Abstract
Background
Benign vocal fold lesions (BVFLs) can cause voice changes, including reduced loudness and pitch range. In recent times, with progression in endoscopic technology, office-based vocal fold steroid injection (VFSI) has been used as an alternative therapy for BVFLs.
Aims/objectives
In this study, we analyzed the efficacy and safety of VFSI to investigate the mechanism underlying its therapeutic effects and determine the conditions in which VFSI will be most effective.
Materials and methods
In this retrospective cohort study, we included 40 condition-matched patients (8 patients per lesion) with chorditis, vocal nodules, vocal polyps, Reinke’s edema (RE), or vocal scars who received similar regimens of steroid injection using a commercial preparation of triamcinolone acetonide. Their phonological outcomes were evaluated 2 or 3 months after the injection.
Results
Significant improvements were observed in Voice Handicap Index scores, results of laboratory voice evaluation, and voice quality measured using the Grade, Roughness, Breathiness, Asthenia, Strain scale in all participants. In subgroup analysis, VFSI was highly effective against chorditis and vocal nodules, but less effective against RE and vocal scars.
Conclusions
Single-dose VFSI is valuable as an alternative to voice rehabilitation and laryngo-microsurgery, but higher concentrations or repeated injections are required for intractable lesions.
Chinese abstract
背景:良性声带病变 (BVFL) 会导致声音变化, 包括响度降低和音高范围减少。近年来, 随着内窥镜技术的进步, 声带类固醇注射 (VFSI) 已被用作 BVFL 的另一种疗法。
目的:在本研究中, 我们分析了 VFSI 的有效性和安全性, 研究其影响治疗效果的机制, 并确定 VFSI 取得最有效的条件。
材料和方法:在这项回顾性队列研究中, 我们纳入了 40 名条件匹配的患有脊索炎、声带结节、声带息肉、Reinke 水肿 (RE) 的患者, 或使用曲安奈德商业制剂接受类似的类固醇注射的声带疤痕(每种病变 8 名患者)。在注射后 2 或 3 个月评估他们的语音结果。
结果:在语音障碍指数得分、实验室语音评估结果, 以及使用等级、粗糙度、呼吸、虚弱、应变测量的语音质量中观察到所有参与者均有显著改善。在亚组分析中, VFSI 对脊索炎和声带结节非常有效, 但对 RE 和声带疤痕的效果较差。
结论:单剂量 VFSI 作为语音康复和喉显微手术的替代方案是有价值的, 但对于顽固性病变需要更高的浓度或重复注射。
Introduction
Benign vocal fold lesions (BVFLs) such as chorditis, vocal nodules, vocal polyps, Reinke’s edema (RE), and vocal scars can cause voice changes, including reduced loudness and pitch range. The incidence and symptoms of BVFLs increase with social demand. Furthermore, BVFLs frequently occur in the working-age population, resulting in a significant negative impact on the patient’s daily life. The histologic characteristics of BVFLs include inflammation, increased vessel wall thickness, edema, thickening of the basement membrane of Reinke’s space, and elevation of the free edge of vocal cords [Citation1]. BVFLs are generally treated conservatively; primary treatment includes medication, vocal hygiene, and voice therapy. Patients refractory to conservative management typically undergo surgical procedures such as laryngo-microsurgery under general anesthesia.
Recent advances in endoscopic technology have led to the increasing use of office-based vocal fold steroid injection (VFSI) as an effective alternative therapy for BVFLs [Citation2–4]. The advantages of VFSI are its high success rates, stable perioperative hemodynamic profiles, good patient tolerability, and cost-effectiveness [Citation2–6]. VFSI was first performed more than 50 years ago, and several reports, including a systemic review and meta-analysis, have reported its effects on voice quality [Citation7]. Wang et al. reported that both subjective and objective measures of voice showed significant improvements after VFSI in 126 consecutive patients (49, 47, and 30 patients with nodules, polyps, and mucus retention cysts, respectively) [Citation8]. In their randomized controlled trial, Ramavat et al. also showed that VFSI was more effective in reducing the size of BVFLs and improving acoustic parameters than initial laryngo-microsurgery [Citation9]. It has been previously reported that VFSI is a safe and effective treatment for BVFLs [Citation2,Citation7–9]. However, unified treatment regimens and optimal indications have not been established because the number of injections, steroid dose, and the number of patients differed between studies. VFSI is a relatively straightforward procedure that can be performed under local anesthesia. It has been associated with early voice recovery without postoperative scar formation [Citation10] or fibrosis in patients with primary BVFLs. However, various injection regimens have been used, leading to mixed results. Furthermore, BVFLs have been strongly associated with environmental, occupational, and psycho-emotional factors [Citation2,Citation8–10], and the therapeutic effects may also be affected by these factors. Therefore, to demonstrate the mechanism underlying the therapeutic effects of VFSI, determine the lesions most amenable to it, and develop a novel therapeutic strategy, we analyzed the data of sex-, age-, and environment-matched patients with various BVFLs that was extracted from a dysphonia patient database.
Materials and methods
Study participants
This retrospective cohort study was approved by the Institutional Review Board of the International University of Health and Welfare (19-S-2). We enrolled participants from our dysphonia patient database, which contains the data of more than 3000 patients with dysphonia who visited our institutions. To ensure consistency in the background of patients with different lesions, we included 40 patients (8 patients per lesion) with chorditis, vocal nodules, vocal polyps, RE, or vocal scars who were treated with similar regimens of steroid injection. All patients had undergone multiple sessions of behavioral counselling and voice therapy by skilled speech–language–hearing therapists, but without any effect. Medical therapy was also not shown to be effective. The voice therapy also was performed after injection on a regular basis. Chorditis was defined as a chronic exudative lesion spanning the entire vocal fold without nodules and/or polyps at the free edges and with suppression of vibration. All participants were women and were age-matched and grouped according to lesion type. Individuals who smoked or used their voice user professionally (e.g. singers and actors) were excluded. Individuals who smoked in the past but had quit smoking more than three years ago were only included in the RE group. The age ranges (mean age) of the patients with each disease were as follows: chorditis, 25–73 (45.9) years; nodules, 24–70 (45.6) years; polyps, 25–74 (47.3) years; RE, 35–76 (48.1) years; and scars, 36–82 (52.0) years. All vocal scars were postoperative scars. Two or three independent phonosurgeons categorized the patients by referring to their clinical history and pre-treatment stroboscopic findings.
Injection procedure
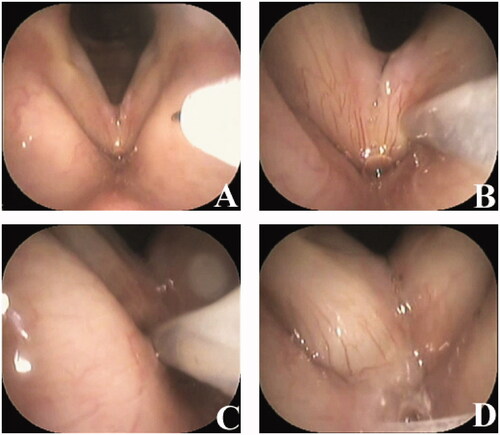
We used a commercial form of triamcinolone acetonide with a depot solution to prolong the development period (KENACORT-A; Bristol-Myers Squibb K. K., Tokyo, Japan). The KENACORT-A was injected under local anesthesia. In brief, the pharynx and larynx were completely anaesthetized with 4% lidocaine. Three milligrams of triamcinolone acetonide dissolved in 0.3 ml per side of depot solution was injected into and spread onto the superficial lamina propria (SLP), and not into the muscle layer, using a 23-gauge injection needle (Varixer; TOP Corp., Tokyo Japan) under transnasal fiberscopic monitoring of the larynx (). The triamcinolone acetonide dose was determined by referring to the manufacturer’s instructions and a previous study [Citation11]. All the participants received the injection bilaterally. To detect possible allergic reactions such as vocal fold edema, the vocal folds were checked using a fiberscope one hour after the injection. Participants received a single dose of injection, which was not repeated. Participants were instructed to rest their voices on the day of the injection but were allowed to use their voices from the following day onwards according to our previously reported injection protocol [Citation12].
Figure 1. Vocal fold steroid injection procedure. Three micrograms of triamcinolone acetonide dissolved in 0.3 ml of solution are injected into the superficial lamina propria using a 23-gauge injection needle. (A) Before vocal fold injection. (B) Injection of the left vocal fold. (C) Injection of the right vocal fold. (D) After vocal fold injection.
Phonological evaluation
Vocal outcomes were evaluated two to three months after the injection. The Japanese version of the Voice Handicap Index (VHI) was used, and total (VHI-T) and individual domain (physical domain [VHI-P], functional domain [VHI-F], and emotional domain [VHI-E]) scores were determined. The maximum phonation time (MPT), mean airflow rate (MFR), pitch range (PR), jitter percentage (jitter%), shimmer percentage (shimmer%), speech fundamental frequency (SFF), noise-to-harmonic ratio (NHR), and sound pressure level (SPL) were assessed. MFR and SPL were measured using a PS-77E phonatory function analyzer (Nagashima Medical Instruments Co., Ltd., Tokyo, Japan). Jitter%, shimmer%, and NHR were assessed using a computerized speech lab (KayPENTAX, Montvale, NJ, USA). PR and SFF were semi-objectively measured using a keyboard and pitch meter.
Statistical analysis
The differences between the means of pre- and post-injection findings for each lesion were analyzed using the Wilcoxon matched-pair signed-rank test.
Results
Adverse effects
No patients experienced allergic reactions or severe adverse effects. Most of the patients experienced vocal fold hyperemia and/or hematoma and roughness of voice for a couple of weeks, but they recovered within a month. Muscle atrophy, which is the most severe adverse effect, was not observed.
Representative case
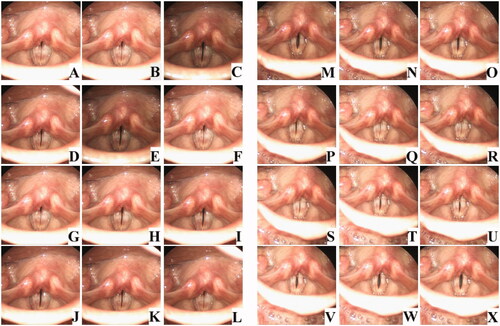
and Supplementary Video 1 shows the stroboscopic findings of a representative case. The patient, a 20s woman, presented with severe dysphonia. Stroboscopy at the first visit showed chorditis (); the free edges of the vocal folds were erythematous and swollen, and there was glottic insufficiency and decreased vibration. VFSI was performed for the bilateral vocal folds. Three months after VFSI, the patient’s voice significantly improved. Stroboscopy showed reduced vocal-fold swelling and glottic insufficiency and improved vocal-fold vibration (). The MPT improved from 5.5 s to 8.0 s, and the VHI score reduced from 93 to 28. As in this patient, the VHI score, laboratory voice evaluation results, and voice quality of most of the patients improved.
Figure 2. Stroboscopic vocal fold examination of a representative patient with chorditis. The patient, a 20s woman, presented with severe dysphonia. Stroboscopy at the first visit showed chorditis (A–L); the free edges of the vocal folds were erythematous and swollen, and there was glottic insufficiency and decreased vibration. VFSI was performed for the bilateral vocal folds. Three months after VFSI, the patient’s voice significantly improved. Stroboscopy showed reduced vocal-fold swelling and glottic insufficiency and improved vocal-fold vibration (M–X).
Post-injection improvements in Voice Handicap Index Scores
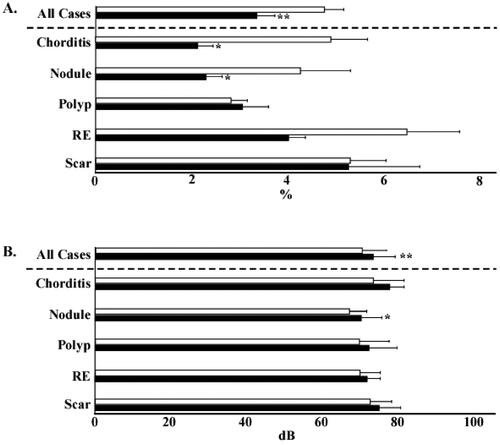
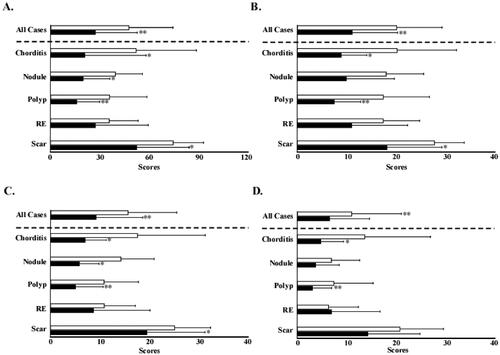
The VHI-T score of all the patients significantly improved from 47.5 to 27.0. In subgroup analysis, the patients with chorditis, nodules, polyps, and scars showed significant improvement from 51.8 to 20.8, 39.3 to 19.6, 35.9 to 15.6, and 74.5 to 52.1, respectively. However, no significant improvement was observed in the patients with RE (). The VHI-P score of all the patients significantly improved from 20.1 to 11.5. In subgroup analysis, the patients with chorditis, polyps, and scars showed significant improvement from 20.2 to 8.9, 17.4 to 7.5, and 27.8 to 18.1, respectively. However, no significant differences were observed in the patients with nodules or RE (). The VHI-F score of all the patients significantly improved from 15.7 to 9.2. In subgroup analysis, the patients with chorditis, nodules, polyps, and scars showed significant improvement from 17.6 to 7.0, 14.3 to 5.8, 10.8 to 5.1, and 25.3 to 19.6, respectively. However, no significant difference was observed in the patients with RE (). The VHI-E score of all the patients significantly improved from 11.3 to 6.7. In subgroup analysis, the patients with chorditis and polyps showed significant improvement from 13.8 to 4.8 and 7.6 to 3.5, respectively. No significant differences were observed in the patients with nodules, RE, or scars ().
Figure 3. Pre- and post-injection Voice Handicap Index scores. (A) Total Voice Handicap Index scores before and after injection. (B) Physical domain scores before and after injection. (C) Functional domain scores before and after injection. (D) Emotional domain scores before and after injection. Open bars indicate pre-injection scores, and closed bars indicate post-injection scores. *p<.05. **p<.01. RE: Reinke’s edema.
Post-injection improvements in laboratory voice evaluation results
Aerodynamic measurements
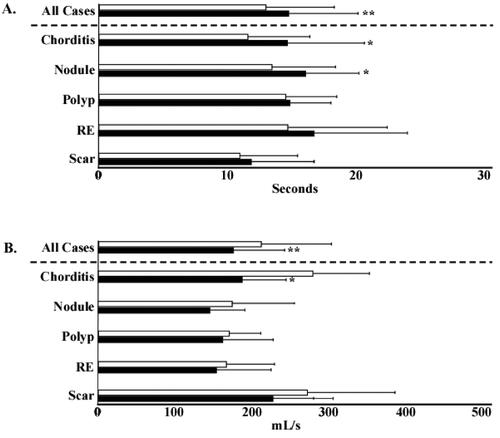
The MPT of all the patients significantly improved from 13.0 to 14.8 s. In subgroup analysis, the MPT of the patients with chorditis and nodules significantly improved from 11.6 to 14.6 s and 13.4 to 15.9 s, respectively. No significant differences were observed in the MPTs of the patients with vocal polyps, RE, or vocal scars (). The MFR of all the patients significantly decreased from 213.9 ml/s to 176.8 ml. In subgroup analysis, the MFR of the patients with chorditis significantly decreased from 281.5 ml/s to 188.4 ml. No significant differences were observed in the MFR of the patients with other lesions ().
Figure 4. Maximum phonation time and mean airflow rate. (A) Pre- and post-injection values of maximum phonation time. (B) Pre- and post-injection values of mean airflow rate. Open bars indicate pre-injection scores, and closed bars indicate post-injection scores. *p<.05. **p<.01. RE: Reinke’s edema.
Acoustic measurements
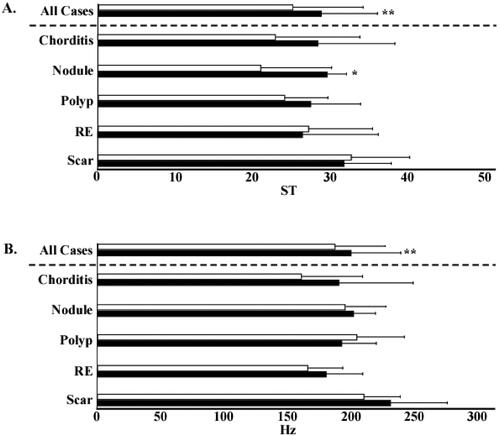
The shimmer% of all the patients significantly decreased from 4.7 to 3.4%. In subgroup analysis, the shimmer% of the patients with chorditis and nodules significantly decreased from 4.9 to 2.1% and 4.3 to 2.3%, respectively. The patients with vocal polyps, RE, or vocal scars did not show any significant differences in shimmer% (). The sound pressure level (SPL) of all the patients significantly increased from 70.2 dB to 73.2 dB. In subgroup analysis, the SPL of patients with nodules significantly increased from 66.8 dB to 70.0 dB. However, no significant differences were observed in the SPL of the patients with other lesions (). The PR of all the patients significantly increased from 25.1 to 28.7 ST. The PR of the patients with vocal nodules significantly increased from 21.0 to 29.5 ST. No significant differences in PR were observed in the patients with other lesions (). Although the SFF of all patients significantly increased from 186.2 to 178.3 Hz, no significant differences were observed on subgroup analysis ().
Post-injection improvement in voice quality assessed using the grade, roughness, breathiness, asthenia, strain scale
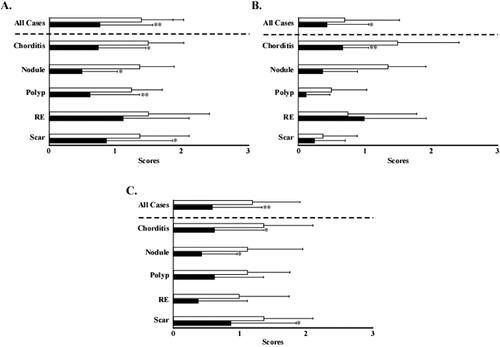
Improvement in voice quality was assessed using the Grade, Roughness, Breathiness, Asthenia, Strain (GRBAS) scale, which is a psychoacoustic measure of voice quality. The Grade score (G score) of all the patients significantly improved from 1.4 to 0.78. In subgroup analysis, the patients with chorditis, nodules, polyps, and vocal scars showed significant improvement from 1.5 to 0.75, 1.38 to 0.5, 1.25 to 0.6, and 1.4 to 0.9, respectively. However, no significant difference was observed in the patients with RE (). The Roughness score (R score) of all the patients significantly improved from 0.71 to 0.43. In subgroup analysis, the patients with chorditis showed significant improvement from 1.5 to 0.58. However, no significant differences were observed in the patients with other lesions (). The Breathiness score (B score) of all the patients significantly improved from 1.2 to 0.59. In subgroup analysis, the patients with vocal chorditis, nodules, and vocal scars showed significant improvement from 1.3 to 0.6, 1.1 to 0.37, and 1.37 to 0.87, respectively. However, no significant differences were observed in the patients with vocal polyps or RE ().
Figure 7. Voice quality evaluation using the Grade, Roughness, Breathiness, Asthenia, Strain scale. (A) Pre- and post-injection Grade scores. (B) Pre- and post-injection Roughness scores. (C) Pre- and post-injection Breathiness scores. Open bars indicate pre-injection scores, and closed bars indicate post-injection scores. *p<.05. **p<.01. RE: Reinke’s edema.
Discussion
The advantages of VFSI are the higher regional steroid concentration and minimal systemic effects [Citation2,Citation3,Citation7]. By performing flexible-fiberscope-guided needle placement and combining triamcinolone acetonide with a depot solution to prolong the development period [Citation11], VFSI may be performed as an in-office procedure [Citation13]. VFSIs have been performed immediately after laryngo-microsurgery to prevent scar formation following traumatic damage during surgery [Citation10]; they have also been performed as pitch-raising surgery in transgendered individuals. VFSI has been reported to be effective for treating vocal nodules, vocal polyps, RE, vocal scars, mucus retention cysts, and laryngeal granuloma [Citation2,Citation4,Citation7–9,Citation11,Citation13,Citation14]. However, most studies recruited patients with a variety of lesions, and the lesion in which VFSI will be most effective has not been determined. For this purpose, we recruited eight sex-, age-, and environment-matched patients for each lesion type who received similar regimens of VFSI. Phonological factors, including VHI-T, VHI-P, VHI-F, and VHI-E scores; MPT; MFR; G, R, and B scores; shimmer%; SPL; PR; and SFF, significantly improved after VFSI in all the patients. As in previous studies, we observed improvements in the results of subjective and objective laboratory measurements [Citation7–9,Citation13]. The improvements in the total and domain VHT scores are especially noteworthy because the VHT has been established as a screening tool. The VHI-T, VHI-P, and VHI-F scores; MPT; G, R, and B scores; and shimmer% significantly improved in patients with chorditis. The VHI-T, VHI-P, VHI-F, and VHI-E scores; MPT; MFR; G, R, and B scores; shimmer%; SPL; and PR significantly improved in patients with vocal nodules. The VHI-T, VHI-P, VHI-F, and VHI-E, G, and B scores significantly improved in patients with vocal polyps. The VHI-T, VHI-P, VHI-F, G, and B scores significantly improved in patients with vocal scars. However, no factor showed significant improvement in patients with RE. Based on the number of phonological factors showing improvement, vocal nodules were the most amenable to VFSI, followed by chorditis, polyps, and scars. The differences in the therapeutic effects of VFSIs on different types of lesions remain to be elucidated. VFSIs could provide a high steroid concentration in Reinke’s space while avoiding systemic adverse effects. Most BVFLs are caused by inflammation due to infection, smoking, gastric acid, phonotrauma, and/or vocal fold injury [Citation4,Citation8,Citation10,Citation15]. The effects of steroids on the vocal folds include the reduction of cellular permeability, proinflammatory cytokine production, collagen deposition, fibroblast proliferation, and collagen synthesis [Citation12,Citation16,Citation17]. Consequently, VFSI might reduce inflammation in Reinke’s space and the mucosa overlying it [Citation18] and counter the effect of basic fibroblast growth factor in patients with vocal fold atrophy [Citation12]. Furthermore, the anti-inflammatory effect of steroids might depend on the status (acute or chronic) and extent of inflammation. This leads to differences in the therapeutic effects of steroids in patients with different BVFLs. Here, we investigated the therapeutic effects of VFSI on chorditis, vocal nodules, vocal polyps, RE, and vocal scars. VFSI led to improvements in phonological efficiency and voice quality in patients with chorditis, but not in those with RE. This may be due to a difference in inflammatory status. There is a greater increase in fibrin and vasculature and greater accumulation of gelatinous fluid in Reinke’s space in RE than in chorditis.
Vocal nodules were the most amenable to treatment with VFSI. Previous studies on the efficacy of VFSI for treating vocal nodules [Citation2–4,Citation7,Citation8,Citation13] have reported complete resolution rates of approximately 40% and partial regression rates of more than 50% on laryngoscopic examination [Citation3]. Many studies have reported improvements in the results of laboratory voice evaluations after VFSI [Citation8,Citation13,Citation19]. Post-VFSI improvements have been reported in MPT and MFR by Tateya et al. [Citation13]; in MPT and jitter% by Wang et al. [Citation8]; and in MPT, jitter%, and NHR by Lee et al. [Citation19] in patients with vocal nodules; this is consistent with our findings. However, the therapeutic mechanism of VFSI has not been adequately explained. The VHI can be self-administered and reflects subjective symptoms, which are important in the treatment of dysphonia. Here, all vocal nodules were not completely reduced following VFSI. However, reduction rate was not significantly correlated with VHI score, possibly due to the effects of VFSI on vocal fold thickness, edema, vibration efficiency, and glottic insufficiency.
Patients with vocal polyps showed improvements in fewer measures of voice quality after VFSI than those with vocal nodules or chorditis. The therapeutic effect of VFSI on vocal polyp size differs from that on vocal nodule size [Citation19]; 33.3% of patients with vocal polyps showed a complete response, as opposed to 53.3% of patients with vocal nodules [Citation19]. Generally, hemorrhagic vocal polyps are large and organized, and greater voice improvement may be achieved by reducing the vocal fold thickness to improve vibration efficiency than by decreasing the polyp size. Here, complete remission was not observed, but subjective symptoms and voice quality improved.
Vocal scars are generally caused by injury during intubation, radiation therapy or laryngo-microsurgery [Citation20]. Vocal scarring occurs via fibrous tissue proliferation in the lamina propria, which results in decreased mucosal pliability and increased stiffness [Citation20]. VFSI has been associated with reduced vocal strain and better vocal fold vibration. Here, laryngo-microsurgery-induced vocal scars showed improvement on five measures following VFSI. While voice quality as measured using the GRBAS scale improved, phonological efficiency did not. Although we obtained encouraging results about the efficacy of VFSI for treating vocal scars, additional therapeutic strategies were required for scar-induced dysphonia.
It is well-known that laryngo-microsurgery under general anesthesia is effective for BVFLs, but it requires a couple of days of hospitalization and voice rest after injection. In contrast, VFSI is an easy and a safe technique as demonstrated in this study. All participants who had undergone multiple sessions of behavioral counselling and voice therapy by skilled speech-language-hearing therapists, but with any effect, were selected. Therefore, though the primary treatment modality for BVFL is voice therapy, behavioral management, and voice hygiene, VFSI should be performed in mild-to-moderate BVFLs refractory to voice therapy, or in patients who are expected to show improvement with voice therapy but immediate improvement is desirable.
Limitations
This study had a few limitations. First, it is a retrospective study with a small sample size, and detailed laryngeal examination could not be conducted in a time-dependent manner because the recording conditions differed between participants. Therefore, the mechanism of improvement could not be sufficiently elucidated. However, previous studies did not perform age, sex, and environment matching, and this study was the first to demonstrate that VFSI was highly effective against chorditis and vocal nodules, but less effective against RE and vocal scars. Second, due to the short follow-up period, the long-term outcomes of VFSI, such as BVFL recurrence rate, could not be determined. The durability/longevity of results is more significant than the short-term results. However, under the Japanese medical insurance system, the duration of hospital visits depends on the patient’s willingness, and many patients drop out of retrospective studies. Therefore, a prospective study should be planned to investigate the long-term results of this treatment. Although repeated injections or higher steroid concentrations may be required for intractable cases, we only performed single injections. However, we obtained valuable results were the first to standardize the injection protocol and schedule of data collection.
Conclusion
We treated 40 patients (8 patients per lesion) with similar regimens of VFSI. Significant improvements occurred in both subjective and objective measures of voice quality. In subgroup analysis, VFSI was highly effective against vocal nodules and chorditis, but less effective against RE and vocal scars. Single-dose VFSI is easy to perform as an outpatient procedure and is valuable as an alternative to voice rehabilitation and laryngo-microsurgery. However, higher concentrations or repeated injections may be required for intractable lesions. Further studies with subgroup analyses of large samples are required to determine the lesion that is most amenable to treatment with VFSI and to develop novel therapeutic strategies for RE and vocal scars.
Supplemental Material
Download MP4 Video (67.7 MB)Acknowledgments
The authors thank the speech-language-hearing therapists from our department—Taisuke Sotome, Nahoko Tashiro, Miyuki Kurihara, Takumi Omae, Yosuke Nakayama, Ayumi Yamamoto, and Ayane Sato—for their support in the data collection and clinical assessments. The authors also thank Editage (www.editage.com) for English language editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Wallis L, Jackson-Menaldi C, Holland W, et al. Vocal fold nodule vs. vocal fold polyp: answer from surgical pathologist and voice pathologist point of view. J Voice. 2004;18(1):125–129.
- Wu CH, Lo WC, Liao LJ, et al. Vocal fold steroid injection for benign vocal lesions in professional voice users. J Voice. 2021:S0892-1997(21)00057-6. doi:https://doi.org/10.1016/j.jvoice.2021.02.002. Online ahead of print.
- Shoffel-Havakuk H, Sadoughi B, Sulica L, et al. In-office procedures for the treatment of benign vocal fold lesions in the awake patient: a contemporary review. Laryngoscope. 2019;129(9):2131–2138.
- Wang CT, Lai MS, Cheng PW. Long-term surveillance following intralesional steroid injection for benign vocal fold lesions. JAMA Otolaryngol Head Neck Surg. 2017;143(6):589–594.
- Bové MJ, Jabbour N, Krishna P, et al. Operating room versus office-based injection laryngoplasty: a comparative analysis of reimbursement. Laryngoscope. 2007;117(2):226–230.
- Yung KC, Courey MS. The effect of office-based flexible endoscopic surgery on hemodynamic stability. Laryngoscope. 2010;120(11):2231–2236.
- Wang CT, Liao LJ, Cheng PW, et al. Intralesional steroid injection for benign vocal fold disorders: a systematic review and meta-analysis. Laryngoscope. 2013;123(1):197–203.
- Wang CT, Lai MS, Hsiao TY. Comprehensive outcome researches of intralesional steroid injection on benign vocal fold lesions. J Voice. 2015;29(5):578–587.
- Ramavat AS, Tiwana H, Banumathy N, et al. Efficacy of intralesional steroid injection in small benign vocal fold lesions. J Voice. 2019;33(5):767–772.
- Cho JH, Kim SY, Joo YH, et al. Efficacy and safety of adjunctive steroid injection after microsurgical removal of benign vocal fold lesions. J Voice. 2017;31(5):615–620.
- Tateya I, Omori K, Kojima H, et al. Steroid injection for Reinke’s edema using fiberoptic laryngeal surgery. Acta Otolaryngol. 2003;123(3):417–420.
- Kanazawa T, Komazawa D, Indo K, et al. Single injection of basic fibroblast growth factor to treat severe vocal fold lesions and vocal fold paralysis. Laryngoscope. 2015;125(10):E338–E344.
- Tateya I, Omori K, Kojima H, et al. Steroid injection to vocal nodules using fiberoptic laryngeal surgery under topical anesthesia. Eur Arch Otorhinolaryngol. 2004;261(9):489–492.
- Ogawa M, Inohara H. Is voice therapy effective for the treatment of dysphonic patients with benign vocal fold lesions? Auris Nasus Larynx. 2018;45(4):661–666.
- Naunheim MR, Carroll TL. Benign vocal fold lesions: update on nomenclature, cause, diagnosis, and treatment. Curr Opin Otolaryngol Head Neck Surg. 2017;25(6):453–458.
- Campagnolo AM, Tsuji DH, Sennes LU, et al. Histologic study of acute vocal fold wound healing after corticosteroid injection in a rabbit model. Ann Otol Rhinol Laryngol. 2010;119(2):133–139.
- Zhou H, Sivasankar M, Kraus DH, et al. Glucocorticoids regulate extracellular matrix metabolism in human vocal fold fibroblasts. Laryngoscope. 2011;121(9):1915–1919.
- Jin HJ, Lee SH, Lee SU, et al. Morphological and histological changes of rabbit vocal fold after steroid injection. Otolaryngol Head Neck Surg. 2013;149(2):277–283.
- Lee SW, Park KN. Long-term efficacy of percutaneous steroid injection for treating benign vocal fold lesions: a prospective study. Laryngoscope. 2016;126(10):2315–2319.
- Hantzakos A, Dikkers FG, Giovanni A, et al. Vocal fold scars: a common classification proposal by the American Laryngological Association and European Laryngological Society. Eur Arch Otorhinolaryngol. 2019;276(8):2289–2292.