Abstract
Background: An adhesively attached bone conduction hearing device has been newly developed. This novel bone conduction hearing device is placed behind the ear and has an audio processor connected to the adapter to transmit sound through vibrations.
Objective: To obtain preliminary results regarding the use of this device, we sought to apply it to patients with various types of hearing loss.
Methods: Nine patients aged over 18 years and with hearing loss due to bilateral middle ear anomaly (n = 1), bilateral aural atresia (n = 3), unilateral aural atresia (n = 2), and single-sided deafness (n = 3) were recruited.
Results: The functional gain provided by the adhesive bone conduction hearing device for the aided side was found to be sufficient. Although the results on speech perception in noise showed significant improvement for patients with conductive hearing loss, no improvement was found for patients with single-sided deafness. Subjective assessment showed that speech and spatial hearing-related issues were improved.
Conclusion: The adhesive bone conduction hearing device was thought to provide a feasible option. Additionally, in patients considering the use of a surgically implanted hearing device, this device is a preferable option for preoperative assessment due to its non-invasiveness.
Chinese abstract
背景:新开发了一种附着式骨传导听力装置。这种新型的骨传导听力装置被放置在耳朵后面, 并有一个音频处理器连接到适配器上, 通过振动传递声音。
目的:为了获得使用该设备的初步结果, 我们试图将其应用于各种类型的听力损失患者。
方法:对9例18岁以上双侧中耳畸形(n =1)、双侧耳闭锁(n =3)、单侧耳闭锁(n =2)、单侧耳聋(n =3)所致听力损失患者进行回顾性分析。
结果:附着式骨传导听力装置可充分提高受助侧的听力功能。虽然传导性耳聋患者在噪声中的语音感知有显著改善, 但单侧耳聋患者的语音感知没有改善。主观评价显示, 言语和空间听觉相关问题均得以改善。
结论:附着式骨传导听力装置是一种可行的选择。此外, 对于考虑使用手术植入的听力装置的患者, 该装置由于其非创伤性, 是术前评估的最佳选择。
Introduction
Bone conduction (BC) hearing aids have typically been used for conductive or mixed hearing loss patients who are unable to use a conventional hearing aid due to aural atresia, microtia, or other pathogens that can impair sufficient amplification. The BC vibrator has to be placed onto the skin with high transcutaneous pressure using a soft band or a steel spring headband; otherwise, the sound cannot be transmitted sufficiently through the skin into the bone. This can cause discomfort and skin defects in some cases, causing the patients to give up on using BC hearing aids. To overcome these disadvantages, bone conductive implants (BCIs) and middle ear implants (MEIs) have been developed since the 1970s and have been widely applied for patients with conductive or mixed hearing loss [Citation1]. Currently, several types of BCI and MEI based on different mechanisms and surgical procedures are available; however, each type of implant has its specific advantages and disadvantages, as well as complications [Citation2]. The bone-anchored hearing aid (Baha) is a well-known BCI that is surgically placed as an external abutment to the temporal bone. The Bonebridge (BB) is similar to a cochlear implant system; it is an active transcutaneous system, in which a vibrating transducer is totally implanted under the skin and temporal bone, with an audio processor worn to provide sound signals through the skin. The most well-known MEI is the Vibrant Soundbridge (VSB), which has a very small vibrating transducer, termed a floating mass transducer (FMT), which is connected to the ossicles or placed on the round window. Both the BB and VSB are positioned on the temporal bone with the skin intact, whereas the Baha needs to be embedded with an external abutment exposed outside the skin. The specific disadvantages or complications of each system include (1) poor aesthetics and the irritation or overgrowth of skin, resulting in loss of the abutment for the Baha [Citation3,Citation4]; (2) the need for adequate space to place the transducer in the mastoid area for the BB [Citation5]; and (3) difficulty in fixing the FMT to avoid facial nerve injury, in the case of middle ear anomalies for the VSB [Citation6,Citation7].
Recently, an adhesively attached BC hearing device that is non-implantable, pressure-free, and non-invasive has been developed. This novel BC adhesive hearing device, termed ADHEAR (MED-EL, Innsbruck, Austria), consists of an adhesive adapter, which is placed behind the ear in contact with the skull, and an audio processor connected to the adapter to transmit sound through vibrations. In this study, we sought to obtain preliminary results regarding the use of ADHEAR by applying it to patients with various types of hearing loss. Here, we present the hearing abilities observed while using the adhesive hearing device and provide details of several notable cases.
Subjects and methods
Participants
Nine hearing loss patients aged over 18 years were recruited for this study. Six patients were men, and three were women. The patient diagnoses were bilateral middle ear anomaly (n = 1), bilateral aural atresia (n = 3), unilateral aural atresia (n = 2), and single-sided deafness (SSD) (n = 3). The patient demographics and details are shown in . Each subject ID was defined by the type of hearing loss as follows; ACB: ADHEAR-conductivebilateral, ACU: -conductive unilateral, and ASU: -sensorineural unilateral. Subjects ACB1 (steal headband type) and ACB2 (eyeglasses type) used conventional BC hearing aids (BC-HAs) for both sides. Subjects ACB3 and ACB4 had tiny ear canals and underwent canal reconstruction surgery, and were somehow able to use a small in-the-canal hearing aid (ITC-HA) for the left ear. The other subjects (ACU1 and 2, and ASU1, 2, and 3) had never continuously used any type of hearing device. The ADHEAR was fitted according to the manufacturer’s recommendations, and the optimal position for adapter placement was ascertained. We provided instructions for the participants and their families so that they were able to use it effectively and adjust the loudness by themselves.
Table 1. Subject demographics.
This study was approved by the Ethics Committee of Shinshu University School of Medicine (IRB approval number: 3880).
Clinical evaluations
All audiological testing was performed prior to and at 1 and 3 months after the start of ADHEAR use. Free-field audiometry, speech perception tests in quiet and noise, and sound localization tests were carried out. To minimize cross hearing with the normal or better-hearing ear (non-device ear) during free-field audiometry, adequate masking was applied by placing an ear muff over the ear and/or inserting an earplug. For the speech perception test, we used the Japanese monosyllable test (67S test). The speech signal was presented at a constant 65dB sound pressure level in the 67S test, whereas the noise signal varied across a signal-to-noise (SN) ratio of SN + 10 dB, SN0 dB, and SN − 5 dB. Tests were performed in a free field, with the subject seated one meter away from the loudspeakers. The spatial configurations for the speech testing were as follows: (1) speech and noise were presented from the front (S0N0); (2) speech from the front and noise from the hearing loss side (i.e. the device side) (S0Naided); and (3) speech from the front and noise from the normal or better-hearing side (S0Nnormal ear).
Sound localization testing was performed in a semi-anechoic chamber, with nine loudspeakers located in a semi-circle of 1-m radius (−90° to 90°). A total of 135 speech-shaped 1-s noise bursts were randomly selected at 60–80 dB.
The subjective benefits of the ADHEAR were assessed using the Speech, Spatial, and Qualities of Hearing Scale (SSQ) [Citation8]. The SSQ is a self-reported questionnaire that is comprised of 50 questions covering the three domains of speech understanding, spatial hearing, and sound quality. This questionnaire was administered before applying the ADHEAR device and at 1–3 months after application. We interviewed and examined the participants monthly, including every visit for the above evaluations, and at any time the participants needed medical consultations regarding the device.
For the speech perception test, sound localization test, and self-reported questionnaire assessment, subjects ACB1 and ACB2 were assessed using the BC-HA, and subjects ACB3 and ACB4 were assessed using the ITC-HA; the results thus obtained were considered to be under “unaided” conditions to compare the subjects’ usual hearing setting with that for ADHEAR use.
Statistical analysis
Statistical analysis was performed using the Prism 7 software package (GraphPad, San Diego, CA). The two groups were compared using an unpaired two-tailed Student’s t-test. A p value < .05 was considered statistically significant.
Results
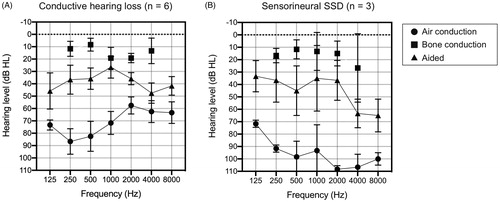
Hearing thresholds and functional gain
The average unaided and aided thresholds for each group; i.e. conductive hearing loss and sensorineural SSD, are shown in . In the conductive hearing loss subgroup, the average pure tone threshold of 500, 1000, 2000, and 4000 Hz for the aided ear was 69 dBHL and 15 dBHL for air conduction (AC) and BC thresholds, respectively. In the sensorineural SSD subgroup, the average AC threshold was 102 dBHL, and BC threshold for the healthy (contralateral) ear was 17 dBHL. With the use of the adhesive hearing device ADHEAR, the average aided thresholds were 36.5 dB and 45.0 dB for the conductive hearing loss subgroup and SSD subgroup, respectively.
Figure 1. The average ADHEAR-aided thresholds and the pure tone average for air conduction and bone conduction thresholds at 3 months after use. (A) In conductive hearing loss patients (n = 6) and (B) SSD patients (n = 3). With the use of the adhesive hearing device, ADHEAR, the aided thresholds were improved on average by 36.5 dBHL and 45.0 dBHL for the conductive hearing loss subgroup and the SSD subgroup, respectively. Bone conduction thresholds in the SSD group indicate hearing levels in the contralateral, healthy ear (B). SSD: sensorineural single-sided deafness.
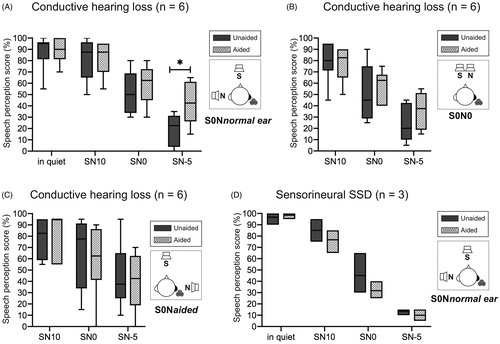
Speech perception in noise
show the results of the three configurations for the speech in noise test in the conductive hearing loss subgroup (n = 6). No differences in the test scores between the unaided and aided conditions were observed in quiet conditions. For the S0Nnormal ear condition, the speech perception scores for the unaided and aided conditions were improved from 52% to 59% at SN0 and from 19% to 43% at SN-5. There were no significant differences in the speech perception scores between the unaided and aided conditions for the S0N0 and S0Naided configurations. For the sensorineural SSD subgroup, no significant differences were observed for any of the SN values, even for the S0Nnormal ear conditions ().
Figure 2. Speech perception tests in multiple noise configurations at 3 months after use. (A–C) In conductive hearing loss patients and (D) SSD patients. For the S0Nnormal ear conditions in the conductive hearing loss subgroup, the speech perception scores for the unaided and aided conditions were improved from 52% to 59% at SN0 and from 19% to 43% at SN-5 (∗p < .05) (A). There were no significant differences in the speech perception scores between the unaided and aided conditions for the S0N0 and S0Naided configurations (B, C). For the sensorineural SSD subgroup, no significant differences were observed for the S0Nnormal ear conditions (D). SSD, sensorineural single-sided deafness.
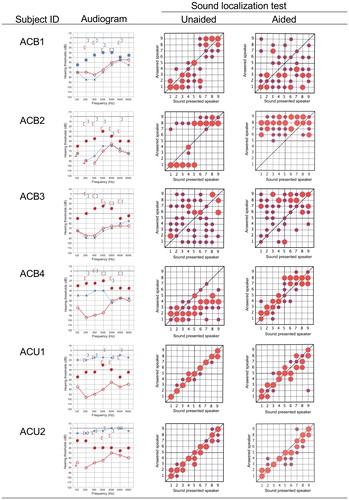
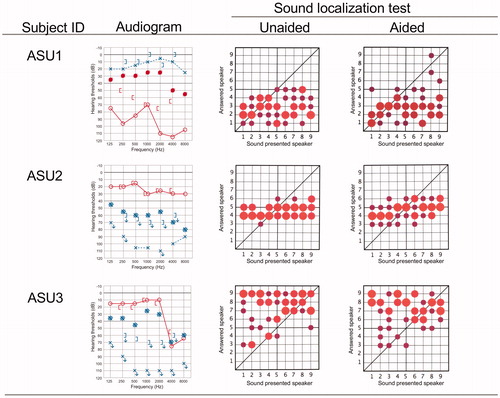
Sound localization
As shown in , the results of the sound localization test varied among the subjects, and no general tendencies among the variations related to the type of hearing loss could be extracted. Improvement in the sound localization ability was found in only one subject, ACB4. Notably, the results for subjects ACU1 and ACU2, who had unilateral conductive hearing loss, showed almost normal sound localization ability without the device, with the results obtained under aided conditions being slightly worse, indicating a disturbance in the accuracy of sound localization with the device.
Figure 3. Sound localization abilities under unaided and aided conditions for each subject at 3 months after use. Pure tone audiograms and aided thresholds are shown on the left. The horizontal axis shows the number of speakers arranged from the –90 to 90 azimuths, and the vertical axis shows the number of speakers from which the sound could be correctly identified. Speaker No. 5 was directly in front of the patient. The size of each circle in the diagram indicates the number of times the patient chose each specific speaker. Larger circles indicate that the patient chose a speaker more frequently, whereas smaller circles indicate that the speaker was less frequently chosen. The position of each circle indicates the position of the sound-presenting speaker chosen by the patient.
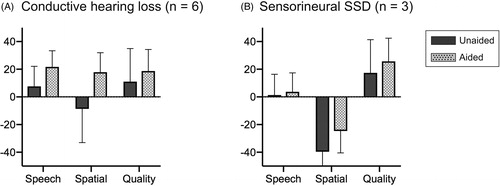
Subjective assessments
As shown in , use of the device for 3 months in the conductive hearing loss subgroup led to improvement in the results of the SSQ questionnaire for all scores in the speech, spatial hearing, and perceptive hearing domains. The mean scores for speech, spatial hearing, and perceptive hearing were 7.6, −8.6, and 11.0, respectively, under unaided conditions (i.e. before starting to wear the device); and these improved to 21.7, 17.8, and 18.7, respectively, after use of the device in aided conditions. For the sensorineural SSD subgroup, the results showed improvement between the unaided and aided conditions from 1.3, −39.9, and 17.3 to 3.7, −24.6, and 25.6, respectively. No statistically significant differences were observed, and this was probably due to the small sample size.
Clinical findings and self-reported experiences
We kept records of the patients’ complaints and/or symptoms during the study period. The adhesive adapter seemed to be more stable and could be placed more easily behind the auricle for subjects who had hard scarring and skin tension due to auricular reconstruction surgery in comparison with those with normal ears. Therefore, the subjects with aural atresia from surgery had longer durability (approximately 4 or 5 days wearing capability), whereas the others with normal auricles had shorter durability of less than 3 days. Therefore, one patient (subject ACU1), who had unilateral atresia, developed mild dermatitis around the adhesive adapter (). A dermatologist diagnosed the problem as skin irritation due to soap or shampoo residue, not from the adapter itself, as the skin under the adapter showed normal findings ().
Figure 5. Mild dermatitis around the adhesive adapter and inappropriate position of the device. Mild dermatitis around the adhesive adapter was observed in subject ACU1. The erythema is observed only in the surrounding area and not directly under the area of contact with the adapter (A, B). Subject ACB2 placed the adapter on the lower mastoid area so he could not obtain sufficient gain from the device due to sound transmission attenuation by the soft tissues.
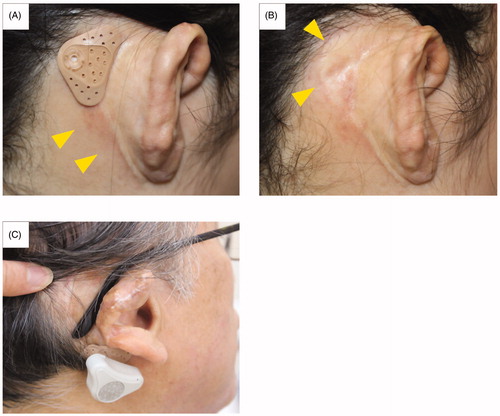
Another patient, subject ACB2, had concerns about eyeglass use with the adapter (). He placed the adapter on the lower mastoid area, so he could not obtain sufficient gain from the ADHEAR due to sound transmission attenuation by the soft tissues.
Discussion
The results from this heterogeneous group of hearing loss patients showed variations according to their hearing status. For all six patients with conductive hearing loss, including those with aural atresia and middle ear anomalies, the audiological results revealed that the adhesive BC hearing device gave adequate hearing gain and improved speech perception in noise. The subjective assessment by the SSQ questionnaire also showed an increase in the hearing ability after using the adhesive hearing device in daily life. In particular, the three patients with bilateral conductive hearing loss reported that the adhesive hearing device was very useful and that they wanted to continue using it even after the 3-month trial period (). Dahm et al. assessed the efficacy of the same device in 12 patients with bilateral or unilateral conductive hearing loss and reported significant functional gain, improvement in word recognition score, and benefits related to improved patient satisfaction [Citation9]. Gawliczek et al. reported and validated the use of the adhesive hearing device in 15 subjects with induced (i.e. occluded with earplugs) bilateral conductive hearing loss [Citation10]. In our study, although two patients with unilateral aural atresia were satisfied with the device for an initial period of 1–2 months, they gradually found it bothersome to wear at 3 months; they wanted to use it only in noisy conditions as they had been used to hearing from only one side for so long. The results of the speech perception test in noise revealed significant improvement in limited conditions in the S0Nnormal ear with high noise. Interestingly, the results of sound localization in both cases were normal, although they could only hear from one side. As shown in the right column of , 3 of 4 subjects with bilateral conductive hearing loss regarded the device as preferable and 2 subjects with unilateral hearing loss felt that the device was unnecessary for use in daily life. However, because of the limited number of subjects with severe unilateral conductive hearing loss in this study, further studies on a larger population may provide more subjective insights, including those from patients with unilateral conductive hearing loss. We believe that appropriate counseling and a sufficient trial period are necessary to gain more complete results for patients with unilateral conductive hearing loss. Moreover, for patients who considered undergoing BCI or MEI surgery for conductive hearing loss, the trial use of the adhesive hearing device may be helpful in preoperative decision-making in order to prevent patients from later becoming BCI or MEI non-users.
The three subjects with sensorineural SSD showed no improvement in speech perception or sound localization, and two of them (ASU2 and 3) did not want to use the device on a daily basis, despite the functional hearing gains transmitted to the contralateral normal cochlea. However, the bone-conduction thresholds of the contralateral ear for these two SSD subjects were 28 and 26 dBHL (on average of 500, 1000, 2000, and 4000 Hz), which were slightly outside the ADHEAR eligibility criteria of below 20 dB on the normal hearing for SSD patients, according to the manufacturer. For another SSD subject, ASU1, although the 10-dB bone conduction threshold of the contralateral ear met the criteria, he was moderately satisfied with the ADHEAR, particular in noisy conditions. We thought that small differences in the hearing levels of the normal ear seemed to influence the benefit afforded by the adhesive hearing device, suggesting a limitation in transmitting output power to the contralateral ear. We enrolled only a few participants; therefore, we had limited results for patients with sensorineural SSD. Nevertheless, the use of the ADHEAR seemed to require careful attention with regard to patient selection. Indeed, two SSD patients decided to proceed with the clinical trial for the cochlear implant program for SSD after the current adhesive hearing device trial. There are many patients who have SSD due to sensorineural or conductive hearing loss, and it is likely that most of these patients do not use any hearing aid in the affected ear. However, they still require assistance in order to hear properly, particularly in noisy conditions. For sensorineural SSD patients, no substantial improvement in speech perception in noise or sound localization were observed after using the adhesive hearing device, although only three subjects were included in the trial. Further, Mertens et al. have investigated the effect of the adhesive hearing device in 17 patients with SSD and reported no significant improvement in speech perception in noisy conditions with the device; however, only slight improvement in sound localization was observed under device-aided conditions [Citation11].
Various BCIs and new BC hearing devices have become available, and the indications for each have considerable overlap. This may appear to be beneficial, as patients have more choices in terms of devices; however, the clinical decision-making, considering the relative advantages and disadvantages of each device, is now more complex [Citation12,Citation13]. Due to the non-invasiveness of the adhesive hearing device, one possible use would be as a preoperative assessment tool. In fact, one patient with bilateral aural atresia, subject ACB3, decided to be implanted with a BB in the left ear after the ADHEAR trial for the left ear and wanted to use the ADHEAR continuously for the right ear, resulting in bilateral hearing through a combination of the two BC hearing devices.
We observed mild dermatitis and inappropriate positioning of the adapter in the subjects. To maximize the benefits associated with the adhesive hearing device, we suggest appropriate counseling and advice on possible adverse events and unsuccessful hearing outcomes, as well as examinations for the patients.
In conclusion, we presented the preliminary results of a new adhesive BC hearing device for patients with various types of hearing loss. The adhesive hearing device was thought to be a good option for patients who could not use conventional hearing aids. Additionally, for patients considering the use of surgically implanted hearing devices, the use of ADHEAR may be a preferable option for preoperative assessment due to its convenience and non-invasiveness.
Ethics approval
This study was approved by the Ethics Committee of Shinshu University School of Medicine (IRB approval number: 3880).
Disclosure statement
For this study, MED-EL supplied the adhesive hearing device, ADHEAR, as this hearing device had not yet been approved by the Ministry of Health of Japan during the study period. The authors disclosed the above conflict of interest in this clinical research to the Ethics Committee of Shinshu University School of Medicine and received the committee’s approval.
References
- Mudry A, Tjellstrom A. Historical background of bone conduction hearing devices and bone conduction hearing aids. In: Implantable bone conduction hearing aids. Vol. 71. Basel: Karger Publishers. 2011. p. 1–9.
- Reinfeldt S, Hakansson B, Taghavi H, et al. New developments in bone-conduction hearing implants: a review. Med Devices (Auckl). 2015;8:79–93.
- Dun CA, Faber HT, de Wolf MJ, et al. Assessment of more than 1,000 implanted percutaneous bone conduction devices: skin reactions and implant survival. Otol Neurotol. 2012;33(2):192–198.
- Kiringoda R, Lustig LR. A meta-analysis of the complications associated with osseointegrated hearing aids. Otol Neurotol. 2013;34(5):790–794.
- Riss D, Arnoldner C, Baumgartner WD, et al. Indication criteria and outcomes with the Bonebridge transcutaneous bone conduction implant. Laryngoscope. 2014;124(12):2802–2806.
- Lesinskas E, Stankeviciute V, Petrulionis M. Application of the Vibrant Soundbridge middle-ear implant for aural atresia in patients with Treacher Collins syndrome. J Laryngol Otol. 2012;126(12):1216–1223.
- Ikeda R, Hidaka H, Murata T, et al. Vibrant Soundbridge implantation via a retrofacial approach in a patient with congenital aural atresia. Auris Nasus Larynx. 2019;46(2):204–209.
- Gatehouse S, Noble W. The Speech, Spatial and Qualities of Hearing Scale (SSQ). Int J Audiol. 2004;43(2):85–99.
- Dahm V, Baumgartner WD, Liepins R, et al. First results with a new, pressure-free, adhesive bone conduction hearing aid. Otol Neurotol. 2018;39(6):748–754.
- Gawliczek T, Munzinger F, Anschuetz L, et al. Unilateral and bilateral audiological benefit with an adhesively attached, noninvasive bone conduction hearing system. Otol Neurotol. 2018;39(8):1025–1030.
- Mertens G, Gilles A, Bouzegta R, et al. A prospective randomized crossover study in single-sided deafness on the new non-invasive adhesive bone conduction hearing system. Otol Neurotol. 2018;39(8):940–949.
- Monini S, Filippi C, Atturo F, et al. Is the Bone conduction HeadBand test useful for predicting the functional outcome of a round window active middle ear implant? Otol Neurotol. 2013;34(7):1329–1335.
- Spielmann PM, Roplekar R, Rae C, et al. Is the use of a bone conduction hearing device on a softband a useful tool in the preoperative assessment of suitability for other hearing implants? J Laryngol Otol. 2018;132(06):505–508.