Abstract
Background
There were heterogeneous or even conflicting data regarding the ability of platelet-to-lymphocyte ratio (PLR) for predicting the prognosis of laryngeal/hypopharyngeal squamous cell carcinoma (LHSCC). The discrepancies were found to be largely due to the cutoff value of PLR.
Aims
The aims of this study were to rationally select an optimal PLR cutoff value and to analyze the relationship between pretreatment PLR and the prognosis.
Methods
A total of 180 male patients were eligible for this retrospective study. We included another 180 healthy male individuals as controls. The relationship between PLR and age in patients and the controls was determined. The optimal cutoff values of PLR were identified. PLR value was then dichotomized into two categories, and the relationship between PLR and the clinicopathologic parameters were calculated. Kaplan–Meier curves were used to evaluate the overall survival (OS), and the association between PLR and the OS was analyzed.
Results
The linear regression analysis showed a positive correlation between age and PLR in the control group, but not the patients. The optimal cutoff value of PLR was 112.5. The high PLR value group of patients exhibited significantly decreased OS. PLR was related to prognosis, as revealed by the univariate Cox regression.
Conclusion
Patients with LHSCC have abnormal high PLR, and a high pretreatment PLR portends adverse survival.
Chinese abstract
背景:关于血小板与淋巴细胞比率(PLR)是否能够预测喉/下咽鳞状细胞癌(LHSCC)预后, 存在着有异议的甚至矛盾的数据。差异主要是由于PLR的临界值。
目的:本研究的目的是合理选择最佳的PLR临界值, 并分析治疗前PLR和预后之间的关系。
方法:共180名男性患者符合这项回顾性研究要求。还招纳了180名健康男性作为对照。确定了PLR患者和对照组与年龄的关系。确定了PLR的最佳临界值。然后将PLR值分为两类, 并计算了PLR与临床病理参数之间的关系。 Kaplan–Meier曲线用于评估总生存期(OS), 并分析了PLR与OS之间的相关性。
结果:线性回归分析显示, 对照组与年龄之间呈正相关, 而患者不是如此。 PLR的最佳临界值为112.5。高PLR值的患者表现出明显降低的OS。单变量Cox回归分析显示, PLR与预后相关。
结论:LHSCC患者的PLR异常高, 治疗前PLR较高预示着生存几率不佳。
Introduction
Larynx and hypopharynx are neighboring structures that are functionally integrated and surgically related. According to the GLOBOCAN 2018 report by the International Agency for Research on Cancer, worldwide incidence of the laryngeal and hypopharyngeal cancers is about 1% and 0.4% of all sites, respectively [Citation1]. As the most common pathological type, the squamous cell carcinoma (SCC) has a poor overall survival (OS) rate [Citation2]. Compared to females, male patients have a circa sevenfold cumulative risk [Citation3]. Fully assimilate the nature of laryngeal/hypopharyngeal SCC (LHSCC) mainly relies on pathology of the surgical specimen including tumor invasion, nodal involvement, perineural and lymphovascular invasion, and distant metastases. However, the main drawback is that these indexes can only be assessed after surgery.
A cancer-related inflammatory microenvironment can be reflected in the peripheral blood. The basic hematologic changes are neutrophilia, lymphocytopenia, and thrombocytosis. Different inflammatory cells stand for different meanings: neutrophils reflect host response to systemic inflammation, lymphocytes reflect host antitumor forces, while platelets reflect the pro-tumor and neoangiogenic effect. The platelet-to-lymphocyte ratio (PLR) is defined as the ratio of the platelet count divided by the lymphocyte count. The elevation of platelet counts results from tumor-cell-induced megakaryopoiesis, subsequent thrombopoiesis and aggregation [Citation4]. The reduction of lymphocyte counts was largely due to the reduced T-cell subtype [Citation5].
The appropriate cutoff value of PLR for predicting LHSCC prognosis remains a mystery, as different methods were used. Also, there was a lack of analysis of reference PLR values when interpreting results in previous reports. This study aimed to present an alternative perspective on finding an optimal PLR cutoff point and further evaluated the prognostic value of preoperative PLR in patients with LHSCC.
Materials and methods
Study population and data collection
This retrospective study was conducted on male LHSCC patients presenting to our service who underwent surgery between April 2009 and November 2019. The inclusion criteria of the study were as follows: a history of tobacco use, male, and primary LHSCC. The exclusion criteria of the study were as follows: (1) history of other metastasis or other malignancies on the initial visit and (2) history of concurrent hematological/infectious/autoimmune diseases, as PLR can be potentially affected in all of above comorbidities. A total of 180 eligible patients enrolled in this study, with 180 controls.
All of the clinical data were retrieved from the medical records of our university hospital. Clinicopathological parameters included age, gender, primary tumor site (larynx or hypopharynx), TNM stage (I–IV), and pathological differentiation (low/moderate/high). The 8th edition of American Joint Committee on Cancer (AJCC) TNM staging system for laryngeal/hypopharyngeal cancer was applied. The blood samples were analyzed by the automated blood counter and obtained within 5 days before surgery. The PLR was calculated on the basis of peripheral blood counts of platelets (P; ×109/Liter) and lymphocytes (L; ×109/Liter). This study was approved by the Institutional Review Board of the Affiliated Drum Tower Hospital of Nanjing University Medical School and conducted in accordance with the ethical principles of Declaration of Helsinki.
Statistical analysis
The statistical analysis was carried out with Statistical Package for Social Sciences (SPSS, version 24, IBM Inc., Chicago, IL, USA). The descriptive data were presented with median and mean ± standard deviation (SD). Linear regression analysis was performed to explore the correlation between age and PLR distribution in patients and controls. The optimal cutoff values of PLR were generated by the X-tile 3.6.1 software (Yale University, New Haven, CT), SPSS, and online Cutoff Finder (based on R language). The relationship between clinicopathologic parameters and PLR categories was evaluated by Pearson chi-square test. The endpoint analyzed was the OS. Kaplan–Meier curves were used to compare the OS with log-rank tests. The impact of the PLR and other potential prognostic factors on the OS was calculated using the univariate Cox proportional hazards regression analysis. The level of statistical significance was set to p < .05.
Results
The relationship of PLR and age
PLR according to age of patients with LHSCC and the control group is shown in . The distribution of PLR was tested to have no normality (Kolmogorov–Smirnov test, data not shown). We carried out the linear regression analysis and found a positive correlation between the age and PLR in the healthy control group (linear equation: R = 0.214, p = .004), but not the patients (linear equation: R = 0.126, p = .091) ().
Figure 1. Linear regression analysis of PLR and age. (a) The regression analysis revealed that, there was no positive correlation between age and PLR in the patient group (red) (linear equation: R = 0.126, P = .091; quadratic equation: R = 0.130, P = .219; exponential equation: R = 0.105, P = .166). (b) There was a positive correlation between age and PLR in the control group (black) (linear equation: R = 0.214, P = .004; quadratic equation: R = 0.245, P = .006; exponential equation: R = 0.197, P = .008).
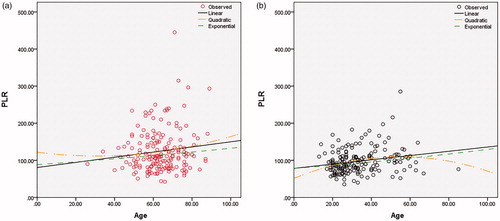
Table 1. Correlation between age and PLR in patients and healthy individuals.
Patients’ characteristics
The clinical features of patients are depicted in . The patients included were adults from 26 to 89 years of age (median 63 years). 37.2% of them were below 60 years. Sites of primary malignancy were specifically limited to the larynx (supraglottis 11%; glottis 73.3%; and subglottis 5%) and hypopharynx (10.6%). Patients underwent total/partial laryngectomy by open or CO2 laser surgeries for curative intent. 27.2% patients were stage I, 13.3% were stage II, 28.3% were stage III, and 31.1% were stage IV. Percentage of low, moderate, and high differentiation were 22.2%, 59.4%, and 18.3%, respectively.
Table 2. Clinical and pathological features of patients stratified by PLR.
PLR cutoff value prediction
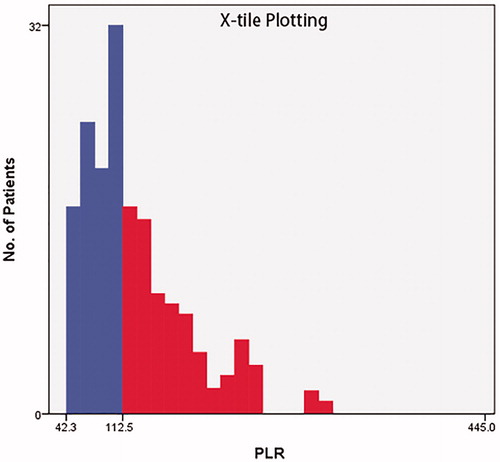
The optimal PLR cutoff value was generated by X-tile to be 112.5 (). This cutoff value had an utmost predictive power for the OS. According to the cutoff value, patients were assigned into two groups: low (≤112.5) and high (>112.5) (). Statistically different numbers of patients distributed in PLR subgroups of TNM stage (χ2 = 8.143, p = .043) and pathological differentiation (χ2 = 6.902, p = .032), but not age (χ2 = 0.022, p = .882), or tumor site (χ2 = 2.385, p = .497). Other PLR cutoff value prediction methods (including the reference PLR value) were also applied and compared in the discussion section.
Higher PLR indicates poorer survival outcomes
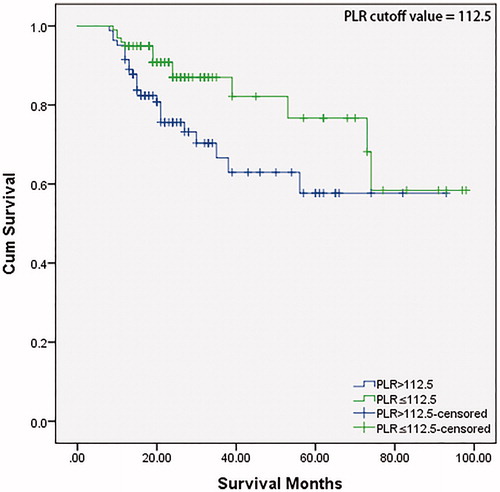
The median time of follow-up was 23 months (range 8 − 98 months) in our LHSCC patient cohort. Kaplan–Meier with log-rank test was utilized to investigate the significance of PLR for predicting the OS of patients with LHSCC (). The OS decreased significantly in the high (>112.5) PLR value group (p = .027).
PLR is related to prognosis as evidenced by univariate cox regression
We further investigated whether PLR was related with prognosis of LHSCC patients by the univariate Cox regression hazards model (). The following possible prognostic factors were included: age, location, stage, differentiation, and PLR. In the univariate analysis, PLR had a statistical difference (≤112.5 vs. >112.5: hazard ratio (HR) = 0.480, 95% confidence interval (CI) = 0.246 − 0.936, p = .028). However, as many of the parameters were not significant in the univariate analysis of our small data set, a multivariate Cox proportional hazards regression model predicting independency was unapplicable.
Table 3. Univariate Cox regression analysis of factors for the prediction of OS.
Discussion
The value of PLR as a predictor for head and neck maligancies
PLR is quite different from TNM system as it can be assessed before any treatment. Cumulating evidences show that patients with high pretreatment PLR level have poor OS in many types of cancer, especially the advanced types [Citation6]. Bardash et al. [Citation7] showed that an elevated PLR in head and neck cancer was significantly associated with poorer OS in their meta-analysis.
Considering SCC, surprisingly in Yang et al.’s large meta-analysis of 6847 head and neck SCC patients, the association between PLR and OS was not statistically significant. We reviewed this report and found that only 3 cohorts involving 736 patients had data about the relationship of PLR and OS [Citation8].
Yang et al.’s result was contradicted to 273 patients’ results in an earlier article by Rassouli et al, which revealed PLR >170 was associated with higher mortality and PLR was an independent predictor. Primary sites in this report were integrated, as not only pharynx, larynx, and oral cavity, but also the nasal cavity, paranasal sinuses, and salivary glands were included [Citation9]. Bojaxhiu et al published their results showing a non-significant association between higher PLR and lower OS, and over 75% primary sites of their 186 cases were oral cavity or oropharynx [Citation10]. The above literatures indicated variation in tumor primary sites caused giant heterogeneity.
In our cohort, only laryngeal and hypopharyngeal patients were enrolled. Both Kaplan–Meier method and the univariate Cox regression hazards model showed that, high pretreatment blood PLR level had poor OS. This phenomenon was in accordance with several groups [Citation11–14].
Selection of PLR cutoff value could influence on stratifying patients
There were heterogeneous or even conflicting data regarding the ability of PLR for predicting the prognosis, resulting in controversial views. One of the most important reasons for these discrepancies is the selection of the PLR cutoff value. An optimal PLR cutoff should consistently and independently differentiate patients to better and worse prognosis groups with desired sensitivity versus specificity. However, till now there was no golden-standard cutoff value of PLR. Without such standardization, it is difficult to determine the exact significance of PLR ratio.
Currently, several available software can determine the cutoff thresholds: SPSS, X-tile, or online Cutoff Finder. In our study, we also tested SPSS and online Cutoff Finder. Optimal cutoff values of PLR generated by SPSS was 109.75 (sensitivity: 53.3%, specificity: 73.9%, the area under the receiver-operating characteristic (ROC) curve: 0.655), and 112.5 (by X-tile) had a sensitivity and specificity around 46.1% and 77.2% in SPSS, separately. The online Cutoff Finder generated a cutoff value of 113, which was quite close to 112.5. The main difference between these three methods is that SPSS analysis relies not only on the patient group but also a control group simultaneously, while the latter two do not.
However, the PLR cutoff value in SPSS can be greatly deranged by different control groups (data not shown); consequently, there may be confusing and even confounding results when evaluating survival data based on PLR. Disappointly, there were extraordinarily limited large and longstanding population-based cohort studies about the reference values of PLR. Fei et al analyzed a total of 26,242 Chinese males together with 11,934 females and found the median PLR was 111.26 and 122.9, respectively [Citation15]. Several groups reported quite consistent a range of PLR cutoff values for laryngeal SCC patients, such as 109.54, 110.94, 111, 112, 114, and 119.55 [Citation11,Citation13,Citation14,Citation16–18]. Since our patients were all male and had a median PLR of 110.61, it was reasonable to use 111.26 as a reference. We consider 109.75, a value lower than 111.26, is inappropriate to be used as a practical cutoff, since a higher PLR is related to higher risks. PLR cutoff value for the hypopharynx was higher than their laryngeal counterparts, but due to limited hypopharyngeal cases, a critical appraisal was not separately made [Citation19,Citation20].
Conclusion
PLR has a positive correlation with age of the healthy individuals, but not the LHSCC patients. The high PLR group of patients exhibits significantly decreased OS. The PLR cutoff values vary by different methods and the selection of the cutoff value can influence on patients stratifying and thereby clinical decisions. Largescale high-quality evidence-based prospective trials are fruitful for validating the significance of PLR and helping to make a definite conclusion. Using PLR as a simple and economic parameter to guide our clinical approaches is encouraging, and future analysis of the immune and genomic landscape of LHSCC may bring profound changes to our contemporary understanding.
Acknowledgment
We thank the statistician Biyun Xu, for her helpful discussions.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Perri F, Ionna F, Longo F, et al. Immune response against head and neck cancer: biological mechanisms and implication on therapy. Transl Oncol. 2020;13(2):262–274.
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–1953.
- van Es N, Sturk A, Middeldorp S, et al. Effects of cancer on platelets. Semin Oncol. 2014;41(3):311–318.
- Marchi F, Missale F, Incandela F, et al. Prognostic significance of peripheral T-cell subsets in laryngeal squamous cell carcinoma. Laryngoscope Investig Otolaryngol. 2019;4(5):513–519.
- Song PP, You CG. Clinical application values of neutrophil to lymphocyte and platelet to lymphocyte ratios in multiple cancers. Clin Lab. 2020;66:1027–1032.
- Bardash Y, Olson C, Herman W, et al. Platelet-lymphocyte ratio as a predictor of prognosis in head and neck cancer: a systematic review and meta-analysis. Oncol Res Treat. 2019;42(12):665–677.
- Yang L, Huang Y, Zhou L, et al. High pretreatment neutrophil-to-lymphocyte ratio as a predictor of poor survival prognosis in head and neck squamous cell carcinoma: systematic review and meta-analysis. Head Neck. 2019;41(5):1525–1535.
- Rassouli A, Saliba J, Castano R, et al. Systemic inflammatory markers as independent prognosticators of head and neck squamous cell carcinoma. Head Neck. 2015;37(1):103–110.
- Bojaxhiu B, Templeton AJ, Elicin O, et al. Relation of baseline neutrophil-to-lymphocyte ratio to survival and toxicity in head and neck cancer patients treated with (chemo-) radiation. Radiat Oncol. 2018;13(1):216.
- Chen L, Zeng H, Yang J, et al. Survival and prognostic analysis of preoperative inflammatory markers in patients undergoing surgical resection for laryngeal squamous cell carcinoma. BMC Cancer. 2018;18(1):816
- Tham T, Wotman M, Chung C, et al. Systemic immune response in squamous cell carcinoma of the head and neck: a comparative concordance index analysis. Eur Arch Otorhinolaryngol. 2019;276(10):2913–2922.
- Song S, Chen H, Dong W, et al. The prognostic value of preoperative derived neutrophil-to-lymphocyte ratio in patients undergoing total laryngectomy with laryngeal carcinoma. Acta Otolaryngol. 2019;139(3):294–298.
- Cai H, Zhang ZH, Zhou YJ, et al. The prognostic value of preoperative plasma fibrinogen and neutrophil-to-lymphocyte ratio in patients with laryngeal squamous cell carcinoma. Ear Nose Throat J. 2020. DOI: 10.1177/0145561320920746
- Fei Y, Wang X, Zhang H, et al. Reference intervals of systemic immune-inflammation index, neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, mean platelet volume to platelet ratio, mean platelet volume and red blood cell distribution width-standard deviation in healthy Han adults in Wuhan region in central China. Scand J Clin Lab Invest. 2020;80(6):500–507.
- Hsueh C, Tao L, Zhang M, et al. The prognostic value of preoperative neutrophils, platelets, lymphocytes, monocytes and calculated ratios in patients with laryngeal squamous cell cancer. Oncotarget. 2017;8(36):60514–60527.
- Wang J, Wang S, Song X, et al. The prognostic value of systemic and local inflammation in patients with laryngeal squamous cell carcinoma. Onco Targets Ther. 2016;9:7177–7185.
- Mao Y, Fu Y, Gao Y, et al. Platelet-to-lymphocyte ratio predicts long-term survival in laryngeal cancer. Eur Arch Otorhinolaryngol. 2018;275(2):553–559.
- Lau HC, Hsueh CY, Chen Q, et al. Prognostic values of preoperative platelet-to-lymphocyte ratio and platelet-related indices in advanced hypopharyngeal squamous cell carcinoma. Clin Otolaryngol. 2020;45(2):221–230.
- Sun W, Huang JQ, Chen L, et al. Peripheral inflammation markers of chemosensitivity to induction chemotherapy in hypopharyngeal cancer patients. ORL J Otorhinolaryngol Relat Spec. 2019;81(2–3):82–91.