Abstract
Background
The rate of genetic deafness in Chengdu is still underestimated.
Objective
To investigate patients’ molecular etiology with profound hearing loss and facilitate genetic counseling for their families, we screened deafness-related genes of profound hearing loss in the population.
Methods
A total of 1427 unrelated patients with profound hearing loss containing all age groups in the administrative area of City Chengdu (Sichuan, China) were enrolled in this study, and the average examination rate is 81.13%. Nine loci of four deaf-associated genes (GJB2, GJB3, SLC26A4, and mitochondrial 12SrRNA gene) were analyzed. Then we examined all the deaf-associated mutations and compared them between groups.
Results
The average age of all subjects is 48.537 ± 19.077 years, peak range in 41–70 years (985/1427, 69.03%). The positive mutation rates of patients in GJB2, SLC26A4, and 12S rRNA are respectively 8.90%, 4.84%, and 5.96%, and GJB3 none. In group A the GJB2 and SLC26A4 mutation rate is 14.17% (36/254), which is remarkably higher than group B (6.14%, 72/1173). The frequency of 12SrRNA mutations is 3.15% (8/254) in group A, which is significantly different (χ2 = 4.34, p < .05) from that of group B (6.56%, 77/1173).
Conclusions and significance
The mutation rate of mtDNA 12SrRNA is higher than SLC26A4 gene in our study, which is different from other parts of China. And the deaf-related gene mutation spectrums have a distinct age difference.
Chinese Abstract
背景:成都的遗传性耳聋率仍被低估了。
目的:为了探讨重度听力损失患者的分子病因, 以便为他们的家人提供遗传方面的咨询, 我们筛选了严重听力损失人口的耳聋相关基因。
方法:成都(中国四川)共 1427 名所有年龄的没有亲缘关系的重度听力损失患者被纳入本研究, 平均检测率为81.13%。分析了四个耳聋相关基因(GJB2、GJB3、SLC26A4 和线粒体 12SrRNA 基因)的九个基因座。为探讨遗传性听力损失是否与年龄相关, 我们将 1427 例患者按 30 岁分为两个年龄组(A 组, 年龄小于30岁;B组, 年龄大于30岁)。然后, 我们检查了所有与耳聋相关的突变并对此在组间进行了比较。
结果:所有受试者的平均年龄为 48.537±19.077 岁, 峰值范围在 41-70 岁(985/1427, 69.03%)。 GJB2、SLC26A4和12S rRNA患者的阳性突变率分别为8.90%、4.84% 和 5.96%, GJB3患者则无阳性突变。 A组GJB2和SLC26A4突变率为14.17%(36/254), 明显高于 B 组 (6.14%, 72/1173)。 A组12SrRNA突变频率为3.15%(8/254), 与B组(6.56%, 77/1173)有显著差异(v2 ¼ 4.34, p < .05)。
结论与意义:我们的研究表明, 线粒体 12SrRNA 基因的突变率高于SLC26A4基因。这与中国其它地区不同。和耳聋相关的基因突变谱具有明显的年龄差异。
Introduction
An incrementing number of findings demonstrate that hearing loss is attributed to genetic, and more than 50% of the cases are hereditary [Citation1]. About 70% of them are classified as non-syndromic hearing loss since it is the only symptom, while 30% are syndromic hearing loss associated with other clinical features [Citation2]. Hereditary mode of non-syndromic hearing loss can follow the pattern of autosomal dominant, autosomal, or X-linked recessive, and mitochondrial inheritance [Citation3]. Over 200 genes have been found to associate with sensorineural hearing loss [Citation4]. In these genes, GJB2 and SLC26A4 are the most common ones of disease-causing chromosome genes, and mitochondrial DNA12SrRNA is the most common in mitochondrial genes in the Chinese population [Citation5].
The most common molecular defect in non-syndromic autosomal recessive deafness involves the GJB2 gene, which encodes Connexin 26, a gapjunction protein 6,7, and 235delC, the most frequent mutation in the Chinese population [Citation6]. Defects in SLC26A4, which encodes the anion transporter pendrin, can cause non-syndromic DFNB4 deafness with enlargement of the vestibular aqueduct and Pendred syndrome [Citation7]. Mutation of SLC26A4 is the second most common cause of deafness in China [Citation8], and IVS7-2A > G is the common mutation [Citation9]. Moreover, well-studied mutations related to aminoglycoside susceptibility are A1555G and C1494T in the mitochondrial 12SrRNA gene [Citation10]. Non-syndromic inherited hearing loss caused by mutations in GJB2, SLC26A4, and mtDNA 12SrRNA accounts for 33.8% of all the deaf from Special Education School within China [Citation8], and the hotspot mutation detection becomes a much more available method. Based on the large-scale epidemiological multi-center study data across 28 provinces and municipalities all over China, nine hot gene loci of four deaf-associated genes have been found in the Chinese population. Thus, we focus on detecting the nine hotspots simultaneously using microarray chip technology in inherited hearing loss [Citation11].
The National Sample Survey on Disability in 2007 revealed approximately 27.8 million residents who suffer from hearing loss in China, and about 10,000 of them are from Chengdu (Sichuan province) [Citation12]. Carrier frequencies of common hotspot mutation associated with hearing loss in the region have not been reported. The only study was an analysis from 17,000 newborns, and each compound mutation has only one to several to be checked out, lacking prevalence distribution of common hotspot mutation for whole deaf residents. This study screened the nine loci of GJB2, GJB3, SLC26A4, and mitochondrial 12SrRNA genes for all profound hearing loss residents (hearing loss level of the better ear is worse than 80 dB) and contained all age groups in the administrative area of the region. This study aims to investigate the molecular etiology for residents with profound hearing loss to provide practical risk assessment and genetic counseling for hearing loss patients and their families in the above region.
Methods
Subjects and DNA samples
Subjects who suffer from profound hearing loss (>80 dB, the better ear) from the city Chengdu were collected to perform mutation detection between January 2012 and December 2012. 1759 cases of profound hearing loss registered in the Chengdu Federation of the Disabled in the five regions, and 1427 cases consented to the detection. The different regions’ examination rate varies from 70.68% to 92.73%, and the average examination rate is 81.13%.
All subjects are profound bilateral sensorineural deafness on audiograms, whose average hearing loss was 81 dBHL or greater, and all of them hold a household register of Chengdu and a certificate of Chinese Hearing Disability. They were recruited to participate in the study by signing a written informed consent, and the participants donated a blood sample (1–3 ml) for detection. The ethical committee approved the ethical permission of West China Hospital and the national project: Standardization and popularization of prevention and intervention of defects in deafness. All procedures performed in studies were under the committee’s ethical standards and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
To explore whether hereditary hearing loss is associated with age or not, we divided the 1427 cases into two age groups by age 30. All patients in group A were younger than 30 years old, while group B patients were older than 30.
Molecular screening
The detection of nine hotspot mutations in four most common pathologic genes in China, including GJB2 (35delG, 176del16 235delC, 299delAT), GJB3 (538C > T), SLC26A4 (2168A > G, IVS7-2A > G), mitochondrial 12S rRNA (1494C > T, 1555A > G), which were detected in Capital Bio Medical Laboratory.
Genomic DNA was extracted from peripheral blood using a commercially available DNA isolation kit (Tiangen whole blood genome DNA extraction Kit and nine deafness gene detection kit, Tianjin Biotech Corporation, Beijing, China), and then conducted polymerase chained reaction, chip hybridization, and chip scan for detection of nine hotspots mutations simultaneously. The test results were determined based on the fluorescent hybridization signal and the distribution of the microarray probe (LuxScanTM 10K-B Microarray Scanner and Jingxin® Deafness Gene Detection and Analysis System, Microarray method).
Blank control and standard control were set for each batch of samples to verify the reliability of the inspection results. And also, for quality control, 50% of the positive-result DNA samples were randomly redetected.
Statistical analysis
We used software SPSS 19.0 to analyze statistics (SPSS Inc., Chicago, IL). The intergroup difference in frequency was compared using the two-tailed chi-square test. A p-value less than .05 was considered statistically significant.
Results
Characteristics of the subjects
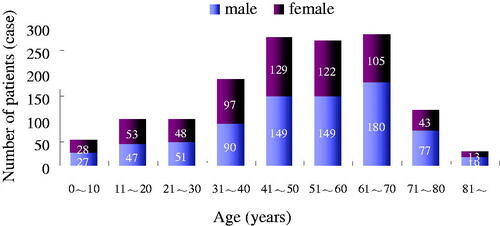
The 1427 cases included 789 males and 638 females, and their ages ranged from 3 months to 97 years old (average age 48.537 ± 19.077, median age 50). All the cases were Han Chinese. summarizes their distribution by age and gender.
The prevalence of deaf-associated genetic mutations
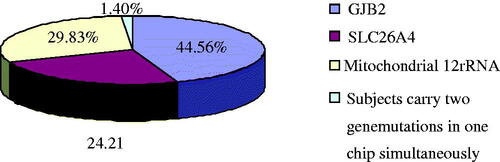
summarizes the frequencies of these deaf-associated gene mutations. In these positive samples, the group with GJB2 mutations is the largest, and then the mitochondrial 12SrRNA mutations, while the group with SLC26A4 mutations is the fewest (). And there is no GJB3 538C > T mutation. One patient carries two genetic mutations in a chip with GJB2 235del heterozygote + SLC26A4 IVS7-2A > G heterozygote; another patient GJB2 carries 176del16 heterozygote + Mitochondrial 12SrRNA 1555A > G mutations; other two patients carry GJB2 299delAT heterozygote + Mitochondrial 12SrRNA 1555A > G mutations simultaneously.
Table 1. Prevalence of deaf-associated gene mutations.
The frequency of hereditary deafness in profound hearing loss
and summarize the genotypes of GJB2 and SLC26A4 genes in positive samples with profound hearing loss. Besides, 85 patients carry homogeneous mitochondrial 12SrRNA mutation, include 1555A > G mutation in 79 cases (79/85, 92.94%) and 1494C > T mutation in 6 cases (6/85, 7.06%).
Table 2. Genotypes of GJB2 in positive samples with profound hearing loss.
Table 3. Genotypes of SLC26A4 in positive samples with profound hearing loss.
Different frequencies of hereditary hearing loss across age groups in Chengdu
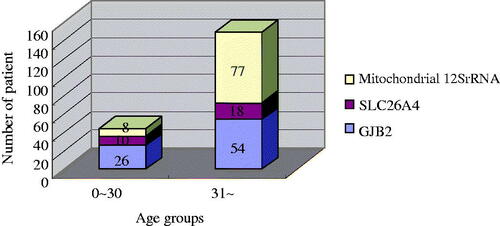
We divided the 1427 cases into two age groups. and summarize the frequencies of deaf-associated gene mutations in two groups. shows that group A’s different mutations are significantly different from group B’s (p < .05).
Table 4. Prevalence of GJB2, SLC26A4, mtDNA 12S rRNA mutations in age groups.
Table 5. Between-group difference for GJB2, SLC26A4 and mtDNA 12S rRNA mutations.
Discussion
The mutation rate of mtDNA 12SrRNA is higher than the SLC26A4 gene in our study, and the percentage of mtDNA 12S rRNA mutation in this study is different from other parts of China in previous reports [Citation13]. This genetic difference perhaps is from the variation in different residences of Han Chinese. Our region is the southwest part of China, close to Tibet. It has been somewhat similar to Tibetan results, with more 12SrRNA mutation and rare GJB2 and SLC26A4 [Citation14]. However, further study is needed.
In group A, the GJB2 and SLC26A4 mutation rate is 14.17%, similar to the typical areas of China [Citation13]. But it is remarkably higher than group B (χ2 = 19.27, p < .05), so GJB2 and SLC26A4 mutations in group B were lower than in previous reports. This result suggests a big part of patients with non-congenital hearing loss in group B. The etiology of hearing loss for the elderly is multiple and more complex, so the association between hearing loss and gene mutations is poorer. It may affect the mutation ratio of group B. On the other hand, the included number of group A is smaller and may cause bias.
The frequency of 12S rRNA mutations seems higher in the older population. Similar to GJB2 and SLC26A4 gene mutations, the association between mtDNA 12S rRNA and profound deafness also has a unique age difference in our study (χ2 = 4.34, p < .05). Besides, the mutation rate in our study is much higher than in other reports. The rate of 12S rRNA was detected about 3% in Japanese [Citation15], 1–2.4% in European or Arabian[Citation16], and in some typical areas of China [Citation17]. But it is close to the finding in Indonesian (5.3%) [Citation18] and the investigations in the south part of China (7.7–8.5%) [Citation19]. This is probably due to the different gene origin or overuse of antibiotics in the South part of China, as we all know that the 12S rRNA is the primary targeting site for aminoglycosides [Citation20]. The patients with 12S rRNA mutations may use aminoglycoside, resulting in hearing loss, and the rate of aminoglycosides treatment is increased with age. But this speculation has not yet been tested in a large sample. Thus, further study is needed.
Conclusion
Our results demonstrate that the mutation spectrums of GJB2, SLC26A4, and mtDNA 12S rRNA in the Chengdu population with profound hearing loss are similar to most cities in China. But the mutation rate of mtDNA 12SrRNA is higher than SLC26A4 gene in our study, which is different from other parts of China. And the deaf-related gene mutation spectrums have a distinct age difference.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Morton NE. Genetic epidemiology of hearing impairment. Ann NY Acad Sci. 1991;630:16–31.
- Friedman TB, Schultz JM, Ben-Yosef T, et al. Recent advances in the understanding of syndromic forms of hearing loss. Ear Hear. 2003;24(4):289–302.
- Epstein FH, Willems PJ. Genetic causes of hearing loss. N Engl J Med. 2000;342(15):1101–1109.
- Lalwani A, Castelein CM. Cracking the auditory genetic code: non-syndromic hereditary hearing impairment. Am J Otol. 1999;20:115–132.
- Yang W. Summarized Research Progress of Hereditary Deafness and Clinical Application of Genetic Diagnosis of Deafness. Paper presented at the Ninth National Conference on Otolaryngology (Sep. 2006, Guiyang), Head and Neck Surgery of the Chinese Medical Association; 2007.
- Dai P, Yu F, Han B, et al. GJB2 mutation spectrum in 2,063 Chinese patients with nonsyndromic hearing impairment. J Transl Med. 2009;7:26.
- Royaux IE, Suzuki K, Mori A, et al. Pendrin, the protein encoded by the Pendred syndrome gene (PDS), is an apical porter of iodide in the thyroid and is regulated by thyroglobulin in FRTL-5 cells. Endocrinology. 2000;141(2):839–845.
- Yuan Y, You Y, Huang D, et al. Comprehensive molecular etiology analysis of non-syndromic hearing impairment from typical areas in China. J Transl Med. 2009;7(1):1–12.
- Dai P, Li Q, Huang D, et al. SLC26A4 c.919-2A > G varies among Chinese ethnic groups as a cause of hearing loss. Genet Med. 2008;10(8):586–592.
- Dai P, Liu X, Han D, et al. Extremely low penetrance of deafness associated with the mitochondrial 12S rRNA mutation in 16 Chinese families: implication for early detection and prevention of deafness. Biochem Biophys Res Commun. 2006;340(1):194–199.
- Li CX, Pan Q, Guo YG, et al. Construction of a multiplex allele-specific PCR-based universal array (ASPUA) and its application to hearing loss screening. Hum Mutat. 2008;29(2):306–314.
- Chen K, Zheng X. Data analysis report of the second national sample survey of disabled people. Beijing (China): HuaXia Publishing House (Beijing); 2008.
- Ji YB, Han DY, Lan L, et al. Molecular epidemiological analysis of mitochondrial DNA12SrRNA A1555G, GJB2, and SLC26A4 mutations in sporadic outpatients with non-syndromic sensorineural hearing loss in China. Acta Oto-Laryngol. 2011;131(2):124–129.
- Yuan Y, Zhang X, Huang S, et al. Common molecular etiologies are rare in nonsyndromic Tibetan Chinese patients with hearing impairment. PLoS One. 2012;7(2):e30720.
- Usami S, Abe S, Akita J, et al. Prevalence of mitochondrial gene mutations among hearing impaired patients. J Med Genet. 2000;37(1):38–40.
- Mkaouar-Rebai E, Tlili A, Masmoudi S, et al. New polymorphic mtDNA restriction site in the 12S rRNA gene detected in Tunisian patients with non-syndromic hearing loss. Biochem Biophys Res Commun. 2008;369(3):849–852.
- Qu C, Sun X, Shi Y, et al. Microarray-based mutation detection of pediatric sporadic nonsyndromic hearing loss in China. Int J Pediatr Otorhinolaryngol. 2012;76(2):235–239.
- Malik SG, Pieter N, Sudoyo H, et al. Prevalence of the mitochondrial DNA A1555G mutation in sensorineural deafness patients in island Southeast Asia. J Hum Genet. 2003;48(9):480–483.
- Xin F, Yuan Y, Deng X, et al. Genetic mutations in nonsyndromic deafness patients of Chinese minority and Han ethnicities in Yunnan, China. J Transl Med. 2013;11:312.
- Carlberg B, Asplund K, Hägg E. The clinical and audiologic features of hearing loss due to mitochondrial mutations. Otolaryngol Head Neck Surg. 2013;148:1017–1022.