Abstract
Background
The treatment of T1b glottic carcinomas with invasion of the anterior commissure (AC) is still a challenge in larynx oncology. The diversity in treatment protocols is due to the difficulty in achieving safety margins of resection, especially in the AC.
Objective
The treatment success rate of frontolateral vertical partial laryngectomy (FVPL) for the treatment of stage T1b squamous cell carcinoma of the glottic larynx infiltrating the AC.
Material and Methods
Clinical data of patients, who were diagnosed with stage T1b squamous cell carcinoma of the glottic larynx and who underwent a FVPL from 01/2003 to 12/2016 in our ENT clinic were retrospectively evaluated. Clinical and oncological outcomes were analyzed.
Results
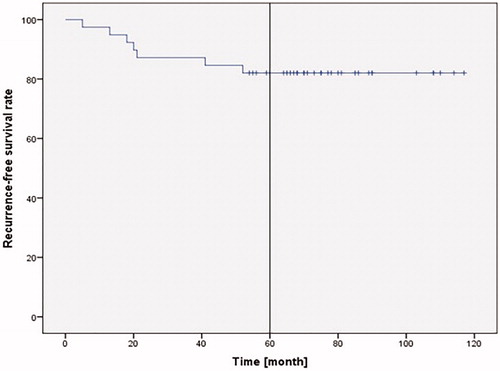
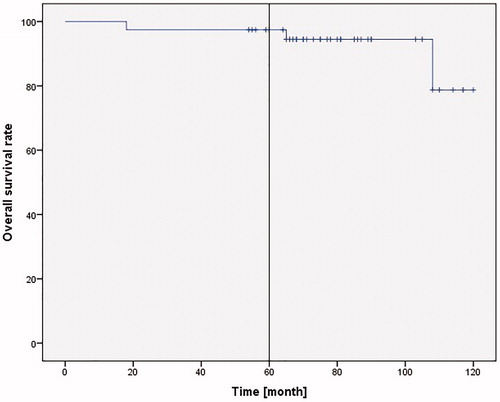
39 patients were included in this study. The mean follow-up duration was 79.95 ± 20.59 months. Intraoperative R0 resection was achieved in all patients. In 33.3% patients, documented complications were tissue granulation and synechia formation in the glottic area. The 5-year recurrence-free survival was 82.1%, the 5-year overall survival rate 97.4%, and the 5-year laryngeal preservation rate 94.8%.
Conclusion
Our clinical data demonstrate that T1b glottic carcinomas with invasion of the AC can be effectively treated with FVPL. The outcome is similar to other methods such as transoral laser microsurgery, supracricoidal partial laryngectomy, and radiotherapy.
Chinese abstract
背景:T1b声门癌合并前联合部(AC)侵犯的治疗仍然是喉肿瘤学的一个挑战。治疗方案的多样性是由于达到切除安全范围的难度, 尤其是AC病例。
目的:用前外侧垂直部分喉切除术(FVPL)治疗声门喉浸润AC的T1b期鳞状细胞癌的成功率。
材料和方法:回顾性评估了01/2003到12/2016期间在我们的耳鼻喉科诊所被诊断为声门喉癌的T1b期鳞状细胞癌并接受了FVPL的患者的临床资料。分析了 临床和肿瘤学结果。
结果:本研究纳入39例患者。平均随访时间为79.95±20.59个月。所有患者均获得了术中R0切除。在33.3%的患者中, 记录的并发症为声门区组织肉芽形成和粘连。 5年
无复发生存率为82.1%, 五年总生存率为97.4%, 五年喉保存率为94.8%。
结论:我们的临床数据表明, 伴有AC侵犯的T1b声门癌可以用FVPL进行有效治疗。其结果与其它方法如经口激光显微外科手术、经舌弓上部分喉切除术和放射疗法相似。
Introduction
Laryngeal carcinoma is the second most common malignant disease of the upper aerodigestive tract. Squamous cell carcinomas make up 85% to 95% of laryngeal malignancies [Citation1]. Glottic carcinoma is the most common type of larynx cancer and often affects the anterior two thirds of the vocal folds. The typical symptoms are hoarseness and dysphagia.
Anterior commissure (AC) involvement occurs in approximately 20% of all glottic carcinomas and is generally associated with a poorer prognosis compared to glottic carcinomas without AC involvement [Citation2]. The tumor can directly invade this area of the thyroid cartilage, because the inner perichondrium is absent here [Citation3].
T1b glottic carcinoma can usually be treated with various larynx-preserving strategies such as transoral laser microsurgery (TLM), radiotherapy (RT), and open partial laryngectomy (OPL) such as frontolateral vertical partial laryngectomies (FVPL), supracricoidal partial laryngectomy with cricohyoideopexy (SCPL-CHP) or cricohyoideoepiglottopexy (SCPL-CHEP), and modified supracricoidal laryngectomy (MSCL) [Citation4]. The diversity in treatment protocols is due to the unique characteristic of this tumor-stage, namely defining the margin of resection, especially in the AC.
All the aforementioned protocols have advantages and disadvantages. TLM has made great progress in recent years in treating larynx carcinoma, especially early stages of glottic carcinoma [Citation5]. However, here the challenge remains determining the adequate resection in the AC with involvement of Broyles' s tendon, especially in a narrow or prominent larynx [Citation6]. Therefore, about 70% of all recurrences of T1b glottic carcinoma occur in the AC [Citation3]. RT provides an ideal option for patients who cannot undergo surgery and anesthesia. In addition, it can be performed without a tracheotomy. However, the disadvantages of RT lie in its many side effects such as mucositis, laryngeal edema, dysphagia, xerostomia, and an increased risk of cartilage necrosis, as well as the difficulty in detecting recurrences [Citation1]. In addition, the cost of RT is much higher, for example four times higher than the cost of TLM [Citation7].
Frontolateral vertical partial laryngectomies (FVPL), a type of OPL, which was first described by Leroux-Robert in 1956 [Citation8] has evolved over time with many modifications. Leroux-Robert describes the resection of the AC and the medial triangle of the thyroid cartilage for the treatment of T1b-T2 glottic carcinoma. Tapia and Leroux-Robert introduced the frontal anterior partial resection for the treatment of T1b and T2 with infiltration of both vocal folds [Citation9]. One of the most important advantages of this method is the safe removal of the tumor under direct vision. Disadvantages, which are not exclusive to this method, include the usually longer hospital stay and the need for a temporary tracheotomy, as well as the complications after surgery such as the formation of granulation polyps in the AC and the consequently worsening of the voice quality [Citation6].
In the context of OPL, some centers perform supracricoidal partial laryngectomy with cricohyoideopexy (SCPL-CHP) or cricohyoideoepiglottopexy (SCPL-CHEP) to treat T1b-T2 glottic carcinoma with very good oncological results [Citation10] and resect the entire thyroid cartilage as well as the paraglottic area. The cricoid cartilage, hyoid bone, most of the epiglottis, and at least one arytenoid cartilage are preserved [Citation11]. The functional results can be improved by MSCL in selected cases [Citation4]. Here, the two sternohyoid muscles are used to reconstruct the neoglottis. However, both arytenoids must be preserved.
In this study, we focus on FVPL. It is the only protocol we use in our center (certified by the German Cancer Society) to treat early stage T1b glottic carcinomas with invasion of the anterior commissure (AC) [Citation12]. During this operation, the tumor can be removed with an adequate safety margin, provided that the cancer has not extended to the ventricle and paraglottic space. Due to the development of laser microsurgery equipment, FVPL has become less frequently used. However, in our experience, this method allows R0 resection in all T1b stage glottic cancer, especially in cases where the endolaryngeal endoscope adjustment is difficult. It is part of the standardized therapy of T1b glottic cancer in our certified head-neck tumor center. Another advantage is that it can be performed even in underdeveloped countries that do not have advanced TLM techniques [Citation13].
This study retrospectively evaluated whether FVPL is a safe treatment to reduce the recurrence rates in patients with T1b N0 stage glottic cancer. The clinical and histological findings, the occurrence of complications, the 5-year recurrence-free survival, the 5-year overall survival, and the 5-year laryngeal preservation rate were investigated over a period of 14 years, and were compared to results in the literature for other methods.
Patients and methods
Patient inclusion criteria and treatment
We retrospectively analyzed the medical records of patients who presented from 01/2003 to 12/2016 in our head-neck tumor center with stage T1b N0 M0 squamous cell carcinoma of the glottic larynx with involvement of the AC. Our center is certified by the German Cancer Society. The patients had no previous treatment for squamous cell carcinoma stage T1b and were treated in our tumor center exclusively by FVPL. Inclusion criteria for the study were patients with confirmed T1b N0 glottic carcinoma with AC involvement and FVPL treatment. A standard FVPL was performed according to Tapia and Leroux-Robert [Citation8,Citation9].
Preoperative examination and follow-up
As part of the tumor staging, patients underwent panendoscopy with sampling, computed tomography of the neck and thorax, and sonographic examination of the neck and abdomen.
After presenting the cases to our tumor board, we discussed the diagnosis, our recommendation for FVPL, as well as possible alternative therapies such as laser surgical resection and primary radiation in detail with the patients. The disadvantages and advantages of all methods mentioned above were explained. Based on the experience in our tumor center, we recommend FVPL as the therapy of choice for T1b glottis carcinoma. All 39 patients with T1b glottic carcinoma gave informed consent to the FVPL procedure.
Patients’ follow-up was performed monthly in the first year after FVPL, then every 2 months in the second year, every 3 months in the third year, every 4 months in the fourth year, every 6 months in the fifth year, and once a year from the sixth year on. Patients had a complete ENT examination including an echographic examination of the neck and a fiberoptic laryngoscopy for tumor follow-up.
Data analysis
The 5-year recurrence-free survival rate, the 5-year overall survival rate, and the 5-year laryngeal preservation rate were analyzed. The Kaplan Meier survival curves were calculated using the function ‘Kaplan-Meier’ from the ‘Survival’ package in SPSS 25.0.0.0. Percentages, means, and standard deviations for were calculated using Excel 2010.
Results
During the 14-year inclusion period, 385 new cases of laryngeal carcinoma were diagnosed in our head-neck tumor center. Of these, 39 patients (10.12%) with T1b N0 glottic carcinoma with AC involvement and FVPL treatment were included in this study. In our center certified by the German Cancer Society, FVPL is the standardized treatment for all patients with this indication. No other treatment options for this stage glottic cancer are performed at our head-neck tumor center. Most patients were male, between 61 and 70 years of age, had been or were smokers, and had moderately differentiated squamous cell carcinoma stage T1b N0 M0. The average stay in the hospital was 14 ± 4 days (n = 39). The patients’ demographics and characteristics are shown in .
Table 1. Patients’ demographics and characteristics.
Surgery to close the tracheotomy fistula was required within 2 weeks after FVPL. The tracheostomy fistula was surgically closed in the operating room in all patients. It was performed under local anesthesia in 16 patients and under general anesthesia in the other 23 patients. In most cases (36/39 patients, 92.3%), it was possible to close the fistula within 30 days after tumor resection. In 20 patients (51.28%), the period was 7 to 14 days after surgery, while the remaining 16 patients (41.02%) had to be readmitted to the hospital to close the tracheotomy fistula. For the remaining 3 patients (7.69%), the operation to close the fistula was not possible until 32–36 days after FVPL. The average time between the surgery and the tracheostoma closure was 17.3 ± 7.0 days (n = 39).
The postoperative follow-up period was 54 to 120 months (mean 79.95 ± 20.59 months, n = 39). One month postoperative 34 of 39 of the patients (87.2%) had no complications, while 5 (12.8%) had wound healing problems and 4 (10.3%) developed fistula after closing the tracheotomy fistula. Patients with fistula formation had successful intravenous antibiotic treatment and topical antibiotic wound dressing for a week. Within 2 years after FVPL, granulation tissue formation und synechia were seen in 13 of 39 patients (33.3%). A localized granulation tissue in the area of the former AC was found in 9 patients. In 4 patients, the granulation tissues were more extensive with connections between the two vocal folds in the anterior third in the form of a synechia. These diagnoses were made during regular tumor follow-ups in the first 3 months after the operation. As soon as this diagnosis was established, microlaryngoscopy was used to exclude tumor recurrence and to remove the tissue changes using a CO2 laser a socalled neoglottic plastic i.e. extension. In patients with synechia, this included synechia cutting. The procedure was sufficient the first time in 6 patients. Unfortunately, 7 patients had neoformation of the granulation tissue and it was necessary to repeat the procedure 3 times within 2 years in 3 patients and 4 times in 4 patients.
After FVPL, patients included in the study complained of hoarseness. However, no one indicated difficulty swallowing. None of the patients needed feeding via a nasogastric tube. The patients’ clinical data are demonstrated in .
Table 2. Study patients’ clinical data (patients with complications and tumor recurrences in bold print).
Tumor recurrence was diagnosed in 5 male and 2 female patients (17.9%). The recurrence occurred in one patient (83 years old) in the first year (at 5 months), in 4 patients in the second year (at 13, 18, 20, and 21 months), and in 2 patients after the third year (at 41 and 52 months) following FVPL. The recurrences were treated using different protocols depending on the location and staging of the recurrence.
Patient 2 who had a total laryngectomy and bilateral modified radical neck dissection (MRND) on both sides and then RCT to treat rpT3, rpN1 tumor recurrence, died 2 years later (65 months after FVPL) from distant lung metastases. Patient 16 had a local recurrence rpT4a 5 months after FVPL. Treatment was tumor debulking using TLM and then radiochemotherapy (RCT). This method was chosen, because this patient refused a total laryngectomy. She died at 18 months of respiratory failure with distant lung metastases. Patient 24 died 108 months after FVPL from bleeding esophageal varices after liver cirrhosis due to chronic hepatitis C. The five-year recurrence-free survival was 82.1% ().
The 5-year laryngeal preservation rate was 94.8%, and 5-year overall survival rate in our collective was 97.4% (38 of 39 patients; ).
Discussion
FVPL, SCPL, TLM, and RT have high success rates in treating early stage T1b glottic carcinomas. The choice of the optimal procedure depends on the patient’s condition, the depth of the tumor’s infiltration, as well as the center’s experience in treating these cases. The specific difficulty of T1b glottic cancer is the infiltration of the AC. This is a negative prognostic factor when RT or TLM is performed [Citation12,Citation14,Citation15]. Therefore, in these cases, even if it causes poorer functional results, it is recommended to resect the corresponding cartilaginous framework, to achieve a tumor free margin, and thus reduce the recurrence rate [Citation10].
compares the present study, which uses FVPL to studies employing various methods for the treatment of T1b glottic cancer published between 1997 and 2018 [Citation10,Citation16–21].
Table 3. Comparison of outcomes of different methods.
The overall or actuarial survival rates range from 78.5 to 91.7% in the published trials. In our study, the 5-year overall survival rate was 97.4%. The 5-year recurrence-free survival rate was 82.1% in our study, which was e.g. 72.4% for TLM [Citation18] or 93.5 to 98.2% for SPCL-CHEP [Citation10,Citation21]. The 5-year recurrence-free survival reported by Giovanni et al. who also used FVPL for stage T1 glottic tumor was 100% and thus higher than our collective [Citation16]. This could be due to the fewer patients with AC involvement reported by Giovanni et al. (98/127; 77%). These patients were not differentiated according to T1 and T2, which could mean that the proportion of their stage T1 patients with AC involvement was even lower than 77%. In contrast, all our patients had AC involvement, which results in a poorer prognosis [Citation2]. Recurrence was not mentioned in the other published studies. The 5-year larynx preservation rate was 92.2 to 100% versus 94.8% in our study. This demonstrates that using FVPL is at least comparable to the other methods. This is confirmed by Giovanni et al. who found a 5-year survival rate of 91% in 62 patients with T1 N0 who were treated using FVPL [Citation16].
When comparing methods directly, neither Marcotullio et al. who compared TLM, SCPL-CHP, and SCPL-CHEP nor Gioacchini et al. who compared RT, TLM, and OPL found one method to be better over the others [Citation17,Citation20]. Gioacchini et al. reported that recurrences were less frequent in patients treated with RT. Taylor et al. found no significant difference between primary RT and TLM for the treatment of T1b glottic carcinoma [Citation22]. The 2-year recurrence-free survival after primary RT was 85.9%, the 2-year overall survival was 94.8%, and the larynx preservation rate in 2 years was 85.9%. For TLM, the 2-year recurrence-free survival was 88.7%, the 2-year overall survival was 94.1%, and the 2-year larynx preservation rate was 100%. The 2-year recurrence-free survival in our study was 87.2% (34/39 patients; ).
Our data show that FVPL delivers similar results for 5-year recurrence-free survival, 5-year overall survival, and 5-year larynx preservation rate to the other methods used for T1b glottic tumors. Determining the adequate resection in the AC with involvement of Broyles' s tendon, especially in a narrow or prominent larynx is a big challenge [Citation6], and most recurrences of T1b glottic carcinoma occur in the AC [Citation3]. The advantage for resection of T1b tumors is that this method allows R0 resection, especially in cases where the endolaryngeal endoscope adjustment is difficult. The tendency of granulation tissue or synechia to form in the former AC (in our study 33.3%) is a disadvantage of FVPL, which has, however, also been reported for the other methods such as TLM [Citation22].
Conclusion
The therapy of T1b glottic carcinomas with invasion of the AC is still a big challenge and the treatment method controversial. After analyzing our clinical data, T1b glottic carcinomas can be effectively treated with FVPL resulting in good oncology outcomes comparable to other methods. The advantages of this surgical procedure are the safety control of the tumor margins, the intraoperative histologically confirmed R0 resection, and the possibility of reconstruction of the glottic larynx. It is also less costly than other methods such as RT. A disadvantage, which is not exclusive to this method, is the need for tracheotomy and the possible formation of synechia and granulation polyps in the reconstruction area of the neoglottis. Further studies are needed to demonstrate which method is best under the individual patient and center conditions.
Acknowledgment
Many thanks and appreciation to Mr. PD Dr. med. Ingo Todt for his participation in reading and evaluating this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Dhanisetty S, Johnson J. Tumors of the Larynx. In: Lee KJ, editor. Essential otolaryngology: head and neck surgery. New York (NY): The McGraw-Hill Companies; 2012. p. 657–662.
- Rifai M, Khattab H. Anterior commissure carcinoma: I-histopathologic study. Am J Otolaryngol. 2000;21(5):294–297.
- Balica NC, Poenaru M, Ştefănescu EH, et al. Anterior commissure laryngeal neoplasm endoscopic management. Rom J Morphol Embryol. 2016;57(2 Suppl):715–718.
- Allegra E, Lombardo N, La Boria A, et al. Quality of voice evaluation in patients treated by supracricoid laryngectomy and modified supracricoid laryngectomy. Otolaryngol Head Neck Surg. 2011;145(5):789–795.
- Hinni ML, Salassa JR, Grant DG, et al. Transoral laser microsurgery for advanced laryngeal cancer. Arch Otolaryngol Head Neck Surg. 2007;133(12):1198–1204.
- Werner JA, Windfuhr JP, et al. Eingriffe an larynx, hypopharynx und trachea [Interventions on the larynx, hypopharynx and trachea]. In: Rettinger A, Hosemann W, Hüttenbrink K B, editors. HNO-Operationslehre [ENT-Surgery theory]. Stuttgart (Germany): Thieme; 2018. p. 248–258. German.
- Phillips TJ, Sader C, Brown T, et al. Transoral laser microsurgery versus radiation therapy for early glottic cancer in Canada: cost analysis. J Otolaryngol Head Neck Surg. 2009;38(6):619–623.
- Leroux-Robert J. Conservative surgery in cancer of larynx. Boll Soc Med Chir Cremona. 1956;10(4):68–76.
- Dietz A. Kopf-Hals-Tumore; Therapie des Larynx-/Hypopharynxkarzinoms unter besonderer Berücksichtigung des Larynxorganerhalts [Head and neck tumors; therapy of laryngeal/hypopharyngeal carcinoma with special attention to preservation of the larynx organ]. Bremen (Germany): UNI-MED; 2010. p. 91. German.
- Laccourreye O, Muscatello L, Laccourreye L, et al. Supracricoid partial laryngectomy with cricohyoidoepiglottopexy for "early" glottic carcinoma classified as T1-T2N0 invading the anterior commissure. Am J Otolaryngol. 1997;18(6):385–390.
- Laccourreye H, Laccourreye O, Weinstein G, et al. Supracricoid laryngectomy with cricohyoidoepiglottopexy: a partial laryngeal procedure for glottic carcinoma. Ann Otol Rhinol Laryngol. 1990;99(6 Pt 1):421–426.
- Yu Y, Lee NY. T1b glottic cancer. In: Dedivitis RA, Peretti G, Haann E, Cernea CR, editors. Laryngeal cancer: clinical case-based approaches. New York (NY): Thieme; 2019. p. 20–23.
- André VG, Marco AV. Kulscar.T1b glottic cancer, vertical partial laryngectomy. In: Dedivitis RA, Peretti G, Haann E, Cernea CR. Laryngeal cancer: clinical case-based approaches. New York (NY): Thieme; 2019. p. 25–29.
- Maheshwar AA, Gaffney CC. Radiotherapy for T1 glottic car-cinoma: impact of anterior commissure involvement. J Laryngol Otol. 2001;115:298–301.
- Bradley PJ, Rinaldo A, Suárez C, et al. Primary treatment of the anterior vocal commissure squamous carcinoma. Eur Arch Otorhinolaryngol. 2006;263(10):879–888.
- Giovanni A, Guelfucci B, Gras R, et al. Partial fron-tolateral laryngectomy with epiglottic reconstruction for manage-ment of early-stage glottic carcinoma. Laryngoscope. 2001;111:663–668.
- Marcotullio D, de Vincentiis M, Iannella G, et al. Surgical treatment of T1b glottic tumor, 10-years follow-up. Eur Rev Med Pharmacol Sci. 2014;18(8):1212–1217.
- Weiss BG, Ihler F, Pilavakis Y, et al. Transoral laser microsurgery for T1b glottic cancer: review of 51 casesEur. Eur Arch Otorhinolaryngol. 2017;274(4):1997–2004.
- Song JA, Rigby MH, Trites J, et al. Outcomes of transoral laser microsurgical management of T1b stage glottic cancer. J Laryngol Otol. 2017;131(5):433–441.
- Gioacchini FM, Tulli M, Kaleci S, et al. Therapeutic modalities and oncologic outcomes in the treatment of T1b glottic squamous cell carcinoma: a systematic review. Eur Arch Otorhinolaryngol. 2017;274(12):4091–4102.
- Allegra E, Saita V, Azzolina A, et al. Impact of the anterior commissure involvement on the survival of early glottic cancer treated with cricohyoidoepiglottopexy: a retrospective study. Cancer Manag Res. 2018;10:5553–5558.
- Taylor SM, Kerr P, Fung K, et al. Treatment of T1b glottic SCC: laser vs. radiation- a Canadian multicenter study, Taylor et al. J Otolaryngol Head Neck Surg. 2013;42:22.