Abstract
Background: Sinonasal inverted papilloma (IP) is a benign tumor with a high risk of local recurrence and a potential to malignify and Human papillomavirus (HPV) has been suggested an etiological factor. p16INK4a (p16) overexpression is considered a surrogate marker for HPV, but whether p16 and HPV correlate to IP is uncertain. Besides, a prognostic role of tumor infiltrating lymphocytes (TILs) are observed in many tumors, however their role in IP is sparsely studied.
Aims/objectives: We hence analyzed IPs for the presence and the prognostic role of HPV and p16 overexpression together with CD8+ and FoxP3+ TILs in a population-based study.
Material and methods: 98 IP patients diagnosed 2001–2010 were identified from the Swedish Cancer Registry and analyzed for HPV by PCR and p16, CD8 and FoxP3 was by immunohistochemistry.
Results: In total, 12.2% of the IPs were HPV-positive (nine HPV-11, two HPV-6 and one HPV-45). Patients with HPV-positive lesions were younger (p = .003) and tended to present with more dysplasia. No correlation was observed between TILs and prognosis.
Conclusions and significance: Our data suggests that patients with HPV-positive IPs present with different clinical characteristics, suggesting possibly different disease entities. Moreover, recurrences may occur >5 years, which should be considered in the follow-up.
Chinese abstract
背景:鼻腔内翻乳头状瘤(IP)是一种良性肿瘤, 局部复发风险高, 有潜在的恶性化。人乳头状瘤病毒(HPV)被认为是其病因。p16INK4a(p16)过表达被认为是HPV的替代标记, 但p16和HPV是否与IP相关尚不确定。此外, 观察到肿瘤浸润淋巴细胞(TIL)在许多肿瘤中的预后作用, 但其对IP的作用却很少被研究。
目的:在一项基于人群的研究中, 我们分析了人乳头状瘤病毒以确定HPV和p16过表达以及CD8+和FoxP3+TIL的存在和预后作用。
材料和方法:从瑞典癌症登记处对2001-2010年确诊的98例IP患者进行了鉴定, 并通过聚合酶链反应(PCR)对其进行了HPV分析, 通过免疫组织化学对p16、CD8和FoxP3进行了分析。
结果:12.2%的IP为HPV阳性(9例HPV-11, 2例HPV-6, 1例HPV-45)。HPV阳性病变患者年龄较轻(p =.003), 并倾向于出现更多的发育不良。TIL与预后无相关性。
结论和意义:我们的数据表明, HPV阳性IP患者具有不同的临床特征, 提示可能存在不同的疾病实体。此外, 复发时间可能超过5年, 应在随访中予以考虑。
Introduction
Inverted papilloma (IP), often referred to as Schneiderian papilloma, is a locally destructive benign tumor of the sinonasal mucosa with a tendency for malignant transformation and a high propensity for local recurrence [Citation1]. IP arises from the Schneiderian membrane (the transitional sinonasal mucosa) and histologically it typically comprises both exophytic and endophytic components. The tumor is most frequently found in patients aged 50–60 years and is more common in males. Treatment consists of surgery, mostly by endoscopic sinus surgery (ESS), but sometimes in combination with open surgery. Recurrences are mainly related to non-radical surgery [Citation2].
The etiology of IP is still unknown and the pathophysiology remains unclear [Citation3]. However, some authors have proposed human papillomavirus (HPV) as a possible etiological factor in these tumors [Citation4]. Nonetheless, previous studies on HPV and IP have shown conflicting results [Citation5]. Overexpression of the tumor suppressor protein p16INK4a (p16), is often considered as a surrogate marker for high-risk HPV in oropharyngeal squamous cell carcinoma (OPSCC), although its validity as surrogate marker outside the tonsils and base of tongue is under debate [Citation6]. How p16 and high-risk HPV correlate in IP if the markers are associated with malignant transformation, recurrence or dysplasia is unclear [Citation7].
It is still uncertain, which factors, other than radical surgery, affect recurrence and malignant transformation [Citation8]. Recent studies have revealed the prognostic role of tumor infiltrating lymphocytes (TILs) in many different tumors including OPSCC [Citation9] Notably, while high numbers of CD8+ TILs have been correlated to a favorable clinical outcome in OPSCC and other head and neck tumors [Citation10], high numbers of infiltrating FoxP3+ T-regulatory cells (Tregs) have been correlated with a worse clinical outcome in some studies and a more favorable in others [Citation11]. The prognostic role of TILs is sparsely studied in IP. Moreover, increased infiltration of immune cells in IP may reflect an active cell-mediated immune response and indicate involvement of a viral infection (such as HPV) in its pathogenesis.
Therefore, the aim of this study was to analyze the presence of HPV and infiltration of CD8+ T cells and FoxP3+ Tregs in IP in a Stockholm cohort and examine their correlation to malignant transformation, recurrence or dysplasia. Secondly, we investigated the value of p16 as a marker for high-risk HPV in IP.
Materials and methods
Patients
Patients were identified from the Swedish Cancer Registry and the study base consisted of all patients (n = 126) diagnosed with IP in Stockholm between 2001 and 2010. Formalin fixed, paraffin embedded (FFPE) blocks with specimens of IP were retrieved from Stockholms Medicinska Biobank (SMB). After histological re-evaluation of the original diagnosis by a qualified pathologist, 98 cases out of 126 were obtained for further analysis.
Patient data (age at diagnosis, gender, recurrence data, follow-up time, and malignant transformation) were retrieved from the medical records. Reported surgical margins were obtained from the surgical notes. Surgery with unsure margins or where the question of radicality was not mentioned were considered as positive surgical margins. Data of reported dysplasia were obtained from the histopathological reports.
The study was approved by the Ethical Committee at the Karolinska Institute, Stockholm, Sweden (2012/49-31/2).
Detection of HPV DNA
Presence of HPV DNA was analysed as previously described [Citation12]. In brief, 2 × 15 μm sections from FFPE IP tissue blocks, with blank controls in parallel to detect cross-contamination, were cut and DNA was purified using the Roche High Pure FFPET DNA Isolation kit (Roche Diagnostics GmbH, Mannheim, Germany). Presence of HPV DNA was analysed with a multiplex LUMINEX assay covering 27 HPV types (HPV 6, 11, 16, 18, 26, 30, 31, 33, 35, 39, 42, 43, 44, 45, 51, 52, 53, 56, 58, 59, 66, 67, 68, 69, 70, 73 and 82) with the house keeping gene β-globin included as a positive control.
p16 immunohistochemistry
p16INK4a overexpression was analysed using the p16 antibody (clone E6H4, CINtec®, Ventana CINtec® p16 Histology, Roche AB, Stockholm, Sweden) using a standard streptavidin-biotin peroxidase method on 4 µm FFPE IP sections [Citation12]. Percent positive cells and staining intensity were evaluated by two independent researchers (AE and AN) blinded for known clinical diagnosis and a consensus was formed for each sample. p16 positivity was defined as a strong and diffuse cytoplasmatic and nuclear staining in >70% of all cells of the lesion, as described for OPSCC elsewhere [Citation12].
CD8 and FoxP3 immunohistochemistry
Staining of CD8- and Foxp3-positive immune cells was performed using the mouse monoclonal antibodies anti-CD8 (dilution, 1 : 40; clone 4B11; Novocastra Laboratories) and anti-Foxp3 (dilution 1 : 100, clone 236 A/E7; eBioscience) with a standard streptavidin-biotin peroxidase method on 4 µm formalin FFPE IP sections, as previously described [Citation13]. Assessment of CD8/Foxp3-positive immune cells infiltrating the lesion was evaluated by one researcher (AN), blinded for clinical and virus data, by counting positive cells in 10 randomly selected high-power fields (40×) per sample. The mean value was reported for each lesion. The ratio of tumor infiltrating CD8- and Foxp3-positive cells was also calculated for each lesion.
Statistical analysis
The Pearson χ2 and the Fisher exact test were used for categorical data. The Mann-Whitney U test was used for continuous data (median values). Time to recurrence was measured from the date of diagnosis until a documented recurrence of disease, when the patients were considered a case. Patients lost to follow-up were censured. Patients were considered as tumor free after surgery, independent of reported surgical radicality. Patients without any follow-up were censored day 0. The Kaplan-Meier estimator was used to estimate survival and differences in survival was assessed using the log-rank test. Hazard ratios (HR) for recurrence were calculated by Cox regression. Multivariate analysis was conducted using the Cox proportional hazard model. In multivariate analysis, the results were adjusted for age, HPV and radicality,
All tests were performed in SPSS (SPSS Statistics for Mac, Version 21.0. Armonk, NY, USA) and STATA (StataCorp, College Station, TX, USA).
Results
Cases were excluded when re-evaluated as non-IP (n = 6—in most cases exophytic papillomas), when diagnosis was unsure at reevaluation (n = 8), when cases were missing or too little material was left to be representative of the tumor (n = 12) or when registered tumors were unidentifiable in the medical records or the SMB (n = 2). Excluded cases did not differ significantly from those included regarding sex and age at diagnosis (data not shown).
HPV prevalence, p16 overexpression, and patients
In total, 12 out of 98 patient samples (12.2%) were HPV DNA-positive, in which eleven were low-risk type positive (nine HPV-11 and two HPV-6) and one was high-risk HPV (HPV-45). Moreover, p16-overexpression (>70% positive cells) was only observed in the one case which was high-risk HPV. None of the HPV negative and none of the low-risk lesions were p16-positive.
Patients with HPV-positive lesions were significantly younger (mean age 45.6 years vs. 59.6 years, p = .003) and their lesions were reported more often to present with mild to moderate dysplasia (25.0% vs. 8.1%), but this difference was not significant (p = .1) (). Only two patients with an inverted papilloma, which were both HPV negative, malignified.
Table 1. Descriptive data on patients diagnosed with inverted papilloma in Stockholm between 2001 and 2010 per HPV status.
CD8 and Foxp3 immune cell infiltration
Patients with HPV-positive lesions had a higher infiltration of Foxp3-positive immune cells although not statistically significant (p = .077), as compared to those with HPV negative lesions (). No differences were observed regarding Foxp3 infiltration and reported dysplasia.
Figure 1. Boxplot with number of FoxP3-positive cells per high-power field in HPV-positive and negative samples.
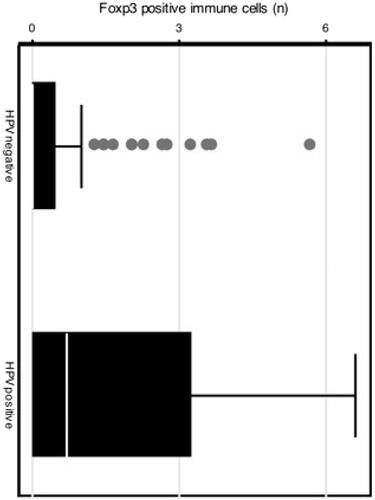
No differences in CD8-positive immune cell infiltration or on CD8/Foxp3 ratio were observed with regard to HPV status or reported dysplasia
Recurrence, HPV status and immune cell infiltration
In our material, the follow-up time ranged from 0–12 years with a median of 5 years. In total 40/98 (40.8%) patients had a recurrence in disease and although 40 patients were lost to follow-up, we still found that 10 patients, 11.2% had late recurrences, after 5 years or more.
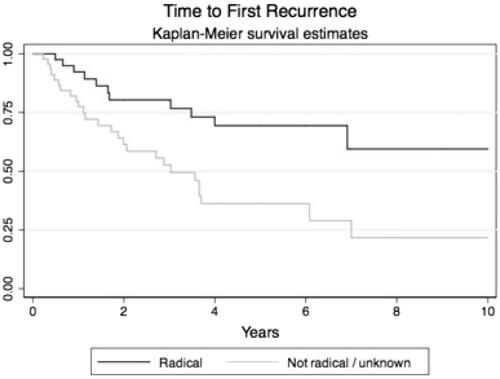
Notably, patients with reported positive or questionable surgical margins had significantly higher recurrence rates as compared to patients with negative or questionable surgical margins (log-rank test p = .001, ). The hazard ratio (HR) for overall recurrence rate for negative compared to positive surgical margins was 0.36 (95% CI 0.18–0.71). Adjustment for age, gender, or HPV did not affect the HR.
Figure 2. Kaplan-Meier curves with time to recurrence in patients treated with radical surgery and patients treated with non-radical surgery.
Moreover, no differences were observed regarding HPV status, CD8 or Foxp3 infiltration and recurrence rates ().
Table 2. Hazard ratios for recurrence in HPV-positive and negative patients.
Discussion
In this study, we report a fairly low proportion of HPV-positive IPs and that p16 overexpression correlated to presence high-risk HPV. Besides, patients with HPV-positive IPs were younger at diagnosis and tended to have higher infiltration of FoxP3+ TILs. Furthermore, the recurrence-rate was higher in patients with negative surgical margins and a considerable proportion of all recurrences occurred after more than five years after surgery.
In our material, we found 12% of patients having HPV-positive lesions, which is lower than many other studies. These differences in HPV prevalence, ranging from 0–100%, may be due to factors such as HPV detection method (in situ hybridization, p16-analysis, PCR), the geographical region/study population, or to the size of the study. Furthermore, it could also possibly be due to a miss-classification of the papilloma type [Citation4]. Here, we found HPV-6 and HPV-11 to be the dominating types—which is similar to previous studies [Citation4]. Moreover, p16-positivity was only found in the specimen with high risk HPV. This correlates well with the findings of Rooper et al. but contrasts with Kim et al. [Citation7,Citation14]. However, in the later, the cut-off for p16 positivity was set at a lower level (10%). Here, a cut-off of 70% was used, which is similar to what is used in OPSCC [Citation12]. Our data implies that p16 overexpression is only related to high-risk HPV in IP and that it may serve as a surrogate marker for high-risk HPV. Since only one specimen was high-risk HPV-positive, conclusions should be drawn with caution.
In our material, patients with HPV-positive IPs were diagnosed at a significantly younger age than patients with HPV negative tumors. They presented also more often with dysplasia (light to moderate) and with a higher infiltration of FoxP3+ T TILs, although these differences were not statistically significant possibly due to the small number of HPV-positive IPs. Patients with HPV-positive IPs had a higher proportion of negative surgical margins, which also might be an indication that their tumors were less extensive or aggressive. This has previously been suggested for sinonasal SCCs [Citation15]. In addition, few studies have investigated the prevalence of HPV in normal sinonasal mucosa. However, some studies have compared HPV prevalence in normal mucosa and in nasal polyps and most of these studies could not detect HPV in normal mucosa [Citation16,Citation17]. Therefore, taken together, the presence of HPV in IP and differences in patient and tumours characteristics, mentioned above, may suggest that HPV is a causative agent in these HPV-positive IPs [Citation17].
For OPSCC, it has been hypothesized that HPV stimulates an effective, prognostically beneficial anti-tumoral response comprising activated CD4+ and especially CD8+ cells and a high CD8+/FoxP3+ ratio [Citation10,Citation13]. It has previously been shown that IP induces a marked immune response [Citation18]. Overexpression of FoxP3 in HPV-positive IPs in our material, even if not statistically significant, may indicate an activation of the immune system, by the HPV virus. However, regarding prognosis, no significant differences in expression of FoxP3, CD8+ or the CD8+/FoxP3+ ratio were found in patients with dysplasia or recurrence compared to those without.
The relatively high rate of recurrence in our material, 40.8%, as compared to previously described recurrences of approximately 20% may be due to the inclusion criteria (where surgery sometimes was for diagnostic purpose thus leading to a limited and subtotal resection of the tumor) and also to the fact that our patients were followed up to twelve years where 10% of the recurrences occurred after five years [Citation19]. As expected, the rate of recurrence was significantly lower in patients who had negative surgical margins. Their still relatively high recurrence rate of 23% may partly be due to the surgical technique. Patients were treated by endoscopic surgery but should, in some cases, possibly have been treated with a combined approach to lower the recurrence rate, as previously has been suggested [Citation19]. It has previously been shown that drilling or cauterization of the underlying bone is of importance when it comes to lowering recurrence rates [Citation2].
We have earlier shown that in a population based study in Sweden the incidence of malignant transformation is 1.35% [Citation20]. In this Stockholm population, the incidence in similar. However, in both of these studies metachronous cancers were more common and there is a risk that in the case of synchronous cancers the inverted papillomas were overlooked and therefore not registered. Therefore, the true malignant potential of IP needs to be further investigated. Nonetheless, in our study, we did not find any statistically significant correlation between HPV and dysplasia, recurrence or malignant transformation or with CD8+ or Foxp3+.
We acknowledge limitations in this study. First, the study material was relatively small and the recurrence rate of the whole study population may be over-estimated, due to the study design—which was initially made to only identify HPV positivity and TILs. Therefore, IPs with diagnostic and not therapeutic surgery were also included. Also, as always, the retrospective design is associated with limitations in data collection, for instance on surgical notes. In this study, surgery was defined as radical when the surgeon clearly reported the opinion of a radical surgery combined with a drilling or resection of the bone underlying the supposed origin of the tumor, in the surgical report. Furthermore, the length and frequency of follow-up differed between patients and it cannot be excluded that this may introduce bias. However, there are also considerable strengths that there was no evident selection bias, since it was population-based and not only based on tertiary referral center patients.
In conclusion, patients with HPV-positive IPs were younger and HPV-positive IPs showed a tendency for higher rate of dysplasia, and a higher infiltration of FoxP3+ cells. Therefore, HPV-positive and HPV negative IPs may be two different entities with different clinical behavior. In addition, recurrences of IP may occur more than five years after surgery and this must be taken in to account in the patients’ follow-up.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Buchwald C, Franzmann MB, Tos M. Sinonasal papillomas: a report of 82 cases in Copenhagen County, including a longitudinal epidemiological and clinical study. Laryngoscope. 1995;105:72–79.
- Healy DY, Jr., Chhabra N, Metson R, et al. Surgical risk factors for recurrence of inverted papilloma. Laryngoscope 2016;126:796–801.
- d'Errico A, Zajacova J, Cacciatore A, et al. Occupational risk factors for sinonasal inverted papilloma: a case-control study. Occup Environ Med. 2013;70:703–708.
- Syrjanen K, Syrjanen S. Detection of human papillomavirus in sinonasal papillomas: systematic review and meta-analysis. Laryngoscope. 2013;123:181–192.
- Jenko K, Kocjan B, Zidar N, et al. In inverted papillomas HPV more likely represents incidental colonization than an etiological factor. Virchows Arch. 2011;459:529–538.
- Hoffmann M, Quabius ES, Tribius S, et al. Influence of HPV-status on survival of patients with tonsillar carcinomas (TSCC) treated by CO2-laser surgery plus risk adapted therapy – a 10 year retrospective single centre study. Cancer Lett. 2018;413:59–68.
- Kim SG, Lee OY, Choi JW, et al. Pattern of expression of cell cycle-related proteins in malignant transformation of sinonasal inverted papilloma. Am J Rhinol Allergy. 2011;25:75–81.
- Wang MJ, Noel JE. Etiology of sinonasal inverted papilloma: a narrative review. World J Otorhinolaryngol Head Neck Surg. 2017;3:54–58.
- Seo N, Shirakura Y, Tahara Y, et al. Activated CD8(+) T cell extracellular vesicles prevent tumour progression by targeting of lesional mesenchymal cells. Nat Commun. 2018;9:435.
- Nordfors C, Grun N, Tertipis N, et al. CD8+ and CD4+ tumour infiltrating lymphocytes in relation to human papillomavirus status and clinical outcome in tonsillar and base of tongue squamous cell carcinoma. Eur J Cancer 2013;49:2522–2530.
- Shah W, Yan X, Jing L, et al. A reversed CD4/CD8 ratio of tumor-infiltrating lymphocytes and a high percentage of CD4(+)FOXP3(+) regulatory T cells are significantly associated with clinical outcome in squamous cell carcinoma of the cervix. Cell Mol Immunol. 2011;8:59–66.
- Marklund L, Nasman A, Ramqvist T, et al. Prevalence of human papillomavirus and survival in oropharyngeal cancer other than tonsil or base of tongue cancer. Cancer Med. 2012;1:82–88.
- Nasman A, Romanitan M, Nordfors C, et al. Tumor infiltrating CD8+ and Foxp3+ lymphocytes correlate to clinical outcome and human papillomavirus (HPV) status in tonsillar cancer. PLoS One 2012;7:e38711.
- Rooper LM, Bishop JA, Westra WH. Transcriptionally active high-risk human papillomavirus is not a common etiologic agent in the malignant transformation of inverted schneiderian papillomas. Head Neck Pathol. 2017;11:346–353.
- Kilic S, Kilic SS, Kim ES, et al. Significance of human papillomavirus positivity in sinonasal squamous cell carcinoma. Int Forum Allergy Rhinol. 2017;7:980–989.
- Sham CL, To KF, Chan PK, et al. Prevalence of human papillomavirus, Epstein-Barr virus, p21, and p53 expression in sinonasal inverted papilloma, nasal polyp, and hypertrophied turbinate in Hong Kong patients. Head Neck. 2012;34:520–533.
- Hoffmann M, Kahn T, Goeroegh T, et al. Tracing human papillomavirus DNA in nasal polyps by polymerase chain reaction. Acta Otolaryngol. 2000;120:872–875.
- Zhao L, Li CW, Jin P, et al. Histopathological features of sinonasal inverted papillomas in chinese patients. Laryngoscope 2016;126:E141–E147.
- Nygren A, Kiss K, von Buchwald C, et al. Rate of recurrence and malignant transformation in 88 cases with inverted papilloma between 1998-2008. Acta Otolaryngol. 2016;136:333–336.
- Elliot A, Marklund L, Hakansson N, et al. Incidence of IP and risk of malignant transformation in the Swedish population 1960-2010. European Arch Otorhinolaryngol. 2017;274(3):1445–1448.
