Abstract
Background: Trismus is a common complication of radiotherapy for head and neck cancer but its impact on survival is unknown.
Aims/Objectives: This prospective study evaluates the incidence of trismus in patients with head and neck cancer receiving radiotherapy and the impact of trismus on 5-year overall survival.
Material and methods: Two hundred forty-four patients with head and neck cancer were included. All patients received instructions on jaw exercises and were evaluated before initiation of radiotherapy and at 2, 6, and 12 months after termination of radiotherapy.
Results: One year after treatment 25% had a reduced maximum interincisal opening (MIO) of 13 mm or more as compared to the pretreatment MIO. Trismus was most prevalent in patients with oral and oropharyngeal cancer. A trend towards worse 5-year overall survival was seen among patients with trismus.
Conclusions: The trismus rate was approximately 30% at 12 months. Jaw exercises should primarily be offered to patients with oral and oropharyngeal cancer who are most likely to benefit. Further studies are required to investigate the effect of trismus on survival.
Significance: This study identifies patients likely to benefit from jaw exercises and provides basis for further research on trismus and survival.
背景:牙关紧闭症是头颈癌放射治疗的常见并发症, 但它对生存的影响尚不清楚。
目的:这项前瞻性研究评估接受放射治疗的头颈癌患者牙关紧闭症的发生率以及其对5年总生存率的影响。
材料和方法:共224名头颈癌患者纳入研究。所有患者均接受了下颌运动的训练, 并在放疗开始前和放疗结束后的2、6和12个月接受了评估。
结果:与治疗前最大切牙间开口(MIO)相比, 治疗一年后, 25%的MIO降低了13 mm或更多。牙关紧闭症在口腔和口咽癌患者中最常见。在牙关紧闭症患者中观察到5年总体生存率下降的趋势。
结论:在12个月时, 牙关紧闭症发生率约为30%。下颌运动应该主要提供给最有可能受益的口腔和口咽癌患者。需要进一步的研究来研究牙关紧闭症对生存的影响。
意义:本研究确定患者可能从下颌运动中获益, 并为进一步研究牙关紧闭症和生存提供了基础。
Introduction
Trismus is a well-known complication after treatment of head and neck cancer (HNC). The reported incidence varies due to heterogeneous study populations, treatment modalities and different methods of measuring and defining trismus. Moreover, this field has lacked prospective studies until recently. The incidence has been reported to be as high as 79% [Citation1] while a number of studies indicate an incidence between 38% and 52% [Citation2–4] where the majority of patients have received 3D conformal radiotherapy (3DCRT). Trismus severity seems to peak at 6 months after treatment and becomes somewhat lower at 12 months after treatment [Citation3,Citation5]. Newer treatment modalities, in particular intensity modulated radiotherapy (IMRT) are reported to have lower risk for trismus development [Citation6,Citation7].
The pathogenesis of trismus in patients with HNC can be related to a number of parameters and varies between individuals. It may involve a single cause or a combination of different factors such as direct tumour interference with jaw opening, radiotherapy (RT)-induced fibrosis in the muscles of mastication (medial and lateral pterygoid, masseter) as well as the temporomandibular joint [Citation8,Citation9]. In addition, surgical scarring can contribute to the development of trismus.
Post-treatment trismus has a significant impact on quality of life that impacts speech, oral hygiene and the intake of food, and inhibits the patient socially [Citation3,Citation10]. Moreover, oncological follow-up controls and dental care can be problematic in patients with severe trismus.
The definition of trismus in the HNC setting has varied but after Dijkstra et al. [Citation11] presented their study in 2006 a cut-off point at 35 mm of maximum interincisal opening (MIO) is widely accepted. The Dijkstra trismus criterion has enabled comparison between studies on trismus and effects of exercise interventions.
Previous studies have indicated that the development of trismus is correlated with tumour size and poor physical function prior to treatment [Citation2]. To our knowledge, until now, no study has prospectively assessed the effect of trismus on overall survival. The objective of the present study was to investigate the relationship between trismus and 5-year overall survival in a cohort of patients treated for HNC.
Materials and methods
Two hundred forty-four patients diagnosed with HNC receiving 3D conformal RT, delivered either primarily or preoperatively, were included in this prospective observational study. The characteristics of the patients are presented in . RT planned target volume was 64–68 Gy and the maximum dose given to the spinal cord was 48–50 Gy.
Table 1. Characteristics of patients with head and neck cancer (total n = 244).
Intervention and evaluation
All the patients were included in an early rehabilitation program and were seen by a physiotherapist before RT started and at follow-ups at 2, 6, and 12 months after termination of treatment. Before start of RT, patients received written and oral rehabilitation instructions about self-care exercises and stretching of structures of mastication to maintain mobility in the area exposed to RT with the aim of avoiding trismus. Patients were instructed to exercise active maximal mouth opening twice daily for 10 × 20 seconds assisted with the Acute Medic Jaw Trainer and Stretcher®. These instructions were given by one physiotherapist. During the course of the study, these instructions were occasionally modified to a ‘hold and release’ technique when patients exhibited decreased mouth opening or did not improve with the standard technique.
Primary outcome
The patient’s MIO ability was measured before start of RT and at all follow-ups. A trismus cut-off by MIO ≤35 mm was used irrespective of dental status according to Dijkstra et al. [Citation11]. Of the 244 patients, a total of 225 (92%) had teeth in the mandible and maxilla, 11 (5%) had either teeth in the mandible or maxilla, 7 (3%) were edentulous, and information about dental status at diagnosis was missing for one patient.
Statistical methods
The association between mouth opening at baseline and 12-month change was estimated using Pearson’s correlation coefficient. Effects on interincisal opening ability by different clinical factors (age, gender, stage, site, radiotherapy, chemotherapy, surgery) were estimated using linear regression models. Results from these models were presented as mean differences together with 95% confidence intervals. p values from these models refer to Wald tests.
Overall survival was estimated using the Kaplan-Meier technique. Differences in survival time distributions were tested using the log-rank test.
The statistical significance level was set to .05.
Ethical considerations
The Regional Ethical Review Board in Stockholm approved the study, judging that no ethical approval was required, as it was identified as a clinical development program (2005/767-31/1-4). The study was performed in accordance with the declaration of Helsinki.
Results
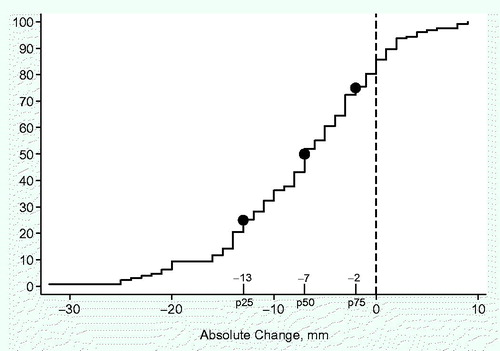
Of the 244 patients who were included in the study, 127 patients (52%) completed the study and were assessed at 12 months. At the 12-month follow-up, 117 patients (48%) had withdrawn from the study. Of these patients, 51 (21%) had experienced cancer recurrence or were deceased while 57 (23%) declined to participate further. Details regarding drop-out are missing for 9 patients. There was an initial decline of MIO and a subsequent slow recovery was seen at 12 months, as shown in . Before treatment 34 patients had trismus (MIO of less than 35 mm) and the mean pretreatment MIO was 46 mm (8–74 mm). The mean MIO capacity reached a nadir at 2 months after treatment, 39 mm (14–69 mm). At 12 months the corresponding figures were 40 mm (5–67 mm). The measured individual change in MIO from baseline to 12 months is presented in . Close to 80% of the patients had a decreased MIO (negative value) at 12 months when compared to the pretreatment value. A MIO reduction of 13 mm or more was seen in 25% of patients at 12 months as compared to the pretreatment value while 30% of the patients were defined as having trismus.
Figure 1. Follow-up of mean maximum interincisal opening (MIO) ability in patients with head and neck cancer (n = 244).
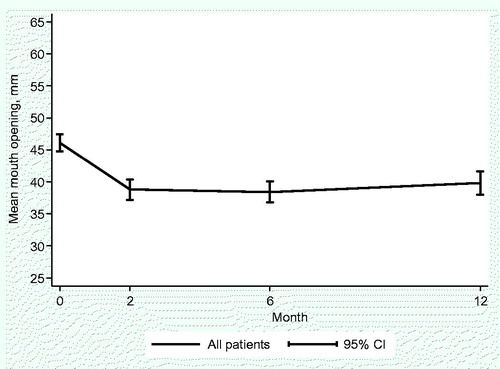
Figure 2. Absolute change in maximum interincisal opening (MIO) ability from baseline to 12 months in millimeters in patients with head and neck cancer.
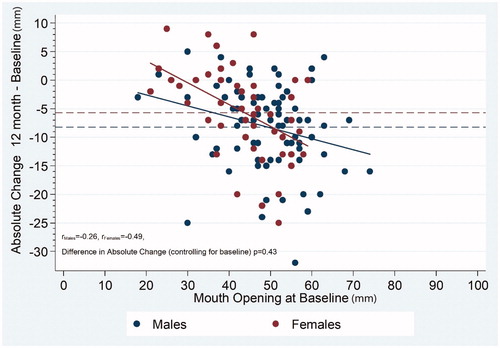
There was a large pretreatment range of MIO, as seen in . A linear regression analysis indicated that patients with the largest MIO before treatment demonstrated the largest reduction of mouth opening at 12 months. This was more pronounced in female patients, although the correlation coefficient showed no statistically significant difference (p = .43).
Figure 3. Maximum interincisal opening (MIO) ability in female and male patients with head and neck cancer. Correlation between 12-month assessment and baseline. The thin dashed lines indicate the average difference at 12 months.
Mean MIO at the 12-month follow-up (n = 127) was analyzed in relation to age, gender, tumour stage, tumour site, and treatment as shown in . Patients with oral cancer were found to have statistically significant more trismus than other subgroups (p = .03). In total 56% of patients with oral cancer, 63% of patients with oropharynx cancer, 94% of patients with larynx cancer and 86% of patients with other HNC had mouth opening of >35 mm.
Table 2. Mean maximum interincisal opening (MIO) ability in 127 patients at 12 month follow-up related to site, stage and treatment.
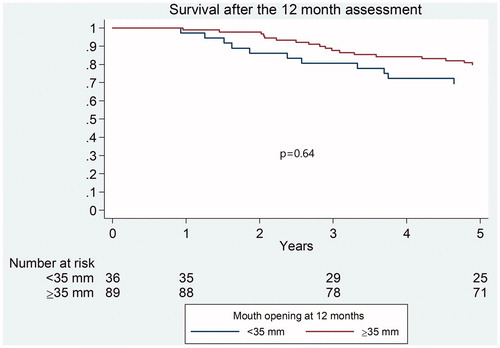
In terms of overall survival after 5 years, patients with MIO ≤35 mm at 12 months tended to fare worse than patients with MIO >35 mm but the difference was not found to be significant (p = .64) ().
Discussion
Development of trismus is a significant problem for patients that have received treatment for HNC. Reduced mouth opening contributes to malnutrition and can have a negative social impact. In addition it is plausible to assume that trismus can affect survival rates.
Previous studies on jaw exercises have shown mixed results in the prevention of trismus. Dijkstra et al. [Citation12] found that in a cohort of 35 patients, trismus related to HNC did not respond as well to preventive treatment as noncancer-related trismus. A study by Loorents et al. [Citation13] showed no difference in MIO reduction between a control group and an intervention group, while Pauli et al. [Citation14] found that patients practicing mouth-opening exercises had greater MIO and better health-related quality of life than the control group.
All patients in the present study were included in an early self-care rehabilitation program and received instructions on mouth-opening exercises in order to prevent trismus. Despite this about 30% had trismus (MIO ≤35 mm) when measured at the 12-month follow-up. In addition, 25% of patients in this study had a reduction of 13 mm or more in MIO at 12 months. It should be noted that compliance was not assessed during the study period.
Trismus related to HNC treatment poses a challenge and is difficult to treat. To be effective, trismus prevention requires dedicated and complying patients and one can conclude that not all patients stand to benefit from mouth-opening exercises. In the present study, patients with oral cancer had significantly more severe trismus at 12-month follow-up, which indicates that this subgroup might benefit the most from mouth-opening exercises. It has previously been shown that both oropharyngeal cancer and oral cancer and its treatment predispose patients to trismus, which our study also indicates [Citation5,Citation15,Citation16]. We therefore suggest that consideration should especially be given to patients with the greatest needs, i.e. mouth opening exercises should primarily be recommended to patients with oral and oropharyngeal cancer. All patients that have undergone RT for HNC should however be evaluated individually with regard to trismus and offered treatment when indicated.
Our findings show that patients with higher baseline MIO experience a greater reduction in mouth-opening after RT. One can postulate that patients with oral and oropharyngeal cancer could benefit from preventive exercises regardless of whether they meet trismus criteria.
It has previously been shown that severe dysphagia is associated with lower survival rates [Citation17]. No studies have previously analyzed the relationship between trismus and overall survival rates after 5 years in a prospective manner. One can speculate that trismus can have a negative effect on survival through dysphagia leading to malnutrition and/or aspiration as well as complicating clinical follow-up, leading to delayed diagnosis of tumour recurrence or second primary cancer. The present study aimed to give information about the influence of trismus on long-term overall survival. Our results could not show any statistically significant difference in 5-year overall survival between patients with trismus and patients with MIO ≥35mm at 12 months. As the Kaplan-Meier curve shows a tendency for a worse overall survival in trismus patients, further studies are warranted, including larger cohorts.
All patients in our study were treated with 3DCRT, which has now been replaced in many centers with modern IMRT. Several reports demonstrate that IMRT is probably less likely to cause trismus [Citation6,Citation18,Citation19]. Further studies on IMRT are required with regard to trismus and our study provides a basis for comparison with 3DCRT. It also has to be considered that additional treatment, surgery and chemotherapy, may increase the risk of trismus. However, that was not seen in the present study.
The strengths of this prospective study include the cohort size and duration of follow-up. This study experienced a high drop-out rate (48%) but that still left us with 127 patients, which is a relatively large cohort compared to earlier studies on trismus.
On the other hand, this study lacks randomization, which might limit it somewhat in that no comparison is possible between different mouth-opening exercises. A potential confounder is the number of patients with pretreatment trismus (n = 34). However in a consecutive study on a cohort of HNC patients one expects to find a certain number of patients with decreased mouth opening at baseline.
To conclude, our hypothesis that trismus has a negative impact on 5-year overall survival could not be confirmed, but a larger study is required to investigate this further. Our study indicates that mouth-opening exercises should mainly be applied to patients with oral and oropharyngeal cancer.
Acknowledgments
Thanks to all the patients who participated.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Lee R, Slevin N, Musgrove B, et al. Prediction of post-treatment trismus in head and neck cancer patients. Br J Oral Maxillofac Surg. 2012;50(4):328–332.
- Johnson J, van As-Brooks CJ, Fagerberg-Mohlin B, et al. Trismus in head and neck cancer patients in Sweden: incidence and risk factors. Med Sci Monit. 2010;16(6):CR278–82.
- Pauli N, Johnson J, Finizia C, et al. The incidence of trismus and long-term impact on health-related quality of life in patients with head and neck cancer. Acta Oncol. 2013;52(6):1137–1145.
- Lyons AJ, Crichton S, Pezier T. Trismus following radiotherapy to the head and neck is likely to have distinct genotype dependent cause. Oral Oncol. 2013;49(9):932–936.
- van der Geer SJ, Kamstra JI, Roodenburg JL, et al. Predictors for trismus in patients receiving radiotherapy. Acta Oncol. 2016;55(11):1318–1323.
- Chen YY, Zhao C, Wang J, et al. Intensity-modulated radiation therapy reduces radiation-induced trismus in patients with nasopharyngeal carcinoma: a prospective study with >5 years of follow-up. Cancer. 2011;117(13):2910–2916.
- van der Molen L, Heemsbergen WD, de Jong R, et al. Dysphagia and trismus after concomitant chemo-Intensity-Modulated Radiation Therapy (chemo-IMRT) in advanced head and neck cancer; dose-effect relationships for swallowing and mastication structures. Radiother Oncol. 2013;106(3):364–369.
- Gebre-Medhin M, Haghanegi M, Robért L, et al. Dose-volume analysis of radiation-induced trismus in head and neck cancer patients. Acta Oncol. 2016;55(11):1313–1317.
- Rapidis AD, Dijkstra PU, Roodenburg JL, et al. Trismus in patients with head and neck cancer: etiopathogenesis, diagnosis and management. Clin Otolaryngol. 2015;40(6):516–526.
- Lee LY, Chen SC, Chen WC, et al. Postradiation trismus and its impact on quality of life in patients with head and neck cancer. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119(2):187–195.
- Dijkstra PU, Huisman PM, Roodenburg JL. Criteria for trismus in head and neck oncology. Int J Oral Maxillofac Surg. 2006;35(4):337–342.
- Dijkstra PU, Sterken MW, Pater R, et al. Exercise therapy for trismus in head and neck cancer. Oral Oncol. 2007;43(4):389–394.
- Loorents V, Rosell J, Karlsson C, et al. Prophylactic training for the prevention of radiotherapy-induced trismus - a randomised study. Acta Oncol. 2014;53(4):530–538.
- Pauli N, Svensson U, Karlsson T, et al. Exercise intervention for the treatment of trismus in head and neck cancer - a prospective two-year follow-up study. Acta Oncol. 2016;55(6):686–692.
- Weber C, Dommerich S, Pau HW, et al. Limited mouth opening after primary therapy of head and neck cancer. Oral Maxillofac Surg. 2010;14(3):169–173.
- Rao SD, Saleh ZH, Setton J, et al. Dose-volume factors correlating with trismus following chemoradiation for head and neck cancer. Acta Oncol. 2016;55(1):99–104.
- Shune SE, Karnell LH, Karnell MP, et al. Association between severity of dysphagia and survival in patients with head and neck cancer. Head Neck. 2012;34(6):776–784.
- Owosho AA, Pedreira Ramalho LM, Rosenberg HI, et al. Objective assessment of trismus in oral and oropharyngeal cancer patients treated with intensity-modulated radiation therapy (IMRT). J Craniomaxillofac Surg. 2016;44(9):1408–1413.
- Kraaijenga SA, Oskam IM, van der Molen L, et al. Evaluation of long term (10-years+) dysphagia and trismus in patients treated with concurrent chemo-radiotherapy for advanced head and neck cancer. Oral Oncol. 2015;51(8):787–794.