Abstract
Background: To date, there have been few conventional algorithms for the treatment of idiopathic sudden sensorineural hearing loss (SSNHL), as there have been only limited reports with high evidence levels.
Objectives: To propose an evidence- and trend-based treatment algorithm for SSNHL.
Methods: We referred not only to the evidence for each treatment, but also to trends related to treatment selection in Japan based on epidemiologic surveys, and considered the balance of the advantages and disadvantages with regard to each patient’s condition.
Results: We propose an algorithm that begins with the grade of SSNHL severity as the prognosis of SSNHL is strongly related to the severity of hearing loss. We selected systemic corticosteroid therapy as the first-line therapy, and Intra-tympanic corticosteroid therapy as salvage therapy. We also proposed the use of prostaglandin E1 with corticosteroids for the treatment of SSNHL patients with severe hearing loss. According to the data obtained from an epidemiologic survey, we decided time limits for the application of each treatment.
Conclusion: An algorithm for the treatment for SSNHL is presented according to the results of epidemiologic surveys in Japan. It is expected that this algorithm can provide a guide to choosing the suitable treatment for SSNHL patients.
Chinese abstract
背景:迄今为止, 治疗特发性突发性感音神经性耳聋(SSNHL)的传统算法很少, 因为只有有限的高证据等级的报道。
目的:提出一种基于证据和趋势的SSNHL治疗算法。
方法:在流行病学调查的基础上, 我们不仅参考了每种治疗方法的证据, 还参考了日本治疗选择的相关趋势, 并考虑了对每个患者来说利弊之间的平衡。
结果:由于SSNHL的预后与听力损失的严重程度密切相关, 我们提出了一种从SSNHL严重程度分级开始的算法。我们选择全身皮质类固醇治疗作为一线治疗, 鼓室内皮质类固醇治疗作为补救治疗。我们还建议使用前列腺素E1和皮质类固醇治疗重度听力损失的SSNHL患者。根据流行病学调查获得的数据, 我们确定了每次治疗的应用时限。
结论:根据日本流行病学调查结果, 提出了一种SSNHL的治疗算法。该算法有望为SSNHL患者选择合适的治疗方案提供参考。
Introduction
Idiopathic sudden sensorineural hearing loss (SSNHL) is a type of sensorineural hearing loss of unknown etiology and which is characterized by sudden onset and mostly unilateral hearing loss. There have been many basic and clinical studies on this disorder; however, the pathogenesis of idiopathic SSNHL remains unclear. Several treatments have been proposed for the possible underlying mechanisms of the disease, such as viral infection, intracochlear membrane rupture, and vascular compromise (local ischemia reperfusion). However, there are only a limited number of high evidence level clinical trials to prove the efficiency of each treatment for idiopathic SSNHL [Citation1,Citation2]. Thus, no clear standard treatment strategy for idiopathic SSNHL has yet been established. Recently, new AAO-HNS guidelines for the diagnosis and treatment of idiopathic SSNHL were published [Citation3], and in these guidelines, initial and salvage corticosteroid therapy including intra-tympanic steroid administration, initial and salvage hyperbaric oxygen therapy and other pharmacological therapies are assessed through a literature review and classified into recommendations, options, and recommendations against based on the evidence for each treatment.
In Japan, nationwide epidemiological surveys of idiopathic SSNHL have traditionally been conducted by the Research Committee for Acute Profound Deafness of the Ministry of Health, Labour and Welfare [Citation4–7]. The epidemiological surveys were conducted five times before 2014. The first survey was conducted for the patients who suffered from SSNHL between 1971 and 1973. The second, third, fourth, and fifth surveys were thereafter conducted in 1987, 1993, 2001, and 2012, respectively. presents a summary of these past surveys. These epidemiological surveys were performed with simple questionnaires and were mainly focused on estimations of patient numbers.
Table 1. Summary of the nationwide epidemiological surveys on SSNHL in Japan before 2014.
In 2017, we performed a nation-wide epidemiological survey based on the electrical data collection system (EDCS), and reported our findings regarding the clinical features, treatment details, and treatment outcomes of idiopathic SSNHL based on an analysis of the epidemiologic survey database [Citation8]. In the 2017 epidemiological survey, 26 university hospitals and 4 medical centers took part, and individual information, such as age, gender, height, weight, alcohol consumption, smoking, associated symptoms, medical history, time from the onset to the start of treatment, pre-treatment hearing thresholds, type of treatment, detailed protocol of treatment, and post-treatment hearing thresholds, was registered with EDCS. A summary of this survey in 2017 is shown in .
Table 2. Summary of the epidemiological survey in 2017.
In this report, we propose a treatment algorithm for idiopathic SSNHL, mainly based on the results of the 2017 epidemiologic survey in Japan as well as the review of previous articles. To establish this treatment algorithm, we also refer to the actual treatments protocols widely used in Japan based on the 2017 epidemiologic survey results.
Material and methods
Epidemiological survey
Idiopathic SSNHL subjects (n = 3419) were registered retrospectively with the above mentioned EDCS database between April 2014 and March 2016. Thirty university hospitals and medical centers participated in this epidemiologic survey. Idiopathic SSNHL was defined according to criteria established by the Sudden Deafness Research Committee of the Ministry of Health and Welfare, Japan (2012). Details of the criteria are shown in [Citation9]. Individual clinical information, including the onset age, gender, height, weight, alcohol consumption, smoking, associated symptoms (vertigo, tinnitus), medical history (diabetes, renal disorders, heart disorders, cerebral infarction, hyperlipidemia), time from the onset to the start of treatment, pre-treatment hearing thresholds, type of treatment, detailed protocol of treatment, and post-treatment hearing thresholds, was registered with the EDCS database. We excluded patients for whom the data were incomplete. We also excluded patients who fulfilled the diagnostic criteria for acute low-tone sensorineural hearing loss described previously [Citation10]. We used the initial hearing loss severity grading () and recovery criteria, as defined by the Ministry of Health, Labour and Welfare in Japan () [Citation7].
Table 3. Criteria for the diagnosis of idiopathic SSHNL.
Table 4. Criteria for grading hearing loss in SSNHL.
Table 5. Hearing improvement criteria for SSHNL as defined by the Ministry of Health and Welfare in Japan.
Literature review
We performed a systematic literature review to assess the evidence for each treatment for idiopathic SSNHL. Articles including “Sudden Deafness Hearing Loss” AND “therapy” in their titles and/or abstracts were selected from the Medline database (accessed through PubMed; http://www.ncbi.nlm.nih.gov/pubmed). We identified 1,908 articles published from 1976 to December 31, 2016. We further curated these articles to include only those in peer-reviewed journals published in the English language and containing the hearing prognosis following treatment with corticosteroids, vasodilators, antiviral drugs, or hyperbaric oxygen therapy. The results of this literature review were summarized in the Japanese guidelines for acute hearing loss published in 2018 [Citation11].
Establishment of a treatment algorithm for idiopathic SSNHL
We selected the treatment for SSNHL in this algorithm mainly based on the results of the epidemiologic survey in Japan in 2017 described in our previous reports [Citation8]. In addition to our previous reports, we conducted an additional analysis based on the 2017 survey to estimate (1) the treatment time-window for initial treatment, (2) the treatment outcome differences between the hospitalization group and outpatient clinic group, (3) the treatment outcomes for salvage intra-tympanic (IT) steroid administration based on the initial hearing loss severity, and (4) the treatment time-window for salvage IT steroid treatment. The results are shown in Figures 2–5 in this article. Statistical analysis was performed using PSAW Statistics version 18.0 (SPSS Inc., Chicago, IL).
In terms of the choice of treatment in our algorithm, we referred not only to the evidence for each treatment (including our survey results and literature review), but also to the actual trends regarding treatment selection in Japan described in our previous report [Citation8], and considered the balance of advantages and disadvantages with regard to each patient’s condition.
Results
shows an outline of the proposed treatment algorithm for idiopathic SSNHL. This algorithm begins with the grade of SSNHL severity based on our large cohorts of epidemiological survey results listed below. (1) The prognosis or treatment outcomes for SSNHL were strongly related to the initial severity of hearing loss. Indeed, 38.3% of patients categorized as Grade 1 and 2 showed poor treatment outcomes whereas 45% of patients in Grade 3 and 4 showed poor treatment outcomes in the epidemiological survey [Citation8]. (2) The use of prostaglandin in combination with steroid administration was associated with a good hearing prognosis in patients with severe hearing loss [Citation12]. (3) The outcomes of salvage intra-tympanic (IT) steroid administration also differed with initial hearing loss severity (shown in Figure 3). From these results, we believed the treatment algorithms for mild hearing loss patients and severe hearing loss patients should be different.
Figure 1. Treatment algorithm for idiopathic sudden sensorineural hearing loss based on an epidemiologic survey of a large Japanese cohort.
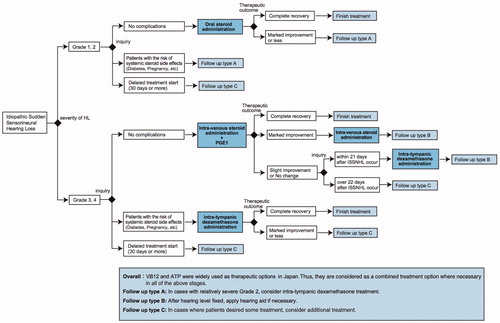
Initial treatment for idiopathic SSNHL
In the 2017 large cohort survey, some type of corticosteroid was administered in the majority of patients (92%), and systemic corticosteroid therapy was used as the most common initial treatment [Citation8], so we selected systemic corticosteroid therapy as the first-line therapy except for patients with high-risk medical conditions such as poorly controlled diabetes, active infection, peptic ulcer disease, prior psychiatric reactions to corticosteroids, or in the early stage of pregnancy. Prednisolone had been used as the most common drug for systemic corticosteroid treatment in the 2017 survey, and the usual maximal dose of prednisolone is 1 mg/kg/day.
For the patients with a high-risk of side effects from systemic corticosteroid treatment, we recommend IT corticosteroid therapy as the first-line therapy in this algorithm. The effect of IT steroid injection is thought to be equal (but not inferior) to systemic steroid treatment as an initial treatment [Citation3,Citation13,Citation14].
However, we limited IT corticosteroid treatment as initial therapy for severe to profound hearing loss (Grade 3/4) cases with a high risk complications or pregnant cases in our algorithm. Because IT corticosteroid injections have a potential risk of persistent perforation of the tympanic membrane, and the potential disadvantage of tympanic membrane perforation outweighs the possibility of hearing recovery in the patients with mild hearing loss. Since dexamethasone or methylprednisolone was used for IT corticosteroid therapy in the majority of past reports [Citation3,Citation14–16], we recommend those drugs for IT administration in this algorithm, and usually inject 0.4–0.8 mL of dexamethasone (4 mg/mL) into the middle ear space up to four injections.
Ng et al. reported that dexamethasone showed more favorable outcomes than did methylprednisolone in the sub-analysis results for a meta-analysis of salvage IT corticosteroid therapy [Citation15].
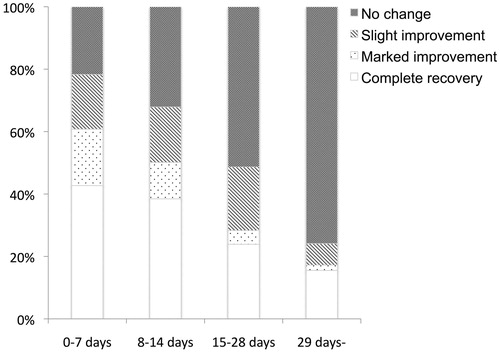
As the time window for initial treatment for idiopathic SSNHL, we compared the hearing improvement of our large cohort results (). As a result, hearing improvement was particularly limited in patients treated at the 29 days or more (4 weeks) from the onset of hearing loss (), so some consideration is necessary whether to treat or not in light of patient preference.
Figure 2. Relationship between the duration from onset of idiopathic SSNHL and hearing recovery (criteria shown in ). The rate of a good improvement (complete recovery or marked improvement) gradually decreased with the increase in the duration from onset. In the 29 days or more group, the criteria of “no change” reached over 75%.
In addition to the corticosteroids, we recommended the use of Prostaglandin E1 (PGE1) in combination with steroid administration as the first-line therapy among patients with severe to profound hearing loss (Grade 3/4). In our results for the large cohort epidemiological survey, PGE1 in combination with steroid administration was associated with a good hearing prognosis in patients with severe hearing loss [Citation12]. Unfortunately, there is no information on the type and dose of PGs administered in the latest epidemiologic survey. In general, we use intravenous drip infusions containing 60 micrograms of PGE1 as reported previously [Citation17].
Salvage treatment for idiopathic SSNHL
As the salvage therapy, we recommended IT corticosteroid administration in this proposed algorithm as, according to the guidelines of the AAO-HNS [Citation3], it is stated that IT corticosteroids should be considered as salvage therapy for the cases refractory to systemic administration.
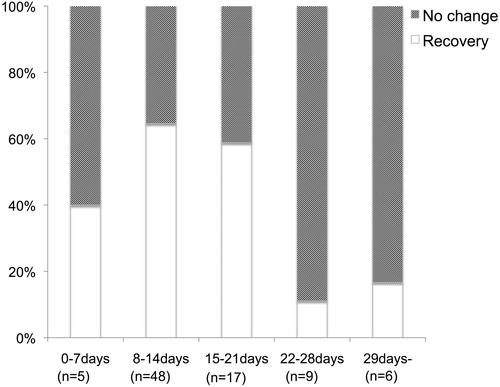
We also restricted salvage IT corticosteroid therapy for patients with severe SSNHL as IT corticosteroid injections are associated with the risk of persistent perforation of the tympanic membrane. Indeed, the outcome of salvage IT corticosteroid injections in mild cases was limited in our epidemiological survey results (). As the time window for salvage IT steroid treatment, we compared the hearing improvement of our large cohort results (). As a result, hearing improvement was particularly limited in patients treated after 22 days from the onset of hearing loss (), so we recommended salvage IT steroid therapy to patients with severe hearing loss and those within 21 days from the onset.
Figure 3. Result of IT steroid therapy as salvage therapy for each grade of hearing loss. The average percent hearing improvement was calculated using following equation: percent hearing improvement = (HLpre-salvage – HLfinal)/(HLpre-treatment – HLcontra). The IT steroid injection was more effective in patients with severe hearing loss (Grade3/4).
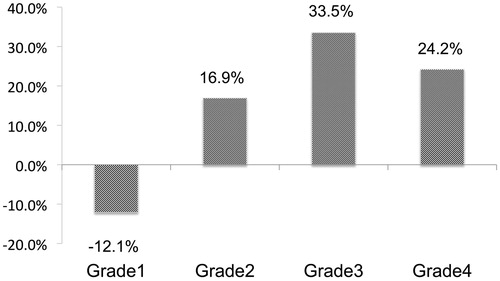
Figure 4. Relationship between the duration from onset of idiopathic SSNHL and IT steroid therapy as salvage therapy. Recovery means a PTA improvement of 10 dB or more from the start of salvage therapy, and no recovery means a PTA improvement of under 10 dB. The treatment effect of IT corticosteroids as a salvage therapy was limited in the patients treated over 21 days from the onset of SSNHL.
In each case, we added the fitting of a hearing aid to the treatment option for the patients with residual hearing after the acute phase of hearing loss and treatment.
Discussion
To date, there has been few conventional algorithms for the treatment of SSNHL as there have been only limited reports with high evidence levels regarding the diagnosis and treatment of SSNHL. In addition, it was very difficult to obtain the clinical condition of each patient. Recently AAO-NHS published new clinical guidelines for the diagnosis and treatment of idiopathic SSNHL [Citation3]. In these guidelines, initial and salvage corticosteroid therapy including intra-tympanic steroid administration, initial and salvage hyperbaric oxygen therapy and other pharmacological therapies were reviewed and integrated into the treatment algorithm. Most of these treatments were assigned as treatment “options” and only salvage IT steroid administration was assigned as a “recommendation” as there were limited numbers of studies with a high-quality level of evidence.
Here we proposed a treatment algorithm for idiopathic SSNHL mainly based on the analysis results of an epidemiological survey for idiopathic SSNHL based on a large nation-wide Japanese cohort and including detailed clinical information for over 3400 patients. We also referred to the results of a literature review and actual treatment protocols used in practical settings in Japan.
Systemic corticosteroid therapy
The efficacy of corticosteroids as a treatment for idiopathic SSNHL has not been sufficiently verified [Citation1,Citation2]. Although, initial corticosteroid treatment is regarded as an “option” in the American Clinical Practice Guidelines for SSNHL [Citation3], corticosteroid treatment was regarded as the de facto standard treatment modality in Japan. Indeed, some type of corticosteroids was administered in the majority of patients (92%) in our survey result [Citation8]. We therefore selected corticosteroids as the first-line therapy for idiopathic SSNHL in this algorithm. Of course, the balance of benefits regarding hearing improvement and the harmful side effects of corticosteroid therapy remains unclear; however, corticosteroid therapy itself was expected to provide equal or better hearing improvement compared to that in non-steroid groups in many previous reports and our results [Citation1,Citation2,Citation8,Citation13]. From these results, we regarded systemic corticosteroid therapy to be a permissible treatment for patients without any high-risk factors, so we recommended initial corticosteroid treatment as the first-line therapy for idiopathic SSNHL. From this “balanced” viewpoint, we separated the patients by severity of SSNHL, systemic complications or medical status indicative of a high risk of side effects related to steroid treatment, and duration of treatment, and recommend different treatment options for each group.
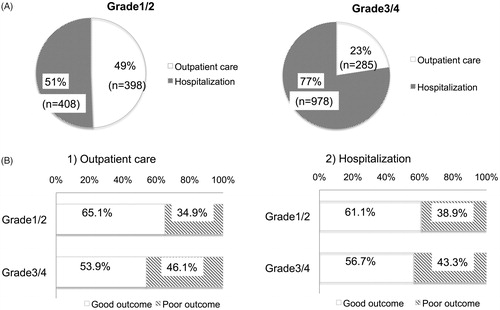
Although there is no study with a high level of evidence to compare the treatment efficacy between outpatient care and hospitalization, we recommended hospitalization during treatment for patients with severe to profound hearing loss according to the results of the epidemiological survey (. Among the patients with severe hearing loss, treatment results were slightly better (but not significantly) in the hospitalization group, although the results were almost equal for the patients with mild hearing loss between outpatient care and hospitalization (. From this finding, we recommended oral corticosteroids administration for mild hearing loss cases and intra-venous corticosteroids for severe hearing loss cases.
Figure 5. Comparison between outpatient care and hospitalization for SSNHL patients. (A) The difference in the selection of outpatient care or hospitalization between grade1/2 and grade3/4 patients with SSNHL. (B) Results of treatment in patients with SSNHL. The upper row of the graph shows the results for patients treated by outpatient care, and the lower row shows the results for patients treated in hospital.
Intra-tympanic corticosteroid injection therapy
The effect of IT steroid injection is thought to be equal (or not inferior) to systemic steroid treatment as an initial treatment [Citation3,Citation13,Citation14], and to be better than other treatments as salvage therapy [Citation3,Citation13–16]. IT steroid injections are recommended as a salvage therapy for the patients who failed to recover after initial treatment in the American Clinical Practice Guidelines for SSNHL [Citation3].
However, the degree and rate of hearing recovery were found to be limited in patients with mild to moderate hearing loss (Grade1/2) in our results (). So the patients with severe to profound hearing loss (Grade 3/4) are thought to be suitable for IT steroid treatment as salvage therapy in this algorithm.
In addition to hearing grade, we limited IT corticosteroid administration as salvage therapy to cases treated within 21 days (3 weeks) from the onset of SSNHL as the treatment effect of IT corticosteroids was limited in patients treated after 22 days from the onset of SSNHL based on our results ().
In the previous report, there are no data to support an absolute time-window for salvage therapy with IT corticosteroid. In several reports on IT corticosteroid therapy designed as a salvage therapy for SSNHL, administration was begun within 7 days after the completion of systemic treatment [Citation3,Citation16]. We set the treatment time-window for salvage IT steroid therapy based on our analysis results and this will provide a useful standard for the treatment time-window of salvage IT steroid therapy.
Prostaglandin E1
In this algorithm, we proposed the use of PGE1 combined with corticosteroids for the treatment of severe idiopathic SSNHL patients (Grade 3/4).
In a meta-analysis, it was proposed that PGE1 was an effective and promising strategy for the treatment of SSNHL [Citation18]. The report assessed the efficacy of PGE1 on SSNHL via a quantitative meta-analysis of 13 randomized controlled studies (total number of 633 patients). The pooled odds ratio was 1.95 and the test for overall effect was 5.31. However, in this meta-analysis, there was no description of the category of the PGE1 treatment, such as single-agent or combination treatment.
In Japan, Kanzaki et al. compared the effects of single-drug treatment including PGE1 (alprostadil) on SSNHL at multiple centers participating in a trial run by the Acute Severe Hearing Loss Study Group in 2003, but there were no statistically significant differences in the recovery rate among any of the drugs tested [Citation19]. However, this trial excluded the patients with profound hearing loss (over 90 dB hearing level). On the other hand, Ogawa et al. performed a prospective randomized trial using PGE1 therapy combined with corticosteroids but there were no statistically significant differences in PTA in comparison with a placebo, but the hearing improvement at higher frequencies was significantly better in the PGE1 group than in the placebo group [Citation17].
In the Japanese epidemiologic survey, which included a large number of patients, Prostaglandins use in combination with steroid administration was associated with a good hearing prognosis in patients with severe hearing loss (over 60 dB hearing level) [Citation12]. The final hearing level and hearing gain of patients treated with steroids + PGs were significantly higher than those of patients treated with steroids alone or no steroids. In addition, the ratio of a good prognosis (complete recovery or marked improvement) in patients treated with steroids + PGs was higher than that in the patients treated with steroids alone or no steroids [Citation12].
Comparison to the AAO-HNS treatment algorithm
In the latest American Clinical Practice Guidelines for SSNHL (published in 2019), an algorithm for diagnosis and treatment has been provided [Citation3]. There are some differences between the AAO-HNS algorithm and that proposed in this paper.
In the AAO-HNS algorithm, the treatment plan is decided mainly based on the period from the onset of hearing loss; however, the algorithm in this paper begins with the grade of SSNHL severity. As a reason for this difference, our treatment algorithm was based on the actual trends in clinical practice in Japan. The treatment plan, including the administration route of corticosteroid (oral, intra-venous, or intra-tympanic), tends to be decided according to the severity of hearing impairment. In addition, the prognosis for each treatment also differed by severity grade. Thus, we believed the treatment algorithm should also consider the severity of SSNHL in each patient.
The use of vasodilators including PGE1 is not recommended in the 2019 AAO-HNS Guidelines as the potential benefits are not clear [Citation3]. In our algorithm, we gave priority to the results of an epidemiological survey of a large cohort that revealed Prostaglandin use in combination with steroid administration was associated with a good hearing prognosis in patients with severe hearing loss (>60 dB hearing level) [Citation12]. This result was also supported by the idea that the treatment should be selected according to the severity of SSNHL.
Limitations of the proposed algorithm
We propose this treatment algorithm based on the results of our epidemiological survey of a large cohort and developed it in line with the actual trends in clinical practice in Japan to make our algorithm useful for evidence-based treatment planning in a practical setting. Thus, some treatment options without a high level of evidence were also included. Further studies to establish a high level of evidence, and/or systematic meta-analysis should be performed to improve this proposed algorithm.
In addition, there is no description of hyperbaric oxygen therapy (HBOT) in this algorithm although HBOT combined with corticosteroid therapy has been described as an initial or salvage treatment option for idiopathic SSNHL in many reports [Citation1,Citation2,Citation20]. The reason why we did not include HBOT into our treatment algorithm was that HBOT is not commonly performed in Japan and only a limited number of patients in our epidemiologic survey had received HBOT. Future studies that include an additional epidemiologic survey including HBOT are needed to include a HBOT option into our algorithm.
Conclusion
In this report, we proposed an algorithm for the treatment of idiopathic SSNHL based on the results of the latest large cohort epidemiologic survey in Japan. It is expected that this algorithm can provide a guide for choosing the most suitable treatment for idiopathic SSNHL patients and also help to extract the task in the conventional treatment of the SSNHL. In the future, we expect to discover new treatment methods for patients in the poor prognosis category using this algorithm.
Disclosure statement
No potential conflicts of interest were reported by the authors.
Additional information
Funding
References
- Conlin AE, Parnes LS. Treatment of sudden sensorineural hearing loss: I. A systematic review. Arch Otolaryngol Head Neck Surg. 2007;133(6):573–581.
- Larwrence R, Thevasagayam R. Controversies in the management of sudden sensorineural hearing loss: an evidence-based review. Clin Otolaryngol. 2015;40:176–182.
- Chandrasekhar SS, Tsai Do BS, Schwartz SR, et al. Clinical practice guideline: sudden hearing loss (Update). Otolaryngol Head Neck Surg. 2019;161(1_suppl):S1–S45.
- Nakashima T, Yanagita N, Ohno Y, et al. Comparative study on sudden deafness by two nationwide epidemiological surveys in Japan. Acta Otolaryngol Suppl. 1994;514:14–16.
- Nakashima T, Itoh A, Misawa H, et al. Clinicoepidemiologic features of sudden deafness diagnosed and treated at university hospitals in Japan. Otolaryngol Head Neck Surg. 2000;123(5):593–597.
- Teranishi M, Katayama N, Uchida Y, et al. Thirty-year trends in sudden deafness from four nationwide epidemiological surveys in Japan. Acta Otolaryngol. 2007;127(12):1259–1265.
- Nakashima T, Sato H, Gyo K, et al. Idiopathic sudden sensorineural hearing loss in Japan. Acta Otolaryngol. 2014;134(11):1158–1163.
- Kitoh R, Nishio SY, Ogawa K, et al. Nationwide epidemiological survey of idiopathic sudden sensorineural hearing loss in Japan. Acta Otolaryngol. 2017; 137(sup565):S8–S16.
- Nomura Y. Morphological aspects of inner ear disease. Tokyo: Springer. 2014.
- Shimono M, Teranishi M, Yoshida T, et al. Endolymphatic hydrops revealed by magnetic resonance imaging in patients with acute low-tone sensorineural hearing loss. Otol Neurotol. 2013;34(7):1241–1246.
- Systematic Review Summary. In: Japan Audiological Society. Clinical Practice Guidelines for the Diagnosis and Management of Acute Sensorineural Hearing loss 2018. Tolyo: Kanehara-Shuppan; 2018. p. 106–124. Japanese.
- Okada M, Hato N, Nishio SY, et al. The effect of initial treatment on hearing prognosis in idiopathic sudden sensorineural hearing loss: a nationwide survey in Japan. Acta Otolaryngol. 2017;137(sup565):S30–S33.
- Crane RA, Camilon M, Nguyen S, et al. Steroids for treatment of sudden sensorineural hearing loss: a meta-analysis of randomized controlled trials. Laryngoscope. 2015;125(1):209–217.
- El Sabbagh NG, Sewitch MJ, Bezdjian A, et al. Intratympanic dexamethasome in sudden hearing loss: systematic review and meta-analysis. Laryngoscope. 2017;127(8):1897–1908.
- Ng JH, Ho RC, Cheong CS, et al. Intratympanic steroids as a salvage treatment for sudden sensorineural hearing loss? A meta-analysis. Eur Arch Otorhinolaryngol. 2015;272(10):2777–2782.
- Zhou Y, Zheng H, Zhang Q, et al. Early transtympanic steroid injection in patients with 'poor prognosis' idiopathic sensorineural sudden hearing loss. ORL J Otorhinolaryngol Relat Spec. 2011;73(1):31–37.
- Ogawa K, Takei S, Inoue Y, et al. Effect of prostagrandin E1 on idiopathic sudden sensorineural hearing loss: a double-blinded clinical study. Otol Neurotol. 2002;23(5):665–668.
- Zhuo XL, Wang Y, Zhuo WL, et al. Is the application of prostaglandin E1 effective for the treatment of sudden hearing loss? An evidence-based meta-analysis. J Int Med Res. 2008; 36(3):467–470.
- Kanzaki J, Inoue Y, Ogawa K, et al. Effect of single-drug treatment on idiopathic sudden sensorineural hearing loss. Auris Nasus Larynx. 2003;30(2):123–127.
- Bennett MH, Kertesz T, Perleth M, et al. Hyperbaric oxygen for idiopathic sudden sensorineural hearing loss and tinnitus. Cochrane Database Syst Rev. 2012;10:CD004739.
