Abstract
Background: Few have investigated long-term effect of treatment of posttraumatic olfactory dysfunction (OD).
Aims/objectives: To explore if sequential treatment with corticosteroids and olfactory training (OT) improved smell in patients with OD after moderate and severe traumatic brain injury (TBI).
Material and methods: Twenty-two patients with persistent OD, mean 62 months after trauma, completed an open uncontrolled intervention study of treatment for 10 d with oral corticosteroids and thereafter for 3 months with OT twice daily. Olfaction was assessed by Sniffin’ Sticks. They were tested at four-time points, with the last assessment 12 months after baseline measurements.
Results: Mean age at trauma was 45 (SD 14) years. Mean threshold, discrimination and identification (TDI) score at baseline was 14.4 (SD 7.3) and increased to mean 20.8 (SD 7.4) after 1 year (minimum −3.0; maximum 19.5, p value <.001). Analysed separately, each TDI component increased significantly after 1 year. Half of the patients (11/22) experienced a clinically significant improvement of ≥6.0 TDI points. Improvement was not associated with any sociodemographic or trauma-related characteristics or with olfactory function at baseline.
Conclusions and significance: Treatment with corticosteroids and OT was promising in persistent OD after TBI and should be further studied.
Chinese abstract
背景:很少有人研究治疗创伤后嗅觉功能障碍(OD)的长期疗效。
目的:探讨糖皮质激素联合嗅觉训练(OT)序贯治疗对中重度颅脑损伤(TBI)后OD患者嗅觉的改善作用。
材料与方法:22例持续性OD患者, 创伤后平均62个月, 采用开放性非对照干预研究, 口服皮质类固醇治疗10天, 然后接受每日2次OT, 持续 3个月。用嗅棒测定嗅觉。他们在四个时间点进行测试, 最后一次评估是在基线测量12个月后。
结果:平均创伤年龄45岁(SD 14)。基线检查时的平均阈值、鉴别和鉴定(TDI)得分为14.4(标准偏差7.3), 1年后增加到平均20.8(标准偏差7.4)(最小值3.0;最大值19.5, p值<.001)。经单独分析, 各TDI组分在1年后显著增加。半数患者(11/22)出现临床显著改善!6.0 TDI点。改善与任何社会人口学或创伤相关特征或基线嗅觉功能无关。
结论及意义:糖皮质激素联合OT治疗TBI后持续性OD是一种有前景的方法, 值得进一步研究。
Introduction
The importance of the sense of smell is increasingly recognized, and olfactory dysfunction (OD) can negatively affect quality of life. OD can be classified as qualitative, involving changes in the quality of odours, or quantitative, involving changes in the perceived strength of odours [Citation1]. The quantitative changes are further classified according to degree of disturbance; in hyposmia, the sense of smell is reduced, and in functional anosmia, there is no useful olfactory function in daily life. Functional anosmia is referred to as anosmia in this article.
In epidemiological studies, the prevalence of OD is reported to be approximately 20%, and this proportion increases with advanced age [Citation2]. The prevalence of anosmia specifically, however, is much lower; 1–5% [Citation3]. The aetiology of OD is heterogeneous and includes, in addition to physiological aging, traumatic brain injury (TBI), congenital anosmia, neurologic diseases, upper airway infections and sinonasal disease. Persistent OD is common after TBI, and many of these cases have a severe degree of OD [Citation1,Citation4]. The background for posttraumatic OD is complex and involves both peripheral and central mechanisms. In a peripheral injury, the olfactory nerve is damaged along the pathway from the olfactory epithelium in the nasal cavity to the olfactory bulb in the anterior skull base. Intracranial haemorrhage, cortical contusions including damage of the olfactory bulb and/or traumatic axonal injury, may affect olfactory centres and olfactory pathways in the brain, and due to its localization, the olfactory nerve system is especially vulnerable to frontal trauma [Citation1]. Spontaneous recovery of posttraumatic OD has been reported in approximately one third of cases, mostly within the first year after the trauma, but not all of these improved to their preinjury sense of smell [Citation5].
Various pharmacological treatments of OD of various aetiologies have been tested, such as zinc gluconate, intranasal insulin, minocycline, vitamin A, vitamin B, Ginkgo biloba, caroverine and corticosteroids, the latter being the most commonly used drugs [Citation1]. According to animal trials, early treatment with corticosteroids may facilitate regeneration of an injured olfactory nerve and regress of scar tissue [Citation6]. When it comes to posttraumatic persistent OD in humans, corticosteroid treatment studies show improved sense of smell in 12–16% of patients [Citation7].
Olfactory training (OT) is a promising treatment for OD of various aetiologies, and OT is currently the therapy for OD with the strongest evidence of effect [Citation8]. The olfactory neuron system is well known for its capacity to regenerate damaged olfactory neurons in the olfactory epithelium, and it is hypothesized that OT may enhance this regeneration [Citation9]. OT may also influence central olfactory structures at the level of the olfactory bulb [Citation10] and induce changes in brain connectivity [Citation11]. Established OT includes sniffing four different odours twice daily for a recommended period of 3 months or more. Regarding persistent posttraumatic OD, studies suggest improved olfaction after OT [Citation12–15], but olfaction was assessed beyond the completed treatment in only one study [Citation14], where the effect declined after 3 months. Based on the existing literature, treating OD after TBI with both corticosteroids and OT might, therefore, be a useful clinical approach. The aim of this study was to explore if sequential treatment with oral corticosteroids and OT for 3 months could improve olfaction in patients with persistent OD after TBI. Olfaction was assessed both after treatment and at a 1-year follow-up. A secondary aim was to explore if sociodemographic and trauma-related factors were associated with the degree of improvement.
Materials and methods
Design
This was an open, uncontrolled intervention study where patients with posttraumatic OD received a sequential treatment with oral corticosteroids and OT. In total, four assessments were performed: T0 at baseline, T1 after oral corticosteroids, T2 after OT and T3 at follow-up, 12 months after the baseline assessment.
Participants
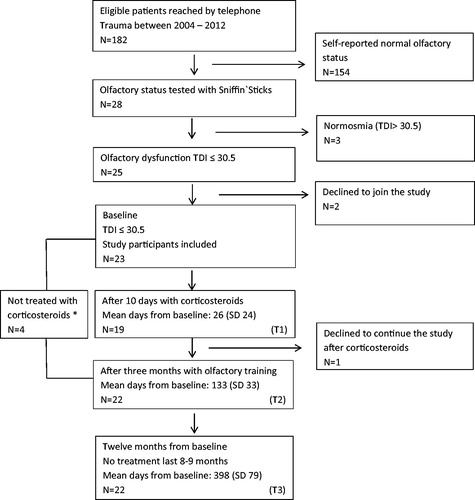
The participants were recruited from the Trondheim TBI study, an ongoing, prospective cohort study of patients with moderate and severe TBI. Patients in the current sample had been injured between 2004 and 2012 and were retrospectively screened by telephone for olfactory function if they were between 18 and 65 years old at the time of injury. Of the 182 patients reached by telephone, 28 reported posttraumatic change of smell and their olfactory function was examined with the Sniffin’ Sticks at the Department of Otorhinolaryngology, for further details, see our previous study [Citation4]. Out of the 25 patients where OD was confirmed, 23 consented to participate in this treatment study, one withdrew after treatment with corticosteroids and 22 completed the study ().
Procedure
The telephone screening was carried out by three members of the Olfactory Research Team, trained by the responsible ear-nose and throat specialist (M.B.). The participants with OD confirmed by Sniffin’ Sticks, were invited to participate in the treatment study. After written consent, the patients were first treated with corticosteroids and subsequently with OT. The Regional Committee for Medical Research Ethics in Mid-Norway approved the study. The procedures were in accordance with the Helsinki Declaration of 1975 as revised in 2008, and the study was registered in ClinicalTrials.gov (NCT02565121).
The treatment
Oral corticosteroids were given daily for 10 d without downscaling using a fixed dose of 30 mg prednisolone, provided no contraindications. OT lasted for 3 months. During OT the patients sniffed concentrates of odorants from four small bottles for four minutes every morning and evening; 1 min per bottle. We used the following odorants: eucalyptus, rose, clove and lemon and the participants were asked to register the training session twice daily in an olfaction diary.
Variables
Educational level had five response options that were dichotomized into ‘higher education’ (‘college’ or ‘university’) or ‘lower education’ (all other options). Occupational activity was dichotomized into ‘working’ (including studying and working part time) or ‘not working’ groups. Based on the patients self-reporting, the presence of asthma, nasal polyps, previous nasal operations and smoking habits were categorized as ‘yes’ or ‘no’. General health was assessed with the following item: ‘How is your health today’, and the four response options were dichotomized into ‘good health’ and ‘not good health’. Satisfaction with life was assessed with the following item: ‘When you think of your current situation in life, are you mostly satisfied with life, or are you mostly dissatisfied?’. The seven response options were dichotomized into ‘not satisfied’ (also including ‘both dissatisfied and satisfied’) and ‘satisfied’.
Nasal endoscopy was performed by a senior consultant (MB) at baseline and at the 1-year follow-up with a 0° or 5° rigid endoscope. An early Lund/Kennedy scoring system was used to register nasal polyps, oedema, secretion, septal deviation, adhesions or crusts. Nasal endoscopy was scored as normal or not normal. Cutaneous allergen tests were performed at baseline with a standard allergy panel that exposed the participant to eight allergens and compared the skin reaction to histamine. The tested allergens were: cat, dog, horse, house-dust mite, birch, timothy, mug wort and moulds. The result was scored as normal or not normal.
Olfactory function was examined with the Sniffin’ Sticks Extended [Citation16] at all assessments. Sniffin’ Sticks assesses three aspects of olfaction: odour threshold (T), odour discrimination (D) and odour identification (I), which are summed up in a TDI score. For further details of the testing procedure, see our previous study [Citation4]. Anosmia was defined as a TDI score ≤16.5, hyposmia as a TDI score higher than 16.5 but ≤30.5 and normosmia was defined as a TDI score >30.5 [2]. The odorants in Sniffin’ Sticks are adjusted for the European population and were validated earlier [Citation2]. Clinically significant improved olfaction was defined as an increase of TDI score ≥6 at 12 months from baseline [Citation17]. In the olfaction training diary, the maximum possible training sessions in 3 months were defined as two daily sessions × 29 d × 3 months = 174 training sessions. The number registered is the number of registrations summarized from the olfaction diary.
Cause of injury was registered as ‘fall’, ‘traffic accident’ or ‘other’ (i.e. all other causes). The Glasgow Coma Scale assessed the degree of impaired consciousness based on observed eye opening and verbal and motor responses to defined stimuli. The Head Injury Severity Scale (HISS) was used to classify injury severity in the acute phase. According to HISS, moderate TBI is defined by a GCS score of 9–13, or loss of consciousness ≥5 min or focal neurological deficits, and severe TBI by a GCS score of 3–8.
Head CT scans were taken and reviewed by radiologists immediately at admission. The CT variables were findings of the most relevant lesion types in the context of OD: (1) skull base fractures defined as fractures visible on CT and engaging the basal part of the ethmoidal, sphenoidal, frontal, temporal or occipital bone; (2) contusions of the brain defined as lesions, where the bulk of the lesion was located along the cortical surface of the brain; and (3) traumatic subarachnoid haemorrhages, including intraventricular haemorrhage. The CT variables were registered as ‘yes’ or ‘no’. Moreover, all patients were scored according to the Rotterdam CT classification with scores of 1–6 (6 being worst). The Rotterdam CT score is based on: visible or compressed/absent basal cisterns, midline shift ≤5 or >5 mm, absence or presence of epidural mass lesion or of intraventricular or traumatic subarachnoid haemorrhage.
MRI of the brain had been performed at a median of 35 d (range 3–528 d) after injury in 20/23 patients in a 1.5-T Siemens Symphony or a Siemens Avanto MR imaging system (Siemens Medical) using a standard clinical protocol including diffusion weighted imaging, T2*-weighted gradient echo imaging and FLAIR imaging. For more details, see a previous publication [Citation18]. One of the co-authors (K.G.M.) reviewed the MRI scans for orbitofrontal lesions. Orbitofrontal lesions were defined as contusions or traumatic axonal injury located in the medial, lateral, anterior and posterior orbital gyrus as well as in the gyrus rectus. The surgical interventions registered were: (1) Mass lesion evacuation (epidural, subdural or intracerebral hematomas or large contusions) and (2) surgery due to facial fracture or soft tissue damage; registered with a yes or no response.
Statistical analyses
Analyses were performed with IBM SPSS Statistics version 23 (IBM SPSS Chicago, IL). Continuous variables are presented as means and standard deviations (SDs). Categorical data are presented as numbers (n) and proportions (%). To analyse change of olfactory function over time, paired sampled T-Test and Nonparametric Wilcoxon’s Signed Ranks Test were used for continuous data (for normal and not normal distribution of the data, respectively) and the McNemar non-parametric two-related-samples test for categorical data. For univariable comparisons of patients having clinically recognizable improvement of olfactory outcome (TDI ≥ 6) with those who do not, the Mann–Whitney nonparametric test for non-normally distributed data and Fisher’ Exact Test were used. Prior to analysis, it was decided that independent variables associated with improved TDI ≥ 6 (versus not) with p < .15, should be included in further multivariable logistic regression analyses. All patients who received any part of the treatment and underwent testing were kept in the analyses, regardless of whether they completed all treatment as prescribed or not. Significance was set to p < .05.
Results
Sample characteristics
Of the 23 patients with posttraumatic OD, 74% was men (). The mean age of all patients at the time of trauma was 45 years, and 44% had severe TBI. The time from trauma to inclusion in the study was mean 62 (SD 25) months. The proportion of patients with anosmia at baseline was 65%. The majority was scanned with MRI (20/23) in addition to CT (23/23), and orbitofrontal lesions were depicted in 90% of the cases where MRI had been performed. The findings in patients with endoscopy ‘not normal’ were mucosal oedema alone (n = 3), mucosal oedema and septal deviation (n = 2), septal deviation alone (n = 3) and mucosal synechia after primary trauma and repositioning (n = 1). Out of the five participants with mucosal oedema, two tested allergy positive and one reported known asthma. Three participants reported previous nasal surgery, two primary repositioning after trauma and one unknown. Four other participants had contraindications or objections to corticosteroids, and oral corticosteroids were omitted, but they participated in the OT.
Table 1. Baseline characteristics of patients with persistent olfactory dysfunction after TBI.
Olfactory outcome
The mean TDI score at baseline was 14.4 (SD 7.3) and after 12 months 20.8 (SD 7.4). The mean increase in TDI points (Diff T3–T0) was 6.5 (SD 6.2) (minimum −3.0, maximum 19.5), p < .01 (). Threshold, discrimination and identification were analysed separately, and all improved significantly from baseline to 12 months. The TDI score after corticosteroids (n = 19) increased significantly from baseline (Diff T1–T0), and the mean increase in TDI score from baseline to termination of OT (Diff T2–T0) was 4.0 (SD 4.3) points, p < .01. In addition, there was a statistically significant increase of 2.5 TDI (SD 4.7) points (p < .05) in the non-intervention period (Diff T3–T2).
Table 2. Olfactory outcome for patients with persistent olfactory dysfunction after TBI tested with Sniffin’ Sticks before and after treatment and at follow-up 1 year from baseline.
A clinically meaningful change, i.e. an increase in TDI points ≥6.0 from baseline to 12 months, was observed in 11 participants (50%). None of the baseline characteristics differed between patients with and without increased TDI ≥ 6.0 with p < .15 () and the pre-set criteria for an adjusted regression analysis were not met.
Table 3. Baseline data comparisons between the two groups with improved TDI ≥ 6.0 and improved TDI < 6.0 when comparing TDI score at 12 months with TDI score at baseline N = 22 participants.
Five patients had oedema of the nasal mucosa at the baseline evaluation. The TDI score for these five improved by 3.5 after steroids compared to 4.2 for the patients without oedema. At 12 months, three of the five patients with oedema had an increase in TDI points ≥6.0
The olfaction diary was submitted after OT by 73% (16/22). Of 174 possible sessions per patient, the mean number of training sessions was 145 (SD 35) per patients. The mean number of sessions did not differ significantly in the two groups with and without a clinically significant increase in TDI score (139 sessions [SD 44] versus 151 sessions [SD 25], respectively) (). Of the six missing diaries, three were from patients with a clinically significant increase in TDI score.
Discussion
Our main finding was that the mean TDI score in patients with persistent posttraumatic OD had improved significantly following sequential treatment of corticosteroids and OT when assessed 1 year from baseline. The improvement in smell was clinically significant in half of the patients 1 year from baseline. There was a significant increase in all three components of the TDI score when analysed separately. None of the baseline characteristics were related to improvement.
In contrast to several of the existing studies reporting effects of treatment of OD, our study comprised only patients with posttraumatic OD. These patients are known to have a severe degree of OD, and their prognosis for a later recovery of olfaction has been regarded as poor compared to OD of other aetiologies. Notably, our patients had signs of structural traumatic damage to the olfactory pathways, as verified by orbitofrontal lesions in the vast majority. In this respect, we find it promising that we could observe a clinically significant improvement in half of the patients with our treatment, especially since the response to monotherapy with either OT or corticosteroids has been more modest. The first study with corticosteroids was conducted in 1995 [7]. In this small, open trial of posttraumatic OD, corticosteroids were either applied topically or given orally, and olfaction improved in 24% of participants. Further, in a study by Jiang et al. olfaction improved after oral corticosteroids in 16% of patients with persistent posttraumatic anosmia [Citation19]. However, since only the odour threshold was targeted in that study, a direct comparison is difficult. The mechanisms, by which steroids might improve olfaction in the chronic phase is not known. A possible explanation, could, however, be that some of the patients had underlying nasal conditions that responded to steroid treatment. We therefore found it important to study if patients with mucosal inflammatory changes responded differently to treatment than the others, as steroids have an anti-inflammatory effect. Five of the patients had mucosal oedema at endoscopy, a key finding in chronic rhinitis. Our results do not indicate that such inflammatory changes affected the outcome, neither after steroids nor after OT. We may speculate if steroids might shrink the mucosa also in patients with either subclinical or no oedema, thus exposing the olfactory receptors more to the stimulation of OT.
Concerning monotherapy with OT, only two studies focused on OT including only patients with posttraumatic OD. In a controlled trial, 42 patients were randomized to OT or no treatment, and OT induced a significant, but transient effect on odour threshold [Citation14]. Another study, also using the Sniffin’ Sticks, found a significant increase in overall TDI score, as well as threshold scores and identification scores, in 37 patients with anosmia or hyposmia. The increase in overall and threshold scores were significant in the subgroup with anosmia, but not hyposmia [Citation15]. No effect sizes were presented, however. A study by Jiang et al. [Citation12] resembles our treatment study regarding posttraumatic patients treated with OT following medical treatment. Jiang et al. introduced OT to patients who remained anosmic after corticosteroids and zinc gluconate. After OT, 24% had improved odour threshold for phenyl ethyl alcohol, compared to 5% of patients in a control group [Citation12].
Our study is the first to introduce a systematic sequential treatment of oral corticosteroids and OT. We emphasize, however, that we cannot infer from our study if one element in the treatment was more helpful than the other, or if the result would be the same if both treatment options were applied simultaneously. Nevertheless, our results suggest that a combination of the two different treatment options, targeting different mechanisms, should be further studied in patients with posttraumatic OD.
In contrast to most previous studies [Citation12,Citation20], in this study, we retested the patients 1 year after baseline, with the last assessment 8 months after ended OT. We observed an increase in TDI score immediately after intervention (T2) followed by a further statistically significant increase after the non-interventional period (T3). This suggests that the improved sense of smell was sustained, at a minimum, for 8 months without treatment. We could speculate that this indicates an increased functionality of the olfactory nerve obtained during treatment. The possibility of a long-lasting alteration in the olfactory nerve system following a limited period of OT should be further explored. The one study of posttraumatic OD that included OT with a follow-up beyond OT found, in contrast to ours, that the increase in olfactory function after OT was temporary and declined after 3 months. Thus, it is important for future treatment studies to explore longer-term olfactory outcome.
We found no difference in baseline sociodemographic or trauma characteristics between participants with clinically significant improved olfactory outcome (increased TDI score ≥6) and participants without (increased TDI < 6). This is in line with some studies on oral corticosteroid treatment that found no association between trauma characteristics and improved olfaction [Citation7,Citation12,Citation19]. In contrast, some studies reported a negative correlation between improved sense of smell and the severity of the head trauma after OT [Citation14,Citation20]. Lower age has also previously been reported to be associated with improved olfaction after treatment [Citation19], while we found no effect of age, in line with one other study [Citation20].
The prospective inclusion and characterization of the patients in the Trondheim TBI study was the strength of this study, as was the length of follow-up after ended treatment. In addition, the study used an olfaction test panel validated for European standards that assessed three aspects of olfaction. That MRI was performed in 90% of patients is another strength in this study, because CT scans have severe shortcomings when it comes to depicting lesions close to the skull base.
Some limitations should be mentioned. The final sample of patients with OD enrolled in this treatment study was small. We were therefore not able to apply a design with a control group receiving placebo treatment. Thus, we do not know if increasing familiarity with the olfaction test panel throughout the year (four tests per participant) influenced the test results. Moreover, although not likely in the chronic stage, a spontaneous recovery cannot be excluded.
Unfortunately, six (27%) of the olfaction diaries were missing at follow-up, and the mean number of training sessions could have been better. However, the number of missing diaries was distributed equally among those with and without improved olfactory outcome.
Conclusion and significance
This study explored a sequential treatment of oral corticosteroids followed by OT with 8 months follow-up for patients with posttraumatic OD. Olfactory function increased gradually during the treatment periods and follow-up, and half of the patients had a clinically significant improved sense of smell after 1 year. The present results indicate that combinations of treatment strategies might be further studied. Larger, randomized, controlled trials with long-term follow-up and risk-benefit analyses are needed in order to further clarify the role of the different treatment options for patients with reduced sense of smell after TBI.
Acknowledgements
We thank the Clinical Research Facility at St. Olavs University Hospital for assistance with the telephone screening and olfactory testing. We thank Ingrid Haavde Strand, Susan Frances Deane and Kjell Arne Kvistad from the Clinic of Radiology and Nuclear Medicine at St. Olavs University Hospital, for investigation of the CT scans.
Disclosure statement
The authors declare to have no conflicts of interests. M.B. and T.S. were supported by grants from the Liaison Committee between the Central Norway regional health authority and NTNU and KGM by NTNU.
References
- Whitcroft KL, Hummel T. Clinical diagnosis and current management strategies for olfactory dysfunction: a review. JAMA Otolaryngol Head Neck Surg. 2019;145(9):846.
- Hummel T, Kobal G, Gudziol H, et al. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects”. Eur Arch Otorhinolaryngol. 2007;264(3):237–243.
- Mullol J, Alobid I, Marino-Sanchez F, et al. Furthering the understanding of olfaction, prevalence of loss of smell and risk factors: a population-based survey (OLFACAT study). BMJ Open. 2012;2(6):e001256.
- Bratt M, Skandsen T, Hummel T, et al. Frequency and prognostic factors of olfactory dysfunction after traumatic brain injury. Brain Inj. 2018;32(8):1021–1027.
- Howell J, Costanzo RM, Reiter ER. Head trauma and olfactory function. World J Otorhinolaryngol Head Neck Surg. 2018;4(1):39–45.
- Kobayashi M, Costanzo RM. Olfactory nerve recovery following mild and severe injury and the efficacy of dexamethasone treatment. Chem Senses. 2009;34(7):573–580.
- Ikeda K, Sakurada T, Takasaka T, et al. Anosmia following head trauma: preliminary study of steroid treatment. Tohoku J Exp Med. 1995;177(4):343–351.
- Patel ZM. The evidence for olfactory training in treating patients with olfactory loss. Curr Opin Otolaryngol Head Neck Surg. 2017;25(1):43–46.
- Hummel T, Rissom K, Reden J, et al. Effects of olfactory training in patients with olfactory loss. Laryngoscope. 2009;119(3):496–499.
- Negoias S, Pietsch K, Hummel T. Changes in olfactory bulb volume following lateralized olfactory training. Brain Imaging Behav. 2017;11(4):998–1005.
- Kollndorfer K, Fischmeister FP, Kowalczyk K, et al. Olfactory training induces changes in regional functional connectivity in patients with long-term smell loss. Neuroimage Clin. 2015;9:401–410.
- Jiang RS, Twu CW, Liang KL. The effect of olfactory training on the odor threshold in patients with traumatic anosmia. Am J Rhinol Allergy. 2017;31(5):317–322.
- Jiang RS, Twu CW, Liang KL. The effect of olfactory training on odor identification in patients with traumatic anosmia. Int Forum Allergy Rhinol. 2019;9(11):1244–1251.
- Langdon C, Lehrer E, Berenguer J, et al. Olfactory training in post-traumatic smell impairment: mild improvement in threshold performances: results from a randomized controlled trial. J Neurotrauma. 2018;35(22):2641–2652.
- Pellegrino R, Han P, Reither N, et al. Effectiveness of olfactory training on different severities of posttraumatic loss of smell. Laryngoscope. 2019;129(8):1737–1743.
- Kobal G, Klimek L, Wolfensberger M, et al. Multicenter investigation of 1,036 subjects using a standardized method for the assessment of olfactory function combining tests of odor identification, odor discrimination, and olfactory thresholds. Eur Arch Otorhinolaryngol. 2000;257(4):205–211.
- Gudziol V, Lotsch J, Hahner A, et al. Clinical significance of results from olfactory testing. Laryngoscope. 2006;116(10):1858–1863.
- Skandsen T, Kvistad KA, Solheim O, et al. Prevalence and impact of diffuse axonal injury in patients with moderate and severe head injury: a cohort study of early magnetic resonance imaging findings and 1-year outcome. J Neurosurg. 2010;113(3):556–563.
- Jiang RS, Wu SH, Liang KL, et al. Steroid treatment of posttraumatic anosmia. Eur Arch Otorhinolaryngol. 2010;267(10):1563–1567.
- Konstantinidis I, Tsakiropoulou E, Bekiaridou P, et al. Use of olfactory training in post-traumatic and postinfectious olfactory dysfunction. Laryngoscope. 2013;123(12):E85– E90.