Abstract
Background
Hearing loss is a common sequela after bacterial meningitis, but risk factors for this are poorly studied, particularly in relation to concurrent acute otitis media (AOM).
Aims
The aim of this study was to investigate incidence and risk factors for hearing loss in patients treated for bacterial meningitis.
Methods
In this retrospective study, medical records for patients admitted to hospital with bacterial meningitis in Skåne county, Sweden, between 2000 and 2017 were retrieved. The association between risk factors and hearing loss was estimated using logistic regression.
Results
During the 18 years, 187 cases of meningitis were identified. Hearing loss was confirmed in 71 of the 119 patients who had done an audiometry. It was significantly more common in adults. There was also evidence of an association between hearing loss and AOM, and between hearing loss and pneumococcal infection.
Conclusion
Age, concurrent AOM and pneumococcal infection were risk factors for developing hearing loss. Despite being recommended in the national guidelines, more than a third of the patients had not done a hearing test after recovering from bacterial meningitis. The findings strengthen the demand for prompt ear examination and – if needed – tympanocentesis in meningitis patients.
Chinese Abstract
背景:听力损失是常见的细菌性脑膜炎后遗症, 但关于其风险因素, 特别是与并发急性中耳炎(AOM)有关的研究很少。
目的:本研究的目的是调查细菌性脑膜炎患者听力损失的发生率和危险因素。
方法:在这项回顾性研究中, 检索了 2000 年至 2017 年间瑞典斯科讷县的细菌性脑膜炎入院患者的病历。用逻辑回归分析评估风险因素和听力损失之间的关联。
结果:在 18 年间, 共发现 187 例脑膜炎病例。在接受了听力测试的119 名患者中, 有 71 名被证实有听力损失。它在成人中更为常见。还有证据表明听力损失与 AOM之间以及听力损失和肺炎球菌感染之间存在关联。
结论:年龄、并发 AOM 和肺炎球菌感染是发生听力损失的危险因素。尽管国家指南推崇, 但超过三分之一的患者从细菌性脑膜炎中恢复后没有进行听力测试。
意义:研究结果强调了对脑膜炎患者及时进行耳部检查甚至是鼓室穿刺术的要求。
Introduction
Meningitis is a life-threatening infection which sometimes occurs as a complication to acute otitis media (AOM). It is not entirely known what percentage of bacterial meningitis is of otogenic origin. A Dutch study found that the most common agents causing community-acquired meningitis was Streptococcus pneumoniae (53%), and Neisseria meningitidis (37%), and that concurrent AOM was a risk factor for negative outcome, indicating the importance of prompt ear examination [Citation1]. A French study of children with bacterial meningitis also identified S. pneumoniae (39%) and N. meningitidis (39%) as the most important pathogens [Citation2].
The most common sequela after bacterial meningitis is hearing loss [Citation3,Citation4]. This is particularly common after pneumococcal meningitis, where as many as 30% of patients suffer from hearing loss [Citation3]. Some studies have found lower incidences (7–12%) of post-meningitis hearing loss in children compared to adults [Citation5,Citation6], whereas others have reported similar incidences [Citation7]. Animal research has shown a correlation between pneumococcal concentration in the middle ear and the development of hearing loss [Citation8], indicating that early diagnosis of concurrent AOM and subsequent myringotomy could be beneficial. Swedish meningitis guidelines include a recommendation to perform otoscopy in all patients with meningitis, and to follow up patients with audiometry [Citation9].
The aim of this study was to investigate the incidence of hearing loss in patients treated for bacterial meningitis in the Swedish county of Skåne (1.4 million inhabitants), between 2000 and 2017 and to investigate risk factors for hearing loss.
Materials and methods
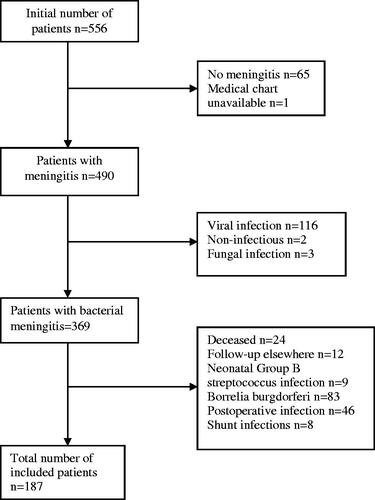
In this retrospective, observational study, medical records from all patients admitted to hospitals in Skåne county with the ICD-code G00 (bacterial meningitis) between 2000 and 2017 were retrieved. Exclusion criteria were neonatal, viral, fungal or non-infectious meningitis, borrelia or nosocomial, postoperative or ventricular shunt-related infections.
Information about gender, age, otoscopy results, CT/MRI signs of middle ear infection, microbiological results, subjective hearing loss and hearing tests were extracted from medical charts. Where pure tone audiograms were available, pure tone averages (PTA4; defined as the average of hearing thresholds at 500, 1000, 2000 and 4000 Hz) were extracted, as was the presence of conductive hearing loss (defined as an air-bone gap of ≥10 dB on at least two adjacent frequencies). Hearing loss was defined as PTA4 ≥ 25 on either ear, and further subdivided into mild (25–40 dB HL), moderate (41–70 dB HL) and severe (>70 dB HL). High frequency hearing was evaluated by calculating the average hearing thresholds at 6000 and 8000 Hz.
By including data over 18 years in one of the most populous counties in Sweden, we hoped to be able to estimate the incidence of bacterial meningitis in Sweden over a long period of time. The proportion of otogenic meningitis cases, time trends, microbiology and outcomes other than hearing loss will be reported separately.
The study was approved by the Ethics Review Authority. The STROBE guideline was used for the preparation of the manuscript.
Statistical analyses
Data were analysed using Stata 16.1 (College Station, TX, USA). The association between sensorineural hearing loss and possible risk factors were investigated using uni- and multivariate logistic regression. Variables showing a substantial association with the outcome in the univariate analysis were kept in the multivariate analysis. Patients were divided into four age groups; children (0–11 years), teenagers (12–21 years), adults of working age (22–65 years), and elderly (over 65 years).
In a substantial number of patients (n = 68), hearing tests were not performed. In order not to overestimate the prevalence of hearing loss, calculations were done in two ways: The first was by including only those who had done some sort of hearing test (analysis A), and the other by including all patients, making the conservative assumption that those who had not done a hearing test did not have a hearing loss (analysis B). As the focus of the study was to investigate sensorineural hearing loss after bacterial meningitis, patients with a purely conductive hearing loss were not included in the hearing loss group.
Results
Initially, 556 patients were identified. After applying exclusion criteria, 187 patients remained (), 106 of whom were men. The age distribution is shown in . Hearing tests were available in 119 cases, 107 of which were pure tone audiometries. Hearing loss was diagnosed in 81 patients, in 13 cases unilaterally, and was more common in adults and elderly (). Three patients had bilateral severe hearing loss, and an additional 13 had unilateral severe hearing loss. Of the 105 patients where data for 6 and 8 kHz were available, hearing thresholds >40 dB on these frequencies occurred frequently in adult patients, however, never in children or teenagers (Table1). The most commonly identified bacteria were S. pneumoniae (58%) and N. meningitidis (12%; ).
Table 1. Age distribution and occurrence of hearing loss in the respective age groups.
Table 2. Bacteriological findings.
In the univariate analysis, there was no evidence of a correlation between gender and hearing loss, however, there was strong evidence of adult and elderly patients having greatly increased odds of hearing loss (OR 11, p = .003 and OR 26, p < .001 in analysis A and OR = 7.3, p = .01 and OR = 8.4, p = .007 in analysis B).
The univariate analysis also supplied evidence that patients with pneumococcal infection had increased odds of hearing loss (OR = 2.3, p = .046 and OR = 4.1, p < .001 for analysis A and B, respectively). On the other hand, patients with meningococcal infections had decreased odds of hearing loss (OR = 0.2, p = .01 in analysis A and OR = 0.2, p = .009 in analysis B). There was also evidence for an association between hearing loss and concurrent AOM (OR = 1.4, p = .4 and OR = 3.3, p < .001 for analysis A and B, respectively).
Age, concurrent AOM and the presence of S. pneumoniae/N. meningitidis was kept in the multivariate analysis (). There was still strong evidence that age was a risk factor for hearing loss. In analysis A, there was no evidence of an association between AOM or pneumococcal infection and hearing loss, however, in analysis B, patients with concurrent AOM had twice the odds of hearing loss (OR = 2.1, p = .04), and for those with pneumococcal infection, the odds increased 3.6-fold (p = .001). In the multivariate analysis, no association was seen between meningococcal infection and hearing loss.
Table 3. Correlation between risk factors and the development of sensorineural hearing loss after bacterial meningitis.
Discussion
In this retrospective study, which comprised data from an entire Swedish county during 18 years, the strongest predictor for post-meningitis hearing loss was age. There was also evidence (analysis B) that pneumococcal infection and concurrent AOM increased the odds of hearing loss. Despite recommendations in the national guidelines, more than a third of patients had not done a hearing test after recovery.
The main outcome in this study was post-meningitis hearing loss. Regrettably, 1/3 of patients had not done a hearing test after recovery. In order not to over-estimate the prevalence of hearing loss, we decided to analyse the data, not only with patients lacking hearing tests censored, but also by using all the patients, assuming that those who had not done a hearing test did not suffer from any new hearing loss. There are several reasons why this is a reasonable assumption to make: First of all, since the Swedish meningitis guidelines pay attention to hearing loss as a common sequela to meningitis, most patients were probably asked about subjective hearing loss before being released from hospital, and there is no reason to believe that anyone complaining of hearing loss would be denied a hearing test. Secondly, in Skåne county, Sweden, where this study was performed, anyone can easily schedule a hearing test if they suspect that their hearing has deteriorated. Lastly, children are regularly followed up with audiometries via child health centres and school health care. All in all, it is therefore reasonable to believe that no significant hearing loss should go undetected.
Of those who underwent a hearing test, 68% had a hearing loss, and the corresponding figure for the entire cohort was 43%. Both these prevalences exceed the 30% which has been described previously [Citation3]. This might, in part, be explained by different definitions of hearing loss, and by some patients in the present study having had previously undiagnosed hearing loss. The extremely high prevalence of 68% hearing loss among the patients who had done an audiometry indicate that it was reasonable to analyse the data in two ways. The conservative assumption that patients lacking audiometries had normal hearing should at least not lead to an over-estimation of the prevalence of hearing loss.
A consistent finding in this study was that age increased the risk of developing hearing loss. Age >70 years has previously been associated with an ‘unfavourable outcome’ [Citation10], Since most patients in this study had not tested their hearing prior to their meningitis, the finding might, at least partly, be explained by the fact that the prevalence of hearing loss increases with age. Some patients might therefore have had previously undiagnosed presbyacusis, an assumption further supported by the large number of patients with high frequency hearing loss in the two older age groups. Undiagnosed presbyacusis should also have been present in some patients with missing hearing tests, where we assumed normal hearing, which should somewhat counteract any such overestimation and might explain why the effect sizes were not as great in analysis B as in analysis A. Missing audiometries were not less common among the older age group (data not shown).
There was a correlation between AOM and hearing loss in analysis B, however, not in analysis A, presumably due to the larger sample size in analysis B. The association seen in analysis B seems to have been partly confounded by pneumococcal infection, since the effect estimate decreased in the multivariate analysis, however, the odds of hearing loss was still twice as high among patients with AOM in the multivariate analysis. A Dutch study also found that the odds of hearing loss increased by 2.6 in patients with concurrent AOM [Citation11].
Streptococcus pneumoniae – a common otopathogen – increased the odds of hearing loss almost four-fold in analysis B after controlling for other risk factors. Analogous to the findings for AOM, this was not seen in analysis A, again, probably due to the smaller sample size in that analysis. That patients with pneumococcal meningitis are more likely to develop long-term hearing loss has been noticed in a previous meta-analysis as well as in a retrospective review on children [Citation3,Citation12]. The negative association between meningococcal infection and hearing loss in the univariate analysis was not present in the multivariate analysis, indicating that the former results were confounded by age; meningococcal infections being almost exclusively found among children and teenagers (data not shown).
This study has several limitations, the greatest of which is the retrospective design, meaning that many patients did not undergo hearing tests. The assumption that patients lacking hearing tests did not suffer from post-meningitis hearing loss (analysis B) seems reasonable to make, as it is unlikely that a patient complaining about a recent hearing loss should not be referred for an audiometry. The one group where this would be more likely to occur is young children, however, the association between age and hearing loss was even more pronounced in analysis A, where only those who had actually done a hearing test were included. In addition, most patients had not done hearing tests before their meningitis, so there was no way of knowing for certain that the hearing loss was caused by the meningitis. The design also means that microbiological PCR tests and serotyping were not performed.
A strength of the study is that it encompasses a whole county of Sweden during a period of 18 years, implying that the results should be generalisable.
Better knowledge of risk factors for post-meningitis hearing loss can hopefully result in better compliance with existing guidelines, leading to more patients undergoing otoscopy when admitted to hospital with bacterial meningitis, and more patients being followed up audiologically after recovery. As discussed above, early diagnosis of concurrent AOM, and subsequent myringotomy might decrease the risk of developing hearing loss.
Conclusion
This study showed that the incidence of hearing loss after bacterial meningitis was strongly associated with age, but also with concurrent acute otitis media and S. pneumoniae infection. In an on-going prospective study on the same population, we hope to be able to confirm the findings more robustly.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- van de Beek D, de Gans J, Spanjaard L, et al. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351(18):1849–1859.
- Bargui F, D'Agostino I, Mariani-Kurkdjian P, et al. Factors influencing neurological outcome of children with bacterial meningitis at the emergency department. Eur J Pediatr. 2012;171(9):1365–1371.
- Edmond K, Clark A, Korczak VS, et al. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(5):317–328.
- Svendsen MB, Ring Kofoed I, Nielsen H, et al. Neurological sequelae remain frequent after bacterial meningitis in children. Acta Paediatr. 2020;109(2):361–367.
- Chandran A, Herbert H, Misurski D, et al. Long-term sequelae of childhood bacterial meningitis: an underappreciated problem. Pediatr Infect Dis J. 2011;30(1):3–6.
- Richardson MP, Reid A, Tarlow MJ, et al. Hearing loss during bacterial meningitis. Arch Dis Child. 1997;76(2):134–138.
- Kutz JW, Simon LM, Chennupati SK, et al. Clinical predictors for hearing loss in children with bacterial meningitis. Arch Otolaryngol Head Neck Surg. 2006;132(9):941–945.
- Perny M, Roccio M, Grandgirard D, et al. The severity of infection determines the localization of damage and extent of sensorineural hearing loss in experimental pneumococcal meningitis. J Neurosci. 2016;36(29):7740–7749.
- Svenska Infektionsläkarföreningen. Vårdprogram Bakteriella CNS-infektioner. 2020. Available from: https://infektion.net/vardprogram/cns-infektioner-bakteriella/#.
- Tubiana S, Varon E, Biron C, et al. Community-acquired bacterial meningitis in adults: in-hospital prognosis, long-term disability and determinants of outcome in a multicentre prospective cohort. Clin Microbiol Infect. 2020;26(9):1192–1200.
- Heckenberg SG, Brouwer MC, van der Ende A, et al. Hearing loss in adults surviving pneumococcal meningitis is associated with otitis and pneumococcal serotype. Clin Microbiol Infect. 2012;18(9):849–855.
- Woolley AL, Kirk KA, Neumann AM, Jr, et al. Risk factors for hearing loss from meningitis in children: the children’s hospital experience. Arch Otolaryngol Head Neck Surg. 1999;125(5):509–514.