Abstract
Background and objective
The main objective of this prospective, open, uncontrolled pilot study was to investigate the safety of administering onabotulinumtoxinA (BTA) towards the sphenopalatine ganglion (SPG) in 10 patients with refractory chronic rhinosinusitis with nasal polyposis (CRSwNP) using a novel injection tool, the MultiGuide®.
Material and methods
A one-month baseline period was followed by bilateral injections of 25 U BTA in the SPG and a follow-up of 12 weeks. The primary outcome was adverse events (AE), and the main efficacy outcome was a 50% reduction in visual analogue scale (VAS) symptoms for nasal obstruction and rhinorrhea in months 2 and 3 post-treatment compared to baseline.
Results
We registered 13 AEs, none of which were serious, however, one patient experienced diplopia which moderately affected his daily activities. The symptoms slowly improved and resolved 4 weeks after injection. Five patients were treatment responders with at least 50% median reduction in the nasal obstruction, and four were treatment responders concerning rhinorrhea.
Conclusions
Injection of BTA toward the SPG using the MultiGuide® in patients with CRSwNP appears to be safe but with a potential for moderately disabling side effects. The study indicates a beneficial effect on nasal obstruction.
Chinese abstract
背景和目标:这项前瞻性、开放、不受控制的试点研究的主要目的是为了研究使用新型注射工具 MultiGuideVR对10例难治性慢性鼻窦炎鼻息肉病(CRSwNP)患者的蝶腭神经节(SPG)注射肉毒杆菌毒素 A (BTA) 的安全性。
材料和方法: 1 个月的基线期后, 对SPG进行双侧注射 25 U BTA, 并有12 周的随访。主要结果是不良事件 (AE), 主要疗效结果是, 治疗后第 2 个月和第 3 个月, 与基线相比, 鼻塞和鼻漏的视觉模拟量表 (VAS) 症状减少 50%。
结果:我们记录到 13 个 AE, 没有一个是严重的。但是, 一名患者出现了复视, 对他的日常活动产生一定的影响。症状缓慢改善并在注射后4周内消退。 5 名患者是治疗反应者, 鼻塞的中位减少至少是50%。4 名患者是鼻漏的治疗反应者。
结论:使用 MultiGuideVR 向CRSwNP 患者的 SPG 注入 BTA似乎是安全的, 但有可能导致中度副作用。研究表明它对鼻塞有作用的。
Introduction
Chronic rhinosinusitis (CRS) is a major health problem worldwide, with an overall prevalence in Europe of 11% [Citation1,Citation2]. A significant burden of CRS on quality of life is well-recognized as is the decrease in work productivity [Citation1,Citation3]. Two clinical phenotypes of CRS exist, where the type with nasal polyps (CRSwNP) is less prevalent (2.6%) than the one without nasal polyps (CRSsNP) (5.8%) [Citation4]. Functional endoscopic sinus surgery (FESS) is the most commonly used treatment procedure, but despite improvement in many patients, a treatment-refractory subpopulation of CRSwNP with frequent relapses remains. While surgery alters anatomy, the abnormalities in the innate immune function of the inflamed mucosa are not cured during surgery. Moreover, despite long-term topical postoperative medical management, patients in this subpopulation often require more extensive or radical revision surgery which may be effective [Citation5].
The sphenopalatine ganglion (SPG) is located in the pterygopalatine fossa, and it contains sensory and sympathetic axons and parasympathetic ganglion cells, all innervating the nasal cavity. The autonomous nervous activity through the SPG plays a critical and delicate coordinating role in regulating vessels and glands in the nasal cavity [Citation6–8]. Parasympathetic activation leads to a nasal glandular secretion. Thus, a possible method for reducing symptoms of CRSwNP, mainly seromucous secretion, nasal obstruction, and vasodilatation in the nose, could be to block the parasympathetic nerves in the SPG.
Vidian neurectomy (VN) or posterior nasal neurectomy (PNN) are well-known surgical procedures for treating different forms of rhinitis; first introduced by Golding-Wood in the 1960s [Citation9]. The aim of the procedure was to correct a postulated imbalance between parasympathetic and sympathetic tone in the nasal cavity, thereby reducing the excess stimulation of goblet cells and mucous glands. The procedure is effective in vasomotor rhinitis and in refractory CRSwNP [Citation10]. A study from 2019 found a 29.6% vs. 44.4% recurrence rate in a patient receiving both FESS and PNN 6 months after the intervention compared to FESS only in patients with allergic rhinitis combined with CRSwNP [Citation11]. VN is usually carried out in general anesthesia and is encumbered with the general risks that follow anesthesia and surgery. Thus, a safer, and the less resource-demanding procedure is desirable. Autonomic nerve fibers from the vidian nerve enter the SPG where the parasympathetic fibers synapse using acetylcholine as a neurotransmitter [Citation12]. OnabotulinumtoxinA (BTA) blocks the release of acetylcholine, and injections of BTA toward the SPG therefore theoretically block parasympathetic output to the nasal structures. The safety of this procedure has been examined in a couple of small pilot studies on other conditions (intractable chronic cluster headache and intractable chronic migraine) [Citation13,Citation14].
To explore whether blockade with BTA may be a safe and possibly effective intervention for CRSwNP, we decided to do a pilot study with BTA injection toward the SPG in patients with treatment-refractory CRSwNP using an image-guided injection device, MultiGuide® (), and to collect pilot data on efficacy to inform and power future potential randomized controlled trials (RCT).
Materials and methods
Study design
A total of 10 patients were treated between December 2016 and October 2018 at the Department of Otolaryngology, Head and Neck Surgery, St. Olavs University Hospital, Trondheim, Norway. The study was designed to evaluate patient-reported outcome measures (PROMs), nasal endoscopy, and physiologic measures (Peak Nasal Inspiratory Flow (PNIF) and acoustic rhinometry (AR)) in patients with CRSwNP 4 weeks (baseline) before injection of BTA and during a follow-up period of 12 weeks. One study month was equal to 28 days. The nasal endoscopy and injection procedures were performed by one surgeon (KAJ) and in the same environment conditions (operation room, assistance) to avoid background biases. CT and MRI scans of the sphenopalatine fossa were obtained before injection. The patients continued the use of nasal corticosteroids throughout the study period and all use of medications was recorded. CT and MRI were also conducted after the procedure for measuring the potential change in nasal anatomy, and these results will be reported in a separate paper.
Study population
The CRSwNP diagnosis was based on the presence of typical symptoms such as nasal obstruction, discharge and reduction of smell, the presence of endoscopically verified nasal polyps, and in accordance with the EPOS 2012 [Citation15]. Patients included were 18–70 years of age, refractory to standard drug therapy, and with refractory CRSwNP despite the previous FESS. Exclusion criteria were any disorder that might complicate treatment; psychiatric illness preventing full participation; pregnancy, nursing, or inability to use contraceptives infertile women; abuse of any pharmacological substance such as narcotics or alcohol; hypersensitivity to short-acting anesthetics such as adrenaline or BTA; and active treatment with pharmacological substances with possible interaction with the study medicament. Further, patients with the systemic disease with potential affection of the nose such as granulomatosis with polyangiitis, cystic fibrosis, primary ciliary dyskinesia, Kartagener’s syndrome, NSAIDs-exacerbated respiratory disease (NERD), sarcoidosis, and with allergy suspected of being the primary cause of nasal symptoms were excluded.
Therapeutic techniques
Navigation-assisted administration of BTA toward the SPG was performed on awake patients, in an outpatient office-based setting with a percutaneous, infrazygomatic approach using the novel injection device MultiGuide®. Pre-treatment planning of the procedure with CT and MRI was performed with Brainlab iPlan 3.0 (Brainlab AG, Feldkirchen, Germany). The SPG was localized and marked on fused MRI and CT scans on both sides. With the patient in a supine position, the skin and deep structures toward the sphenopalatine fossa were anesthetized with 5–7 ml Marcaine-Adrenaline (5 mg/ml-5 μg/ml, AstraZeneca) and a 1–2 mm skin incision was made. Aided by the MultiGuide®, 25 units BTA suspended in 0.5 ml isotonic saline were injected towards the SPG bilaterally (total dose 50 units). The estimated duration of the injection was around 3 min, and for the whole procedure including navigation system setup 20–30 min. In this study, we used the same injection technique as in previous pilot trials on intractable headaches [Citation13,Citation14].
Study outcomes
Primary outcome
The primary outcome was the occurrence of adverse events (AEs). Patients had to keep a daily diary 4 weeks (baseline) prior to and 3 months after the injection, recording AEs. All medical events experienced by the patients from the time of injection and during the 3-month follow-up were evaluated. Information on AEs was collected from each patient during telephone consultation (once each week at weeks 1–8 after injection), at last visit (month 3 after injection), and in the daily symptom diaries (each day had a free text box for AEs). All AEs were evaluated as to whether they were related or unrelated to the intervention and graded according to severity, and whether they were serious and/or unexpected. All AEs were followed until resolved or considered stable.
Secondary outcome
The main efficacy outcome was the change in nasal obstruction and rhinorrhea measured on a 100 mm visual analogue scale (VAS), with 0 representing ‘not troublesome’ and 100 ‘worst thinkable troublesome’ [Citation16], from 28 days preoperatively to weeks 5–8 and 9–12 (predefined in the protocol). The first four weeks after injection were not considered, since the onset of effect may require up to 4 weeks and maximal benefit is expected during month 2, before the usual attenuation of the effect during the 3rd month after injection. A treatment responder was predefined as having at least a 50% reduction in the VAS nasal obstruction or rhinorrhea, from baseline to weeks 5–8 and to weeks 9–12.
The Patient Global Impression of Change (PGIC) and the Sinonasal Outcome Test- 22 (SNOT-22) were reported by patients before and after treatment. The PGIC measures the change in patient-reported overall health status on a seven-point scale (1: very much improved, 2: much improved, 3: minimally improved, 4: no change, 5: minimally worse, 6: much worse, and 7 very much worse), measured at baseline and at month 3 after injection. The SNOT-22 is a validated, 22-item treatment outcome measure applicable to chronic sinonasal conditions. Higher scores on the SNOT-22 survey items suggest higher symptom severity (total score range: 0–110). Scoring is conducted via Likert scale responses (0 = ‘No problem’, 1 = ‘Very mild problem’, 2 = ‘Mild or slight problem’, 3 = ‘Moderate problem’, 4 = ‘Severe problem’, and 5 = ‘Problem as bad as it can be’). Prior analysis of the SNOT-22 has revealed four underlying sub-domains, which were reported in the present study: the four subscales were rhinological symptoms (items 1–5, 7, and 8), ear and facial symptoms (items 9–12), sleep function (items 13–15) and psychological issues (items 17–22). The items ‘cough’ and ‘waking up tired’ were not included in the four subscales [Citation17].
Changes in nasal geometry and nasal airflow were measured by acoustic rhinometry (AR) and Peak nasal inspiratory flow (PNIF), respectively. AR measures nasal volumes and minimal cross-sectional areas and was carried out with RhinoMetrics SRE2100 (Rhinoscan version 2.5, built 3.2.5.0; Interacoustics, Minneapolis, MN) by one trained operator throughout the study and according to published protocols [Citation18]. Briefly, three satisfactory recordings were made from each nasal cavity. The values for each nasal cavity were averaged. Due to the variations caused by the nasal cycle, the mean of the two averages (one for each side) was calculated to obtain the minimal cross-sectional area (MCA, cm2) and nasal cavity volume (NCV, cm3), from 0 to 3 cm labeled MCA1 and NCV1, and from 3 to 5.2 cm labeled MCA2 and NCV2, respectively PNIF was assessed with a Youlten peak flow meter (Clement Clarke International, Harlow, Essex, UK). The average of three satisfactory maximal nasal inspirations was recorded.
Nasal endoscopy was performed with a 2.7 mm, 0° True View II endoscope (Olympus, Japan), and the mucosa in each nasal cavity was quantified using the modified Lund-Kennedy staging system (ranged from 0 to 12 with higher scores representing greater disease severity) and the scoring system adapted from Meltzer et al. (ranged from 0 to 8 with higher scores representing greater disease severity).
PGIC, SNOT-22, AR, and PNIF were registered 28 days before injection and 12 weeks after injection. Pain on the injection site was reported on a VAS from 0 mm (no pain) to 100 mm (intolerable pain) immediately after injection and on the 1st and 7th postoperative days.
Statistical analysis
For statistical analysis, SPSS version 25.0 (SPSS Inc, Chicago, IL, USA) was used. For demographic, medical comorbidity, and disease severity measures, results are given as median (range). In addition, means (±SD) were calculated in order to produce measures enabling comparison with other studies targeting the SPG using the same technique [Citation13,Citation14]. Since the study was an exploratory safety study, no power calculation was performed prior to the study's start.
For the primary endpoint, we analyzed data for all 10 patients. For efficacy outcomes, we analyzed data for 10 patients regarding primary efficacy outcomes and 9 patients for secondary efficacy outcomes. One patient was considered a protocol violator because the patient did not respond to our telephone calls in the follow-up months but nevertheless handed in the diary and AE forms at the end of the study. A protocol violator was defined as a participant with less than 60% of diary days registered or change prophylactic medication during the study. Missing values were estimated using the last observation carried forward methodology. Non-parametrical, 2-sided Wilcoxon Signed Ranks Test was performed to compare nasal obstruction and rhinorrhea measured at baseline and at weeks 5–8 and 9–12 weeks after injection, using a 100 mm visual analogue scale (VAS). The same test was used to determine significant changes in SNOT-22 scores, AR, PNIF, Modified Lund Kennedy, and Meltzer score between two-time points. p < .05 was considered statistically significant.
Results
A total of 11 patients were screened. One patient was considered a screening failure during baseline owing to spontaneous improvement of symptoms and regression of nasal polyps detected by rhinoscopy. One patient was lost to follow-up and no efficacy data could be obtained for this patient. See for the subject characteristics of the sample.
Table 1. Demographics of the sample.
Primary outcome
Nine out of 10 patients experienced AEs, none were serious (). One patient experienced diplopia which moderately affected his daily activities. An ophthalmologist diagnosed a moderate paresis of the inferior rectus muscle with hypertropia in abduction. The symptoms slowly improved and resolved 4 weeks after injection. Two patients experienced nasolabial fold asymmetry, appearing 4 weeks after injection and resolving spontaneously 7 and 12 weeks after injection, respectively. The AE did not require any treatment and was not considered bothersome by the patients. Two patients had pain or swelling at the injection site that resolved within the first month after injection. One of them had to take additional analgesics on the day of injection. Seven patients reported discomfort in the jaw at maximal gaping, which did not interfere with chewing, eating, or speaking and there was no need for analgesics or further treatment. One patient experienced a burning sensation of the tongue that resolved spontaneously within 2 weeks after injection. One patient experienced blurred vision the same evening as the injection, assumed to be due to the local anaesthesia.
Figure 2. Adverse events, demonstrating the number of patients with adverse events resolved 12 weeks after injection.
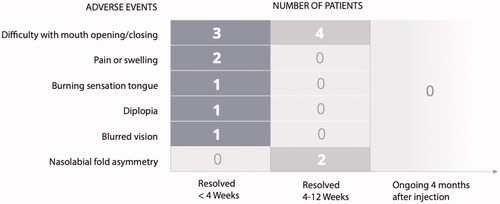
Of the 13 AEs observed; 9 AEs were considered to be secondary to the procedure (pain or swelling at the injection side and jaw problems), 3 AEs to be secondary to BTA (nasolabial fold asymmetry and diplopia), and 1 AE of unknown origin (burning sensation of the tongue). All AEs resolved within 3 months after injection.
Secondary outcomes
For the main efficacy outcome, (), only nasal obstruction and not rhinorrhea reached statistical significance. Five patients were treatment responders () for nasal obstruction, four patients for rhinorrhea, and four patients for both obstruction and rhinorrhea, respectively, when comparing baseline with weeks 5–8. Four patients were treatment responders for nasal obstruction, four patients for rhinorrhea, and three patients for both obstruction and rhinorrhea when comparing baseline with week 9–1.
Figure 3. Graphical representation of main efficacy outcome (a) nasal obstruction and (b) rhinorrhea measured on a 100 mm visual analogue scale (VAS) (y-axis), with 0 representing ‘not troublesome’ and 100 ‘worst thinkable troublesome’, from 4-weeks preoperatively to weeks 1–12 after injection. This figure demonstrates the decreased level of nasal obstruction from baseline to after injection. (a) Nasal obstruction (b) rhinorrhea x-axis: Weeks before and after injection y-axis: VAS: Visual analogue scale.
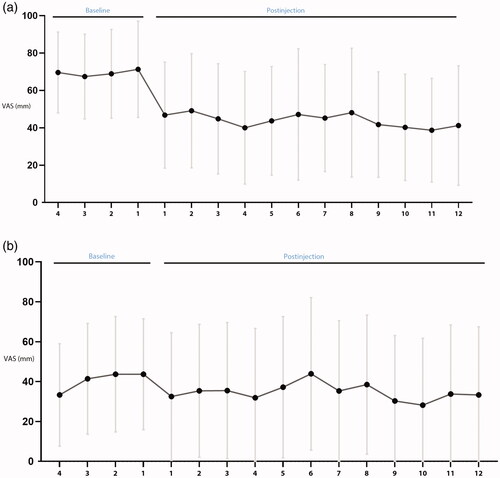
Table 2. Main efficacy outcome (10 patients).
Other subjective measures on VAS () showed in general an improvement, reaching statistical significance for headache and general health. We did not find any significant improvement concerning anosmia from 4 weeks before intervention to 12 weeks after.
Table 3. Secondary efficacy outcome.
For SNOT-22, AR, PNIF, Modified Lund Kennedy, and Meltzer score (), there was no clear trend, and some measures (MCA2 and NCV1) showed a significant worsening after injection.
Three patients had a PGIC of ‘minimally improved,’ 5 ‘no change,’ and 1 ‘minimally worse’ (none ‘very much improved’, ‘much improved’, ‘much worse’ or ‘very much worse’) after injection.
The mean pain at the injection site immediately after the procedure, and on the 1st and 7th postoperative day (VAS 0–100 mm) was 0.8 (SD 0.0–2.6), 1.0 (0.0–3.7), and 0.3 (0.0–0.6) respectively on the right side and 0.9 (0.0–2.5), 1.4 (0.0–6.3) and 0.9 (0.0–6.1) respectively on the left side.
Discussion
This is the first study modulating parasympathetic regulation by blocking the SPG in patients with CRSwNP. The study targeted patients with persistent symptoms despite conventional treatment with intranasal steroids and surgery. We found that injections of BTA toward the SPG in patients with CRSwNP, using a new navigation tool (MultiGuide®), are mainly safe. We registered 13 AEs, none of which were serious in these 10 patients. One patient experienced diplopia which moderately affected his daily activities. The symptoms slowly improved and resolved 4 weeks after injection. There was no need for analgesics towards the pain at the injection site immediately after the procedure and on the 1st and 7th postoperative days. Although the number of patients treated in this study was small, the AE profile is similar to that reported by our group from 3 other pilot trials [Citation13,Citation14,Citation19]. All AEs remitted at the latest 3 months after the treatment as one would expect with BTA.
The novel technique used in this study seems to give mostly mild and transient AEs and no severe AEs. The diplopia in one patient had no need for an eyepatch and was assumed to be caused by the diffusion of BTA through the inferior orbital fissure and affecting the musculus rectus inferior. It may be avoidable through improved technique. The nasolabial fold asymmetry in two patients was most likely caused by the diffusion of BTA toward the zygomatic muscles. One patient experienced blurred vision the same evening as the injection, assumed to be due to the local anaesthesia, and was considered a mild AE.
Some of the AEs can have a moderate disabling potential, however contrary to AEs relating to surgery, the AEs related to BTA in this study were transient.
This study was positive for one main efficacy outcome, nasal obstruction, which was significantly reduced 5 to 12 weeks after treatment, but the efficacy outcome for rhinorrhea was not significant. However, in this particular population 7 of 10 had a high score (VAS >7) for nasal obstruction at baseline whereas for rhinorrhea there were only 2 patients with high scores on the rhinology VAS diary and hence, the statistical power to detect an improvement in rhinorrhea was lower.
Another aspect to consider was the discrepancy between the modest improvement of nasal symptoms reported on the rhinology VAS diary () and the objective findings (AR, PNIF, modified Lund Kennedy and Meltzer score) (). There are four plausible explanations for this: (1) end objective measurements (AR, PNIF, modified Lund Kennedy and Meltzer score) were conducted at week 12–13, which in hindsight probably is after the expected peak BTA effect. In a potential later RCT one should consider performing the objective investigations between weeks 8 and 12. (2) The patients may have discontinued their symptomatic medications (decongestants and antihistamines) even though they were instructed to avoid the change of medications during the study period. (3) There were difficulties measuring MCA, NCV, and PNIF due to severe polyposis and the significant loss of volume in the nasal cavity. (4) The reduction of nasal obstruction on VAS can also be a placebo effect. The lack of improvement of hyposmia () is in accordance with other studies showing that hyposmia is a difficult symptom to treat [Citation20].
The risk of serious and permanent side effects of current surgical procedures shows that novel, minimally invasive, and well-tolerated approaches are needed. A recent study has demonstrated that there is a benefit when combining traditional surgery (FESS) with resection of the parasympathetic innervation [Citation11]. We would therefore expect a more significant reduction in sinonasal symptoms combining the procedure with traditional surgery and biological treatment (monoclonal antibodies) for CRSwNP. Despite patients having severe symptoms related to CRSwNP the present study showed a significant reduction in nasal obstruction without direct surgery. We think the results are promising and believe a larger randomized, controlled study, should be performed. After that, a natural next step could be to combine the procedure with traditional surgery or biological treatment to evaluate the procedure in a more realistic clinical setting.
Strengths and limitations
This was an open-label study, which is adequate for detecting AEs. It adds to the experience we already have about potential AEs using the same technique in other conditions. Despite being a small open-label study, it had strict inclusion and exclusion criteria and used a solid battery of patient-reported outcomes measures (PROMs) and objective measurements. All procedures and investigations were performed in a uniform manner, by the same physician and one research nurse. In our study, we did not include objective investigations of olfactory function which should be considered in future investigations. Any change in symptoms could be a spontaneous improvement or due to the placebo effect.
Conclusion
This study finds that injection of BTA toward the SPG in patients with CRSwNP, using the novel MultiGuide® system, has an acceptable adverse event profile also in this patient group. Although, it is a small potential for inaccuracies of BTA application in the SP fossa that may cause side effects, as in one patient where BTA probably diffused through the inferior orbital fissure causing transient diplopia. The study found an improvement in sinonasal symptoms and suggests a positive effect on nasal obstruction but less on rhinorrhea. Further studies, preferably randomized, controlled trials, examining the efficacy of the procedure for CRSwNP should be performed.
Clinical implications
The injection of onabotulinumtoxinA toward the SPG in CRSwNP appears to be safe.
This study indicates a potential effect on nasal obstruction after injection of 25 U of BTA toward the SPG
Ethics approval
Written informed consent was obtained from all subjects. The study was approved by the Regional Committee for Medical and Health Research Ethics (REK 2015/2018) and the Norwegian Medicines Agency (EUDRACT number: 2015-004377-33). The trial is registered in ClinicalTrial.gov (NCT02784262). Investigations were performed in accordance with the principles of the Declaration of Helsinki.
Consent to participate
All authors agree to participate in this study.
Consent for publication
All authors agree to publish this study.
Author contributions
KAJ, ET, and WMT had the original idea for the manuscript. KAJ, ET, and WMT analysed the data. KAJ reviewed the literature for the introduction and discussion and drafted the manuscript. DFB, JC, IA, LJS, ET, and WMT: assistance for drafting the manuscript and revision of the text. All authors read and approved the final manuscript.
Disclosure statement
Dr. Bratbak is co-inventor of the device used to perform the treatment (patent pending) and may benefit financially from the commercialization of the device. Dr. Tronvik may benefit financially of a commercialization of a proposed treatment targeting the SPG and the intervention device used to perform the treatment through future possible intellectual properties. Dr. Jamtøy dr. Crespi, dr. Thorstensen and dr Stovner have nothing to disclose.
Additional information
Funding
References
- Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhin. 2020;58(1):1–464.
- Hastan D, Fokkens WJ, Bachert C, et al. Chronic rhinosinusitis in Europe-an underestimated disease. A GA²LEN study. Allergy. 2011;66(9):1216–1223.
- Smith KA, Rudmik L. Medical therapy, refractory chronic rhinosinusitis, and productivity costs. Curr Opin Allergy Clin Immunol. 2017;17(1):5–11.
- Ahn JC, Kim JW, Lee CH, et al. Prevalence and risk factors of chronic rhinosinusitus, allergic rhinitis, and nasal septal deviation: results of the Korean national health and nutrition survey 2008–2012. JAMA Otolaryngol Head Neck Surg. 2016;142(2):162–167.
- Wynn R, Har-El G. Recurrence rates after endoscopic sinus surgery for massive sinus polyposis. Laryngoscope. 2004;114(5):811–813.
- Fantozzi R, Masini E, Blandina P, et al. Release of histamine from rat mast cells by acetylcholine. Nature. 1978;273(5662):473–474.
- Anggård A. Parasympathetic influence on the nasal mucosa. Acta Otolaryngol. 1977;83(1–2):22–24.
- Masini E, Rucci L, Cirri-Borghi MB, et al. Stimulation and resection of vidian nerve in patients with chronic hypertrophic non-allergic rhinitis: effect on histamine content in nasal mucosa. Agents Actions. 1986;18(1–2):251–253.
- Golding-Wood PH. Observations on petrosal and vidian neurectomy in chronic vasomotor rhinitis. J Laryngol Otol. 1961;75:232–247.
- Tan G, Ma Y, Li H, et al. Long-term results of bilateral endoscopic vidian neurectomy in the management of moderate to severe persistent allergic rhinitis. Arch Otolaryngol Head Neck Surg. 2012;138(5):492–497.
- Li S, Cheng J, Yang J, et al. Efficacy of posterior nasal neurectomy for allergic rhinitis combined with chronic rhinosinusitis with nasal polyps. Acta Oto-Laryngologica. 2019;139(10):890–894.
- Robbins MS, Robertson CE, Kaplan E, et al. The sphenopalatine ganglion: anatomy, pathophysiology, and therapeutic targeting in headache. Headache. 2016;56(2):240–258.
- Bratbak DF, Nordgård S, Stovner LJ, et al. Pilot study of sphenopalatine injection of onabotulinumtoxinA for the treatment of intractable chronic migraine. Cephalalgia. 2017;37(4):356–364.
- Bratbak DF, Nordgard S, Stovner LJ, et al. Pilot study of sphenopalatine injection of onabotulinumtoxinA for the treatment of intractable chronic cluster headache. Cephalalgia. 2016;36(6):503–509.
- Fokkens WJ, Lund VJ, Mullol J, et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl. 2012;23:1–298.
- Bousquet PJ, Combescure C, Neukirch F, et al. Visual analog scales can assess the severity of rhinitis graded according to ARIA guidelines. Allergy. 2007;62(4):367–372.
- Le PT, Soler ZM, Jones R, et al. Systematic review and meta-analysis of SNOT-22 outcomes after surgery for chronic rhinosinusitis with nasal polyposis. Otolaryngol Head Neck Surg. 2018;159(3):414–423.
- Hilberg O, Pedersen OF. Acoustic rhinometry: recommendations for technical specifications and standard operating procedures. Rhinol Suppl. 2000;16:3–17.
- Crespi J, Bratbak D, Dodick DW, et al. Pilot study of injection of OnabotulinumtoxinA toward the sphenopalatine ganglion for the treatment of classical trigeminal neuralgia. Headache. 2019;59(8):1229–1239.
- Veloso-Teles R, Cerejeira R. Endoscopic sinus surgery for chronic rhinosinusitis with nasal polyps: Clinical outcome and predictive factors of recurrence. Am J Rhinol Allergy. 2017;31(1):56–62.

