-old patients [Citation15]. Other studies have also supported the notion of better hearing preservation among younger patients [Citation11]. Although several studies did not demonstrate any clear evidence regarding the relationship between age and hearing preservation, those study cohorts were based only on adult patients [Citation13]. The hearing level in patients of advanced age seems to deteriorate more than in younger patients after CI surgery, suggesting that the pathophysiology of age-related hearing loss might negatively affect the maintenance of cochlear function. However, we cannot predict the degree to which hearing remains after CI surgery and, as it seems to vary, it might be affected by many factors that have not yet been clarified. Conflicting findings have been reported concerning the effects of longer electrodes on both hearing ability in association with CI and hearing preservation. Both advantages and disadvantages of the longer electrodes have been suggested: i.e., better speech perception with wider cochlear coverage and trauma to the cochlea, respectively [Citation7,Citation16]. In this study, there were no differences in HP rate among the insertion depth angles for each patient, which indicated that electrode length was unlikely to have an impact on HP. We believe that it is important to use longer electrodes as patients receiving EAS may lose hearing during the procedure or long after as part of the natural course of the hearing loss itself, and longer electrodes enable us to stimulate the areas where low frequency hearing might be lost.
Two subjects (Number 7 and 13) did not require amplification as they could combine their natural acoustic hearing in the low frequency with electric stimulation through the standard CI audio processor. In fact, the results for speech perception and sound localization tests appeared relatively better than those for other standard CI listeners (data not shown). Likewise, Dalbert et al. demonstrated that CI recipients with preserved low frequency hearing had significantly better speech perception scores with electric stimulation alone at 18 months or more after surgery [Citation17]. With regard to the amplification of residual low frequency hearing, we previously reported that acoustic amplification might contribute to improved hearing abilities using an EAS audio processor, even within a limited range of low frequencies [Citation18]. We supposed that if a patient who had limited residual hearing could receive CI with a longer electrode than that used for EAS, the combined acoustic stimulation might be beneficial for hearing.
The 106 subjects analyzed here were all aged above 6 years, with 18 of them (17.6%) showing residual hearing in the low frequency. Understandably, many infants with congenital or very early onset hearing loss are regarded as CI candidates; however, it is impossible to measure hearing level at low frequencies in detail at an appropriate time point for CI. Therefore, it is suggested that a relatively high proportion of hearing loss patients, especially infants, appear to have residual hearing in general. HP is important in allowing not only better speech perception with CI but also in expanding future treatment options such as gene therapy or regeneration therapy, particularly in infants.
The heterogeneous nature of hearing loss itself, including the responses to CI surgery, is assumed to result from differences in the etiology of hearing loss disease [Citation19,Citation20]. We identified genetic mutations associated with hearing loss in some subjects (); however, because of the limited study population, we could not draw any conclusions regarding the relevance of the genetic mutations. In terms of further study, if the genetic causes of hearing loss can be identified in a large cohort of CI candidates, it may be possible to predict the hearing loss phenotype and hearing preservation outcomes as well as the appropriate electrode length.
In conclusion, hearing preservation appears to be achievable at a HP rate of 94.4% among patients with residual low frequency hearing, with complete hearing preservation achieved in 50% by the use of longer electrodes. We suggest that the importance of atraumatic CI surgery, even for patients with only limited residual hearing, should be acknowledged and longer electrodes adopted for EAS.
Ethical approval
This study was approved by the Ethics Committee of Shinshu University School of Medicine.
Disclosure statement
No potential conflicts of interest were reported by the authors.
References
- von Ilberg CA, Baumann U, Kiefer J, et al. Electric-acoustic stimulation of the auditory system: a review of the first decade. Audiol Neurootol. 2011;16:1–30.
- Usami S, Moteki H, Tsukada K, et al. Hearing preservation and clinical outcome of 32 consecutive electric acoustic stimulation (EAS) surgeries. Acta Otolaryngol. 2014;134:717–727.
- Causon A, Verschuur C, Newman TA. A retrospective analysis of the contribution of reported factors in cochlear implantation on hearing preservation outcomes. Otol Neurotol. 2015;36:1137–1145.
- Usami S, Moteki H, Suzuki N, et al. Achievement of hearing preservation in the presence of an electrode covering the residual hearing region. Acta Otolaryngol. 2011;131:405–412.
- Tamir S, Ferrary E, Borel S, et al. Hearing preservation after cochlear implantation using deeply inserted flex atraumatic electrode arrays. Audiol Neurotol. 2012;17:331–337.
- Hochmair I, Hochmair E, Nopp P, et al. Deep electrode insertion and sound coding in cochlear implants. Hear Res. 2015;322:14–23.
- Buchman CA, Dillon MT, King ER, et al. Influence of cochlear implant insertion depth on performance: a prospective randomized trial. Otol Neurotol. 2014;35:1773–1779.
- Skarzynski H, van de Heyning P, Agrawal S, et al. Towards a consensus on a hearing preservation classification system. Acta Otolaryngol Suppl. 2013;133:3–13.
- Trieger A, Schulze A, Schneider M, et al. In vivo measurements of the insertion depth of cochlear implant arrays using flat-panel volume computed tomography. Otol Neurotol. 2011;32:152–157.
- Mick P, Amoodi H, Shipp D, et al. Hearing preservation with full insertion of the FLEXsoft electrode. Otol Neurotol. 2014;35:e40–e44.
- Cosetti MK, Friedmann DR, Zhu BZ, et al. The effects of residual hearing in traditional cochlear implant candidates after implantation with a conventional electrode. Otol Neurotol. 2013;34:516–521.
- Van Abel KM, Dunn CC, Sladen DP, et al. Hearing preservation among patients undergoing cochlear implantation. Otol Neurotol. 2015;36:416–421.
- Sweeney AD, Hunter JB, Carlson ML, et al. Durability of hearing preservation after cochlear implantation with conventional-length electrodes and scala tympani insertion. Otolaryngol Head Neck Surg. 2016;154:907–913.
- Wanna GB, O'Connell BP, Francis DO, et al. Predictive factors for short- and long-term hearing preservation in cochlear implantation with conventional-length electrodes. Laryngoscope. 2017;128:482–489.
- Anagiotos A, Hamdan N, Lang-Roth R, et al. Young age is a positive prognostic factor for residual hearing preservation in conventional cochlear implantation. Otol Neurotol. 2014;36:1–33.
- Santa Maria PL, Gluth MB, Yuan Y, et al. Hearing preservation surgery for cochlear implantation: a meta-analysis. Otol Neurotol. 2014;35:e256–e269.
- Dalbert A, Huber A, Baumann N, et al. Hearing preservation after cochlear implantation may improve long-term word perception in the electric-only condition. Otol Neurotol. 2016;37:1314–1319.
- Moteki H, Nishio SY, Miyagawa M, et al. Long-term results of hearing preservation cochlear implant surgery in patients with residual low frequency hearing. Acta Otolaryngol. 2017;137:516–521.
- Miyagawa M, Nishio SY, Usami S. A comprehensive study on the etiology of patients receiving cochlear implantation with special emphasis on genetic epidemiology. Otol Neurotol. 2016;37:e126–e134.
- Usami S, Miyagawa M, Nishio SY, et al. Patients with CDH23 mutations and the 1555A > G mitochondrial mutation are good candidates for electric acoustic stimulation (EAS). Acta Otolaryngol. 2012;132:377–384.