Abstract
Background
Unintended weight loss and nutritional problems are often seen in patients with head and neck cancer, but changes in lipid metabolism are poorly studied.
Aim/Objectives
The present study aimed to explore the longitudinal changes in circulating fatty acid (FA) composition in patients with head and neck cancer.
Materials and methods
This study included 27 patients with head and neck cancer. Treatment consisted of single modality or combined modality treatments. The patients were assessed by repeated blood sampling and body weight assessments before treatment started and on three occasions after the start of treatment. FA profiling included gas chromatography analysis of unsaturated FAs and saturated FAs in serum.
Results
The values of three fatty acids – FA 14:0, FA 18:3n3, and FA 20:3n6 – changed in a specific pattern over the course of the study and the change in FA 14:0 correlated with weight changes.
Conclusions and significance
This study showed altered profiles of both saturated and unsaturated FAs. An improved understanding of the metabolic pathways in patients with head and neck cancer supports the development of better nutritional surveillance and nutritional treatments.
Chinese abstract
背景:头颈癌患者常出现意外的体重减轻和营养问题, 但对脂质代谢的变化研究很少。
目的:本研究旨在探讨头颈癌患者的循环脂肪酸(FA)成分的纵向变化。
材料和方法:这项研究包括 27 名头颈癌患者。治疗包括单一方式或组合方式治疗。患者的评估通过治疗开始前和治疗后的 3 次采血和体重测量。 FA 分析包括血清中不饱和脂肪酸和饱和脂肪酸的气相色谱分析。
结果:三种脂肪酸的值(FA 14:0、FA 18:3n3 和 FA 20:3n6)在研究过程中以特定的模式变化, 并且, FA 14:0 的变化与体重变化相关。
结论和意义:本研究显示饱和和不饱和FA的变化特征。更好地了解头颈癌患者的代谢方式可以帮助开发更好的营养监测和营养治疗。
Introduction
For patients with head and neck cancer, the tumour and treatment may affect the upper aerodigestive tract and cause swallowing problems, and even at the time of diagnosis critical body weight loss (>5% in 1 month or >10% in 6 months) is common [Citation1]. Weight loss is associated with the nutritional status of the patients, which is often impaired because of loss of appetite, dysphagia, and odynophagia. Problems maintaining oral intake usually increase during radiotherapy and combined modality treatment, and this often leads to weight loss [Citation2].
Malnutrition is associated with alterations in a number of metabolic processes [Citation3].
For example, inflammation is reported to be a common aetiological factor for altered metabolism in cancer patients, which leads to a catabolic state and contributes to loss of muscle mass [Citation4]. Cancer-associated malnutrition affects the metabolism of proteins, fats, and carbohydrates, and the most common metabolic abnormality observed in cancer patients is increased mobilisation of peripheral fat and excessive oxidation of fatty acids (FAs). Alterations in lipid metabolism can lead to the depletion of adipose tissue and thus to weight loss [Citation5].
Lipids are a group of water-insoluble molecules that include triglycerides, phospholipids, sterols, and sphingolipids. FAs are the main component in the synthesis of triglycerides, which are mainly used for energy storage. Phospholipids also contain FAs, and together with sterols and sphingolipids they represent the major structural components of biological membranes. Lipids also have essential roles in signalling by functioning as second messengers and as hormones.
Lipids are found in the bloodstream as free FAs bound to albumin or complexed to proteins as low-density and high-density lipoproteins, e.g. cholesterol esters. An FA is a carboxylic acid and is either saturated or unsaturated. Some FAs are obtained from dietary sources, while others are synthesised de novo mainly in the liver, adipose tissue, and lactating breast tissue. Depending on the type of FA, circulating FAs can reflect dietary intake and nutritional status and/or FA metabolism (e.g. de novo lipogenesis and FA desaturation), both of which are pathways that may be altered in cancer patients [Citation6]. Tumour cells show high rates of de novo lipid synthesis, and besides uptake of FAs from blood circulation their ability to synthesize FAs plays a vital role in cancer pathogenesis [Citation7].
The plasma FA profile in patients with cancer has been poorly studied. The present study aimed to explore the longitudinal changes in circulating fatty acids composition in patients with head and neck cancer over the course of one year from the end of treatment. In addition, a secondary aim was to investigate the relationship between circulating fatty acids and the weight loss that is typically seen in the treatment of head and neck cancer patients.
Materials and methods
This study included 27 patients with head and neck cancer who underwent treatment at Uppsala, Umeå and Orebro University Hospitals which are tertiary centres in Sweden. The inclusion criteria were patients with untreated head and neck cancer and a performance status of 0–2 according to the WHO/ECOG Performance status. Patients with previous cancer history in the past 5 years (except skin cancer) and patients with severe alcoholism, dementia, inability to understand Swedish, or any other inability to participate in the study were excluded.
The mean age of the patients was 67 years (range 50–81 years) with a male-to-female ratio of 2.4:1 (19 males, 8 females). The most common tumour site was the oropharynx (n = 10) followed by the oral cavity (n = 9). Treatment consisted of single-modality surgery or radiotherapy or combined modality treatment. The patients’ characteristics are described in .
Table 1. Characteristics of patients with head and neck cancer. Numbers of patients are given (n).
The patients were assessed by blood sampling and body weight measurements before treatment started (i.e. pre-treatment) and at 7 weeks after the start of treatment and at 3 months and 1 year after the termination of treatment. The nutritional status of all patients was followed according to local routines, and supplemental nutritional therapy was offered when indicated. Two patients did not provide blood samples at 7 weeks after the start of treatment. The blood samples at 1 year after the termination of treatment were not collected in three patients. Two patients had died, and one patient was unable to give a blood sample due to fatigue. Body weight was measured in kilograms, and the patients did not wear outdoor clothing or shoes when being weighed. Body mass index (BMI) was calculated using the formula kg/m2. The patients were divided into underweight, normal weight and overweight according to Global Leadership Initiative on Malnutrition (GLIM), as shown in . It was also recorded if the patients had oral intake or nutritional support (total or partial use of enteral nutrition).
Table 2. Weight and nutritional data of patients with head and neck cancer.
FA analysis
Blood samples were stored at −70 °C at the Uppsala University Hospital Biobank, Sweden, until analysis of FA composition. FA profiling included unsaturated FAs 16:1n-7, 18:1n-9, 18:2n-6, 18:3n-6, 18:3n-3, 20:3n-6, 20:4n-6, 20:5n-3, and 22:6n-3 and saturated FAs 14:0, 15:0, 16:0, and 18:0 in serum cholesterol esters using gas chromatography as previously described [Citation8]. For the analyses, 50 μl of centrifuged clarified serum (2000 × g for 10 min) was used as the sample. The values of the FAs are presented as percentages of the total amount of FAs.
Ethical considerations
The Regional Ethical Review Board in Uppsala reviewed and approved the study (No. 2014/447), and written informed consent was obtained from all patients. All blood samples were coded in the Uppsala Biobank (approved RCC 2015-0025). The study is registered at ClinicalTrials.gov (NCT03343236).
Statistical analyses
Generalised estimating equations were used to model the time trends of each FA. Values of the FAs were first transformed from their percentage scale to the log scale, and time was fitted with a second-degree polynomial allowing for non-linear temporal patterns. An AR (1) correlation structure was assumed between the measurements, which expects a higher correlation between measurements from the same individual obtained closer in time than measurements obtained further apart. A comparison of the mean values on the log scale was conducted for each time point versus the time point before, i.e. the mean value at 7 weeks was compared to the mean value at baseline, the mean value at 3 months was compared to the mean value at 7 weeks, and the mean value at 1 year was compared to the mean value at 3 months. The confidence intervals and p-values were adjusted for these multiple comparisons. The freely available statistical software R was used for the statistical analyses. Interaction terms were added to the models to assess the time trends in different pre-defined subgroups, and the p-values were adjusted for the additional tests in those models. An additional analysis was performed to assess the association between FA14:0 and weight change from baseline. The association between FA changes and gender as well as treatment was analysed. A p-value ≤.05 was considered statistically significant.
Results
Changes in FA levels over time
The levels of all studied FAs over the course of the study are shown in . For the whole cohort, FA 14:0 (myristic acid) showed a 15% decrease (p<.001) at 7 weeks, thus supporting a time-dependent association between pre-treatment and at 7 weeks after the start of treatment. At 3 months after the end of treatment, FA 14:0 had decreased even further. The values then increased at 1 year after the end of treatment and were at nearly the same levels as the pre-treatment values, as shown in .
Figure 1. Serum levels of fatty acids (FAs) 14:0, 15:0, 16:0, 16:1n-7, 18:0, 18:1n-9, 18:2n-6, 18:3n-6, 18:3n-3, 20:3n-6, 20:4n-6, 20:5n-3, and 22:6n-3 at pre-treatment (pre), at 7 weeks after the start of treatment (7w), and at 3 months (3 m) and 1 year (1 y) after the termination of treatment in patients with head and neck cancer. The values for the FAs are presented in relation to the total amount of FAs.
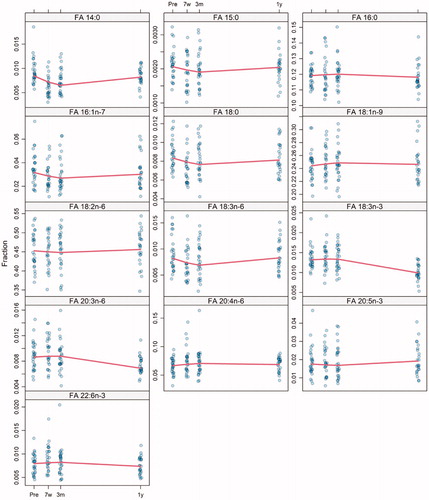
Table 3. Percent change of the mean values of fatty acid 14:0 (FA 14:0) when comparing between 7 weeks (7w) to pre-treatment (pre), 3 months (3 m) to 7 weeks, and 1 year (1 y) to 3 months in patients with head and neck (n = 27).
Another time-dependent association was observed for FA 18:3n-3 (linolenic acid). The values of FA 18:3n-3 decreased by 26% (p<.001) at 1 year after the end of treatment compared to 3 months after the end of treatment. The values of FA 18:3n-3 did not vary between pre-treatment, 7 weeks after treatment start, and 3 months after treatment termination, as shown in . FA 20:3n-6 showed the same trend over time as FA 18:3n-3 with a 21% decrease at 1 year after the end of treatment compared to the value at 3 months (p<.001) ().
The compositions of the other studied FAs in serum were different between patients, and the data do not suggest any clear time-dependent changes.
Weight change over time and nutritional status
At the time of their diagnosis, three patients were underweight (BMI <20 (<22 if ≥70 years)), seven patients were normal weight (BMI = 20–25 (22–27 if ≥70 years)), and 17 patients were overweight (BMI >25 (>27 if ≥70 years)). The mean pre-treatment weight of the patients was 78.1 kg (range 52.6–99.5 kg). There was a significant decrease in mean weight of 3.9 kg between pre-treatment and at 7 weeks, which corresponds to a weight loss of 5%. The percentage weight change from start of treatment to 3 months after the end of treatment was 6.4%. At 1 year after the end of treatment, the mean weight of the patients was 74.3 kg (range 47–94 kg), which was at the same level as the mean weight at 7 weeks after the start of treatment ().
None of the patients needed partial or total parenteral nutrition during treatment or after treatment. A total of seven patients needed enteral nutritional support during the study period as shown in .
Correlation of FA with weight, treatment, and gender
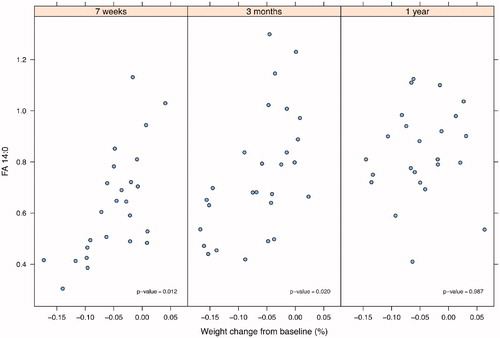
An association was found between FA 14:0 and weight change from baseline. This association was seen at 7 weeks after the start of treatment and at 3 months but not at 1 year after the end of treatment, as shown in .
Figure 2. Correlation between fatty acid 14:0 (FA 14:0) and body weight changes from baseline to 7 weeks after start of treatment and to 3 months and 1 year post-treatment in patients with head and neck cancer (n = 27).
No association was seen in the FA profile between the patients who received radiotherapy and the patients who received chemo radiotherapy.
No association was observed in the FA profile between men and women.
Discussion
The association between FAs and head and neck cancer treatment has not been reported earlier. In the present explorative study, serum levels of FAs were assessed longitudinally up to one year following the end of treatment. Although most of the FAs did not change significantly, FA 14:0, FA 18:3n-3, and FA 20:3n-6 showed more consistent changes and were found to be of specific interest. These three FAs demonstrated a specific pattern over time, and an early decrease of FA 14:0 and late decreases of FA 18:3n-3 and FA 20:3n-6 were seen for the whole cohort of 27 patients with head and neck cancer.
FA 14:0 (myristic acid) is a saturated long-chain FA with a 14-carbon backbone. Myristic acid is found naturally in dairy fat, a major source in the Swedish population, but also in tropical oils including palm oil and coconut oil. FA 14:0 is reported to represent an average of 11% of the dairy intake of FAs and is well known to increase total cholesterol, especially low-density lipoproteins, in the blood [Citation9]. In addition, under lipogenic conditions FA 14:0 may also be produced de novo in parallel with FA 16:0 [Citation10]. FA 18:3n-3 (linolenic acid) is an essential omega-3 FA found exclusively in plant oils, such as rapeseed oil, but it can be converted to very long-chain n-3 polyunsaturated FAs (PUFAs) that are found in seafood. Notably, despite being an essential FA, 18:3n-3 as assessed in serum cholesterol esters does not appear to be a reliable biomarker of self-reported dietary intake [Citation11]. FA 20:3n-6 (dimoho-γ-linolenic acid) is a polyunsaturated omega-6 FA that is produced in the body by elongation and desaturation of FA 18:2n-6 (linoleic acid). Linolenic acid and linoleic acid are the two essential FAs for humans that must be obtained through the diet.
The changes in the FA profile in serum over time in patients with head and neck cancer are most probably multifactorial. The pre-treatment body composition is one factor to take into account, and dysphagia is a frequent contributor to reduced intake of nutrients, including essential FAs, and thus contributes to weight loss. Seventeen patients in the present cohort (63%) were overweight at baseline. Although 7 patients (26%) received enteral nutrition at 7 weeks after start of treatment, only 8 patients (32%) were overweight at 12 months after termination of treatment.
In the present study, FA 14:0 in serum was consistently correlated to weight change both at 7 weeks after the start of treatment and at 3 months after the end of treatment, corresponding to the convalescent phase, but the correlation did not remain at 1 year after the end of treatment, most probably due to the limited sample size. In a study of patients with head and neck cancer, the patients’ mean weight changed over time with a nadir observed at 5–6 months after the start of radiotherapy and thereafter slowly recovered [Citation12]. In the present study, weight loss was most severe at 3 months after the termination of treatment. It can be speculated that the decrease in the serum concentration of FA 14:0 after treatment was associated with a reduced dietary intake of saturated fat (e.g. high-fat dairy foods) as indicated in elderly Swedish populations [Citation13]. In addition to FA 14:0 being a potential marker of saturated fat intake, it is also possible that the reduced FA 14:0 levels to some extent reflect a decrease in de novo lipogenesis due to lowered intake of energy from carbohydrates [Citation14]. Thus, the associations between changes of serum FA 14:0 and body weight observed here may suggest that this FA may reflect the nutritional and consequently the anabolic/catabolic status in these patients post-treatment. Its use as a nutritional marker is of high interest but needs further investigation.
Fourteen patients in the present study received radiotherapy and 9 patients underwent chemoradiotherapy. An increase of pro-inflammatory cytokines is reported in connection to chemoradiotherapy in patients with head and neck cancer, which was correlated to the T-stage of the tumour [Citation15]. It is clear that inflammation and immune reactions play a role in cancer development, progression, and response to treatment [Citation16]. Moreover, the tumour itself may produce pro-inflammatory cytokines that trigger an inflammatory response that may contribute to a decrease in food intake and thus to weight loss.
Lipid metabolism has been more extensively studied in cancer cell progression in pre-clinical studies. Cancer development is dependent on the ability of the cancer cells to produce lipids that are necessary for cell membrane formation, protein modification, and transmission of oncogenic signals [Citation17]. Cancer cells also take advantage of exogenous FAs in order to carry out de novo lipogenesis [Citation18].
In many cases, patients with head and neck cancer need essential nutrients and intake of surplus energy, especially during periods of high energy expenditure, i.e. the treatment phase . Among the various aspects to consider in nutritional surveillance are the nutritional and energy demands of cancer cells in relation to energy intake and storage, which is an area of research that needs to be better explored in clinical studies. The present study indicates that supplementation of the essential FA 18:3n-3 may be especially indicated at 1 year after the termination of treatment.
Although no difference was found in the present study between patients receiving radiotherapy and chemoradiotherapy, the effect of treatment modality on FA profiles cannot be overlooked. It could be speculated that radiotherapy and chemotherapy affect FAs in different ways. An earlier study on three patients with advanced breast cancer suggested that chemotherapy is a possible cause of low levels of plasma phospholipids [Citation19]. A larger study on 42 men with squamous cell carcinoma of the oesophagus demonstrated increased levels of polyunsaturated FAs after platinum-based chemoradiotherapy [Citation20].
The limitations of the study are the size of the cohort and the heterogeneity of tumour site, stage, and treatment. The results of the current study should be regarded as hypothesis-generating, and a larger study cohort should provide confirmatory and more detailed knowledge on the potential importance of FA 14:0, FA 18:3n-3, and FA 20:3n-6 in patients with head and neck cancer.
Conclusion
An altered serum profile of FA 14:0, FA 18:3n-3, and FA 20:3n-6 was observed within a year post-treatment in patients with head and neck cancer. At the group level, FA 14:0 was associated with weight change within the first 3 months after the end of treatment. Whether these FAs reflect nutritional and/or metabolic status is unclear, but of potentially high interest because FAs may be useful prognostic or indicative biomarkers in patients undergoing cancer treatment. Our observations encourage further investigation of the association between FAs and nutritional status in patients with head and neck cancer.
Author contributions
All authors contributed to the work and the final version of the manuscript was approved by all authors. YTE and GL designed the study, YTE collected the data, CC drafted the manuscript, EL analysed the data, and CC, YTE, GL, and UR critically revised the manuscript.
Acknowledgements
Thanks to research nurse Nilla Westöö and specialist nurses Brith Granström and Charlotte Ryman and all participating ENT clinics in the Uppsala-Örebro region and the Northern region. A special thanks also to all participating patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Jager-Wittenaar H, Dijkstra PU, Vissink A, et al. Critical weight loss in head and neck cancer–prevalence and risk factors at diagnosis: an explorative study. Support Care Cancer. 2007; 15(9) :1045–1050.
- Arribas L, Hurtós L, Taberna M, et al. Nutritional changes in patients with locally advanced head and neck cancer during treatment. Oral Oncol. 2017; 71:67–74.
- Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 2005; 9 Suppl 2(Suppl 2):S51–S63.
- Ryan AM, Power DG, Daly L, et al. Cancer-associated malnutrition, cachexia and sarcopenia: the skeleton in the hospital closet 40 years later. Proc Nutr Soc. 2016;75(2):199–211.
- McAndrew PF. Fat metabolism and cancer. Surg Clin North Am. 1986;66(5):1003–1012.
- Vriens K, Christen S, Parik S, et al. Evidence for an alternative fatty acid desaturation pathway increasing cancer plasticity. Nature. 2019;566(7744):403–406.
- Santos CR, Schulze A. Lipid metabolism in cancer. Febs J. 2012;279(15):2610–2623.
- Fisk HL, West AL, Childs CE, et al. The use of gas chromatography to analyze compositional changes of fatty acids in rat liver tissue during pregnancy. JoVE. 2014;(85)51445
- Ohlsson L. Dairy products and plasma cholesterol levels. Food Nutr Res. 2010;54:1–9.
- Roberts R, Hodson L, Dennis AL, et al. Markers of de novo lipogenesis in adipose tissue: associations with small adipocytes and insulin sensitivity in humans. Diabetologia. 2009;52(5) :882–890.
- Iggman D, Ärnlöv J, Cederholm T, et al. Association of adipose tissue fatty acids with cardiovascular and all-cause mortality in elderly men. JAMA Cardiol. 2016;1(7):745–753.
- Einarsson S, Laurell G, Tiblom Ehrsson Y.An explorative study on energy balance in patients with head and neck cancer. Nutr Cancer. 2020;72(7):1191–1199.
- Rosell M, Johansson G, Berglund L, et al. The relation between alcohol intake and physical activity and the fatty acids 14: 0, 15: 0 and 17: 0 in serum phospholipids and adipose tissue used as markers for dairy fat intake. Br J Nutr. 2005; 93(1) :115–121.
- Song X, et al. Dietary long-chain fatty acids and carbohydrate biomarker evaluation in a controlled feeding study in participants from the women's health initiative cohort. Am J Clin Nutr. 2017; 105(6) :1272–1282.
- Astradsson T, Sellberg F, Berglund D, et al. Systemic inflammatory reaction in patients with head and neck cancer-an explorative study. Front Oncol. 2019; 9:1177.
- Bonomi M, et al. The role of inflammation in head and neck cancer. Adv Exp Med Biol. 2014;816:107–127.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674.
- Louie SM, Roberts LS, Mulvihill MM, et al. Cancer cells incorporate and remodel exogenous palmitate into structural and oncogenic signaling lipids. Biochim Biophys Acta. 2013;1831(10):1566–1572.
- Pratt VC, Watanabe S, Bruera E, et al. Plasma and neutrophil fatty acid composition in advanced cancer patients and response to fish oil supplementation. Br J Cancer. 2002;87(12):1370–1378.
- Zemanova M, Vecka M, Petruželka L, et al. Plasma phosphatidylcholines fatty acids in men with squamous cell esophageal cancer: chemoradiotherapy improves abnormal profile. Med Sci Monit. 2016;22:4092–4099.
