Abstract
Background
Universal newborn hearing screening (UNHS) contributes to the early diagnosis of hearing loss. However, not all permanent pediatric hearing impairments can be identified by UNHS.
Aims/objective
To investigate children who have successfully passed the UNHS, but have later-onset hearing loss at an early stage.
Methods
UNHS of children, was reviewed retrospectively from databases at Karolinska University Hospital, Sweden. Gender, age, the reason for contact, the first contact and the most recent audiogram, the hearing diagnosis, the degree of hearing loss when they were enrolled in hearing rehabilitation, and the hearing aids they used were analysed.
Results
63 children who had passed the UNHS at birth and were diagnosed with a hearing impairment at a later stage were included in the study. The average age was 3.3 and 3.9 years old when the children were diagnosed and were finally enrolled in the hearing habilitation, respectively. The reasons for diagnostic evaluation of a suspected hearing loss at present study are preschool hearing tests at the Child Health Care Centres, parents suspect, and/or delayed speech and language development.
Conclusions and significance
Our findings suggest that a passed UNHS does not exclude a future delayed onset of hearing loss, particularly in children with risk factors.
Chinese Abstract
背景:通用新生儿听力筛查 (UNHS) 有助于早期诊断听力损失。然而, 并非所有永久性儿科听力障碍都能通过UNHS 进行识别。
目的:调查曾顺利通过UNHS 检测, 但后来发生早期听力损失的儿童。
方法:回顾性地审查了从瑞典卡罗林斯卡大学医院的数据库中儿童 UNHS检测资料。分析了他们的性别、年龄、接触原因、第一次接触和最近的听力图、听力诊断、他们参加听力训练时的听力损失程度以及他们使用的助听器。
结果: 63 名出生时通过 UNHS检测, 但 后来被诊断出听力障碍的儿童被纳入研究。诊断时的平均年龄和最终参加听力训练的平均年龄分别为 3.3 岁和 3.9 岁儿童。目前研究中诊断性评估疑有听力损失的原因是, 在儿童保健中心进行的学龄前听力测试, 父母怀疑, 和/或延迟的说话和语言发育。
结论和意义:我们的研究结果表明, 顺利通过 UNHS 检测并不能排除后来的听力损失的延迟发生, 对于有危险因素的儿童尤为如此。
Introduction
Hearing loss is one of the most common disability in our society. According to World Health Organization (WHO), approximately 466 million people suffer from hearing loss, which corresponds to over 5% of the global population [Citation1]. In newborns, permanent hearing loss with a hearing threshold ≥40 dB HL, occurs in 1–3 out of 1000 deliveries worldwide [Citation2,Citation3]. Studies have shown better speech and language development among children whose hearing loss is identified and intervened at an early age [Citation4]. Failure to detect or delayed detection of hearing impairment in children may not only result in delays of speech and language development but also subsequent learning problems and social difficulties.
Before universal newborn hearing screening (UNHS) was introduced, the average diagnostic age of hearing loss occurred considerably later, from 1.5 to an age of 3.9 years old in various countries [Citation5–7]. In Sweden, only 5.4% of the children with hearing loss were detected before 6 months of age [Citation8]. Today all newborns are tested and the average diagnostic age occurs much earlier. Against this background, the possibility to identify and intervene in congenital hearing impairments has increased. This improvement contributes to good speech and language development for the children with hearing impairment, and their learning activity and school performance are consequently improved.
Not all permanent hearing impairments in children will be identified by UNHS. Approximately 25% of children, who passed the UNHS, are identified to have a sensorineural hearing loss during the first years of life [Citation9]. The prevalence of hearing loss in children has shown an increase by 2–3 times up to the first year of primary school [Citation10]. That also includes children born with a mild hearing loss that later develops into a more severe hearing loss. Similar to the hearing loss detected by UNHS the postnatal hearing loss (PHL) is caused by genetic factors, various syndromes as well as anatomical malformations, infection, brain trauma and exposure to ototoxic drugs during pregnancy or after birth [Citation11]. In addition, there exist children who develop hearing loss without any known risk factors.
The Huddinge University Hospital in Stockholm has performed UNHS since 1998 [Citation12] and Södertälje Hospital started in 2003, Sweden. In 2005, all paediatric clinics within Stockholm County Council introduced UNHS, leading to that 98% of all newborns in Stockholm County Council undergoing the test [Citation12].
The purpose of the present study is to identify and characterize children who initially passed the neonatal hearing test, but late were enrolled at the Hearing Habilitation department covering the whole Stockholm County Council. The final goal was an attempt to identify risk factors which can gather or differentiate children with a later-onset hearing loss earlier than at the 4-year old age test at the Child Care Centre.
Methods
Databases
The data of UNHS in the complete Stockholm County Council has been registered and collected since 2005 in a database called Audioscreen. Similarly, all children with a hearing loss, referred to the Hearing Habilitation centre, are registered in another database, Audiohab. Except for these two databases the audiogram database, Auditbase, and information from the patient record system Take Care were used for the present study.
Audioscreen
Audioscreen contains hearing test results for all newborns in the Stockholm Council, who have undergone TEOAE, aABR, clinical ABR, or declined to test. The Audioscreen information includes: when and where the child underwent the hearing test; who performed the test and which order of the test. The test results are described as ‘passed’, ‘not passed’, ‘declined’ or ‘not performed’. The resulting file of each ear which passed the test is also stored in the database. ABR thresholds are also included in the Audioscreen database.
Audiohab
The Audiohab register contains all children enrolled in the Hearing Habilitation Centre at Karolinska University Hospital, Stockholm. This is the only provider of hearing services to children in the Stockholm region. The data include the degree and type of hearing loss, classified as mild, moderate, severe, profound or unilateral. Audiohab also holds information about the hearing device, educational setting and whether they suffer from other disabilities.
AuditBase
The Karolinska University Hospital uses AuditBase to store information about performed hearing tests of each individual. In the audiogram, information such as pure-tone average, observational tests are registered.
Take care
Take Care is the patient journal record used in Stockholm County Council. Medical notes, referrals, imaging, and test results can be found in this system.
Screening process
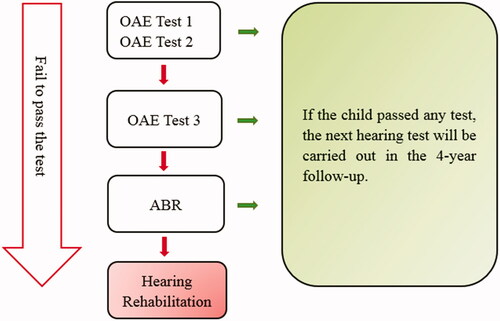
UNHS is usually performed within 2–5 days after birth. If the baby fails the test, a secondary OAE test is scheduled within 5–7 days. If still not passing the test, a third OAE appointment is scheduled within roughly 1 month at the Audiology and Neurotology departments of Karolinska University Hospital. A third failure will lead to an automated ABR (aABR) investigation. If the children do not pass the aABR test at the level of 30 dB nHL, a clinical ABR test will be carried out. However, aABR was not implemented until 2015, consequently, a failed OAE in the present study covering children born between 2005 and 2013, was followed by clinical ABR. Final test results worse than 30 dB nHL in both ears indicate a hearing loss. These children will be referred to the hearing habilitation unit and fitting of hearing aids can be started as soon as possible together with other interventions offered to the children and their families (). Clinical ABR is also the preferred method to assess hearing thresholds at any age when reliable behavioural testing cannot be performed.
Selection of children
The aim of the present study was to investigate children who had passed the UNHS, but later developed a hearing loss with enrolment at the Hearing Habilitation Centre in Stockholm County Council.
Inclusion criteria were as follows:
Children born between 01/12/2005 and 01/01/2013
Children who passed UNHS bilaterally via TEOAE
Children who did not pass TEOAE but passed clinical ABR
Children enrolled at the Hearing Habilitation Centre at Stockholm County Council
During the period 2005-12-01 to 2013-01-01, newborn hearing screening was performed in 196,958 out of 200,281 newborns (98%) in the Stockholm region, according to the register Audioscreen. From this birth cohort, 460 children were registered in Audiohab at the Hearing Habilitation Centre. Of these, 404 children had performed the hearing test in newborns and 56 children had not registered hearing tests in Audioscreen. The number of enrolled children corresponds to about 2.3 ‰ of the total number of children who underwent the UNHS during this period.
The social security numbers (ID) of the 404 children were searched in Audioscreen to check whether they were registered and whether they had passed newborn hearing tests bilaterally. 63 children (∼14%) had passed the UNHS, and 341 had failed to pass the test which was not investigated at the present study. We focused on 63 children who passed the hearing test and figured out why they have PHL.
Data collection
Regarding the 63 children, enrolled in the study, additional data were collected. The medical record system, TakeCare, was scrutinized to obtain further information of the cause and time when the children first contacted hearing care, and which diagnosis of hearing loss the children suffered from. The earliest and most recent hearing test data from Auditbase were also collected. The OAE results of each ear were performed and downloaded and the names of the files were replaced with serial numbers, ‘L’ representing the left and ‘R’ the right ears, respectively.
For each individual, age, gender, the reason for the first contact, the most recent hearing test, hearing diagnosis, degree of hearing and hearing aids used, if tested for CMV infection, connexin and other examinations, OAE and ABR results, other diagnoses and risk factors for hearing loss were also collected.
Ethical considerations
The Ethics Review Act (2003: 460) includes provisions on ethics testing in human studies. The consent has been granted to this study with docket: 2012/354-31/5
Results
Enrolled participants
We presented a flow chart of newborns’ hearing tests. shows the test process in the present study.
Gender distribution
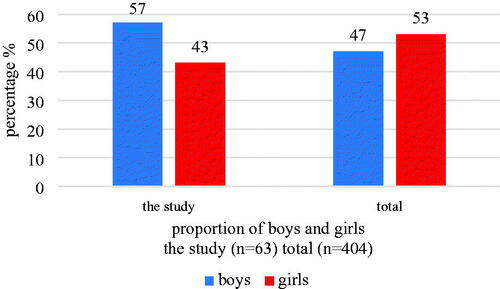
Of the 63 children, males accounted for a greater proportion n = 36 (57%), compared with females n = 27 (43%, ). In the whole study group of children, boys were in minority with 47%.
Contact and age of enrolment
When contacting the hearing care centres for a suspected hearing loss, the average age of the children was 3.3 years and the median age 4.2 years (0.2–6.3 years). The average age of enrolment at the Hearing Habilitation Department was 3.9 years and the median age was 4.3 years (0.6–6.9 years old). The median interval time between first contact and start of the habilitation process was 0.3 years and the longest time was 3.4 years ()
Table 1. Patients’ age of first contact with hearing care and enrollment for habilitation, and interval time between contact and enrollment among 63 children.
Reason for contact
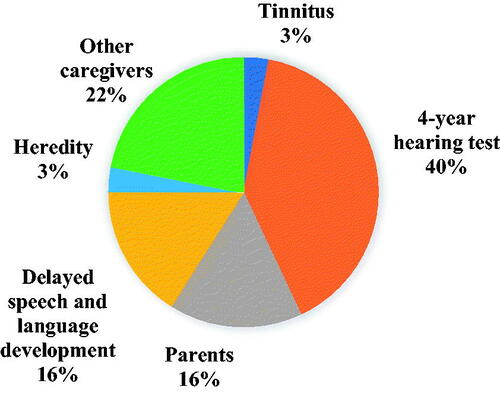
The most common reason for contact with the hearing care, 40%, was a child that failed to pass the 4-year routine hearing tests at the Child Care Centres. Other reasons included a suspected hearing loss by the parents (16%), and a delayed speech and language development of children below 4 years of age (16%). Less common reasons were heredity and tinnitus, and the incidence of both is 3%. 22% of the children were suspected of having hearing loss by other caregivers, including staff at Child Intensive Care Unit, Child Neurologists, Ear-Nose-Throat doctors, Speech and Language Therapists, and Neonatal Department workers when they were treated for other diseases ().
Audiometric tests for newborns
We found that 73% of the ears in the children passed Test 1 (T1) using OAE, 18% of the ears passed Test 2, and 5% of the ears passed Test 3 (T3), 2% (T4) and 2% (T5) respectively ().
Table 2. The number of ears which passed TEOAE test.
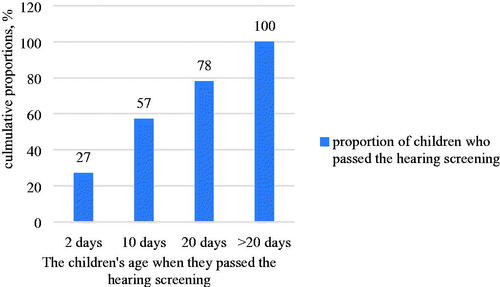
Twenty-seven percent of the 63 children passed the neonatal hearing screening bilaterally at 2 days old of the age, and then the cumulative percentage is 57% and 78% of them passed the test at 10 days and 20 days of the age, respectively. The maximum age of children who passed neonatal hearing tests was at 148 days of age ().
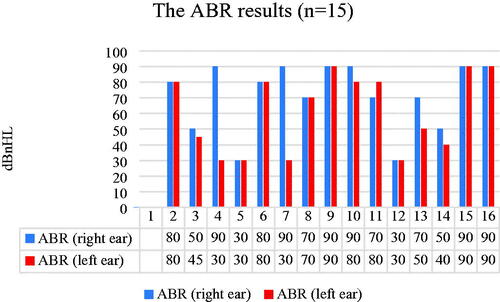
Fifteen (24%) of the 63 children had undergone ABR to determine their hearing thresholds. The results varied, from passed results at 30 dBnHL to non-recordable responses ().
Risk factors
Risk factors were identified in 11 children and included meningitis, craniofacial malformations, heredity, hyperbilirubinemia and syndrome factors. Moreover, children with suspected cytomegalovirus (CMV) infection or with the heredity of infection were found in 16 cases. Cytomegalovirus (CMV) infections were tested among 12 children, 10 of them were negative showing a very low rate of positive results.
Hearing aids
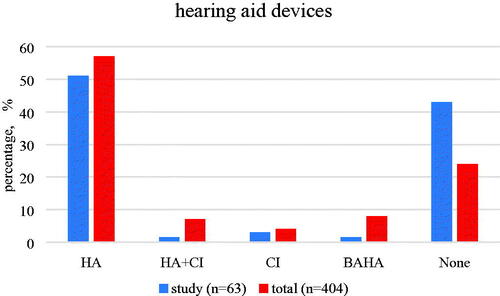
Of the 63 children, 57% were habilitated/treated by hearing aids (HA), cochlear implants (CI) or bone-anchored hearing aid (BAHA) (). In this 57% of children, around 50% of them have hearing aids alone, another 7% of children have CI only, CI plus HA and BAHA.
Discussion
The introduction of UNHS with OAE and aABR has made it possible to identify congenital hearing impairments earlier than before. Only 5.4% of under-6-month-old children with hearing impairments could be diagnosed in Sweden [Citation8] prior to the introduction of the UNHS program. At present, after the introduction of the UNHS program in the Stockholm Council, 84% of the children with hearing loss can be identified at the neonatal screening tests. Still, a certain number of children developing a PHL will not be detected.
In our study, 63 children passed newborn UNHS but later received hearing habilitation. This number corresponded to 15.6% of the total number of 404 children with a verified habilitation demanding hearing impairment. Previous studies have shown the proportion of PHL in the paediatric population to be 14–32% [Citation13–17]. A lower incidence, about 9%, was reported by Uus and David [Citation8]. Our results of PHL are more consistent with that of Dietz et al. [Citation13] and Fitzpatrick et al. [Citation14], which showed 14% and 16.5%, respectively.
Forty percent of the children were found to have PHL through conventional routine hearing control at 4 years old age. Moreover, 16% of the children were found PHL by parents and another16% of them was due to delayed speech and language development. This 32% with PHL could be identified earlier if the child health care staff and parents together give more attention to hearing. It is also worth mentioning that 1/5 of the children with a postnatal hearing impairment were detected at visits for other purposes than hearing impairment at other caregivers.
Risk factors were found in 11 out of 63 children (17%), and suspected risk factors were found in an additional 17 children (27%). Nevertheless, it would fall below 50% of the total number of children. This resembles the results of Weichbold et al. [Citation15] which suggest that, after following up the children with risk factors, more than 50% of the children are still not identified.
In some countries, some questionnaires are used for children to assess their hearing, such as LittlEARS® Auditory Questionnaire (LEAQ) and LittlEARS® Early Speech Production Questionnaire (LEESPQ). LEAQ is also used among children with cochlear implants to monitor their hearing, speech and language development [Citation18]. This tool consists of a questionnaire for children with 35 questions in chronological order for children up to 24 months. LEESPQ is a valid tool for assessing the hearing of children aged 0–18 months [Citation19]. If such tools were used, it is more likely to identify the children’s hearing impairments earlier than at the 4-year examination. Such tools could be used in the Child Care Centre, which can be regularly visited by children during their aging. In addition, it means that the parents would fill in the form when they visit specialists and can become aware of their child’s speech and language development. Furthermore, if the results differ between each other, the child should be referred to the hearing clinic for further investigation.
A Screening and rescreening protocols, which was made in a 2017 International Consensus suggest that children with low risk should be performed either aABR or OAE test. In contrast, children with high risk should receive both aABR and OAE tests. Additionally, clinical ABR or audiometry with a professional person should be done [Citation20] when necessary.
Our study demonstrates that PHL is still a challenge although the primary UNHS program plays an important role to detect congenital hearing impairment. New clinical routines should put more effort into potential PHL in children at-risk children who pass UNHS. Children who have delayed speech and language development should be subjected to new hearing tests.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- World Health Organization [Internet]. Deafness and hearing loss. Geneva (Switzerland): WHO; 2018 [cited 2018 November 3]. Available from: http://www.who.int/mediacentre/factsheets/fs300/en/
- Butcher E, Dezateux C, Cortina-Borja M, et al. Prevalence of permanent childhood hearing loss detected at the universal newborn hearing screen: systematic review and meta-analysis. PLoS One. 2019;14(7):e0219600.
- Bussé AML, Hoeve HLJ, Nasserinejad K, et al. Prevalence of permanent neonatal hearing impairment: systematic review and Bayesian meta-analysis. Int J Audiol. 2020;59(6):475–485.
- Lieu JEC, Kenna M, Anne S, et al. Hearing loss in children: a review. JAMA. 2020;324(21):2195–2205.
- Fortnum H, Davis A. Epidemiology of permanent childhood hearing impairment in Trent region, 1985-1993. Br J Audiol. 1997;31(6):409–446.
- Hadjikakou K, Bamford J. Prevalence and age of identification of permanent childhood hearing impairment in Cyprus. Audiology. 2000;39(4):198–201.
- Uus K, Davis AC. Epidemiology of permanent childhood hearing impairment in Estonia, 1985-1990. Audiology. 2000;39(4):192–197.
- Hergils. Allmän hörselscreening av nyfödda. Statens Beredning för Medicinsk Utvärdering [Internet]. 2004 Mar 31. Available from: https://www.sbu.se/sv/publikationer/SBU-utvarderar/allman-horselscreening-av-nyfodda/
- Niu K, Brandström A, Skenbäck S, et al. Risk factors and etiology of childhood hearing loss: a cohort review of 296 subjects. Acta Otolaryngol. 2020;140(8):668–674.
- Uhlén I, Mackey A, Rosenhall U. Prevalence of childhood hearing impairment in the county of Stockholm - a 40-year perspective from Sweden and other high-income countries. Int J Audiol. 2020;59(11):866–873.
- Dumanch KA, Holte L, O’Hollearn T, et al. High risk factors associated with early childhood hearing loss: a 3-Year review. Am J Audiol. 2017;26(2):129–142.
- Berninger E, Westling B. Outcome of a universal newborn hearing-screening programme based on multiple transient-evoked otoacoustic emissions and clinical brainstem response audiometry. Acta Otolaryngol. 2011;131(7):728–739.
- Dietz A, Löppönen T, Valtonen H, et al. Prevalence and etiology of congenital or early acquired hearing impairment in Eastern Finland. Int J Pediatr Otorhinolaryngol. 2009;73(10):1353–1357.
- Fitzpatrick EM, Dos Santos JC, Grandpierre V, et al. Exploring reasons for late identification of children with early-onset hearing loss. Int J Pediatr Otorhinolaryngol. 2017;100:160–167.
- Weichbold V, Nekahm-Heis D, Welzl-Mueller K. Universal newborn hearing screening and postnatal hearing loss. Pediatrics. 2006;117(4):e631–e636.
- Dedhia K, Kitsko D, Sabo D, et al. Children with sensorineural hearing loss after passing the newborn hearing screen. JAMA Otolaryngol Head Neck Surg. 2013;139(2):119–123.
- Jeong SW, Kang MY, Kim JR, et al. Delayed-onset hearing loss in pediatric candidates for cochlear implantation. Eur Arch Otorhinolaryngol. 2016;273(4):879–887.
- Obrycka A, Lorens A, Padilla García JL, et al. Validation of the LittlEARS auditory questionnaire in cochlear implanted infants and toddlers. Int J Pediatr Otorhinolaryngol. 2017;93:107–116.
- Wachtlin B, Brachmaier J, Amann E, et al. Development and evaluation of the LittlEARS® Early Speech Production Questionnaire - LEESPQ. Int J Pediatr Otorhinolaryngol. 2017;94:23–29.
- Farinetti A, Raji A, Wu H, et al. International consensus (ICON) on audiological assessment of hearing loss in children. Eur Ann Otorhinolaryngol Head Neck Dis. 2018;135(1S):S41–S48.