Abstract
Background
The prevalence of disabling hearing loss is increasing worldwide. However, previous studies on hearing loss prevalence have enrolled small populations or only provided estimates.
Aim
To establish the prevalence of severe-to-profound hearing loss (STPHL) in the adult Swedish population and compare it with the cochlear implantation rate in Sweden.
Material and methods
We established a database containing over 15 million audiograms obtained from regions covering > 99% of the Swedish population by extracting audiogram data from the computer software application, Auditbase. We used this database to calculate the percentage of adult patients with bilateral hearing levels ≥ 70 dB. We collected data regarding cochlear implantations in Sweden from the National Board of Welfare and Health.
Results
The prevalence of STPHL in the adult Swedish population was 0.28%. There were regional variations in the prevalence and rate of cochlear implantation; however, there was no association between both parameters.
Conclusions
This study presents an updated and reliable prevalence figure for STPHL in Sweden.
Significance
Patients with STPHL have extensive rehabilitation requirements; accordingly, it is important to determine the accurate prevalence of STPHL to inform the allocation of adequate resources.
Chinese Abstract
背景:全世界范围内致残性听力损失的患病率正在增加。然而, 以前的关于听力损失患病率的研究只纳入了少数人群或仅提供了估计值。
目的:确定瑞典成年人严重至深度听力损失 (STPHL) 的患病率人口并将其与瑞典的人工耳蜗植入率进行比较。
材料与方法:我们建立了一个包含来自覆盖 99% 以上瑞典人口的地区的超过 1500 万个听力图的数据库, 其方法是从计算机软件应用程序 Auditbase 中提取听力图数据。我们使用这个数据库来计算双侧听力水平为 70 dB 的成人患者的百分比。我们从瑞典国家福利和健康委员会收集了关于人工耳蜗植入的数据。
结果:瑞典成年人口中 STPHL 的患病率为 0.28%。患病率和人工耳蜗植入率具有区域性变化;但两个参数之间并没有关联。
结论:本研究提供了瑞典 STPHL 的最新且可靠的患病率数据。
意义:STPHL 患者有广泛的康复需求;因此, 确定 STPHL 的准确患病率很重要, 以便为足够的资源分配提供信息。
Introduction
Hearing loss (HL) is a major prevalent problem and is ranked as the third most common cause of years lived with disability by the Global Burden of Disease study [Citation1]. In 2018, the World Health Organization (WHO) estimated that there are approximately 466 million patients with disabling HL, with this number being expected to increase to approximately 900 million by 2050 [Citation2]. The severity of HL can be described as mild, moderate, severe, or profound. This study focused on severe-to-profound HL (STPHL). There are different definitions of STPHL. Specifically, the British Society of Audiology defines STPHL as a hearing level ≥70 dB across the frequencies of 0.5, 1, 2, and 4 kHz in the better ear [Citation3], while WHO defines it as a hearing level ≥65 dB across the same frequencies in the better ear in a recently released revised grading system [Citation4]. HL substantially affects patients’ lives by reducing the quality of life through isolation, decreased social activity, and an enhanced feeling of exclusion [Citation5]. Patients with STPHL have high levels of anxiety and depression. Moreover, there are expected variations in the global prevalence of STPHL, which could be attributed to varying factors. Other than the obvious age factors, geographic differences in the prevalence could result from geographical variations in noise exposure, infections, and access to healthcare.
Studies on the prevalence of bilateral STPHL have used different methods and definitions. An extensive population-based study conducted in the 1980s in the United Kingdom found that the prevalence of bilateral hearing level ≥75 dB was 0.7% among individuals aged 18–80 years (sample size, n = 2663) [Citation6]. Turton et al. investigated a British region with 310,000 inhabitants and identified 2199 (0.7%) clinical patients (age ≥20 years) with >70 dB hearing level in the better ear at frequencies 0.5, 1, and 2 kHz [Citation7]. A recent South Korean study on patients registered in the Korean National Health Insurance Service, which covers the entire population, reported that 0.27% of the patients had a bilateral hearing level of ≥70 dB. That study included all age groups, i.e. children and adults [Citation8]. A Finnish study on 850 people (age: 54–66 years) reported that 0.2% of the patients had a bilateral hearing level of ≥70 dB [Citation9]. The Swedish registry for adult patients with bilateral STPHL (≥70 dB) reported an estimated prevalence of ≥0.2% [Citation10]. The Global Burden of Disease 1990–2019 estimated the prevalence of bilateral STPHL (≥65 dB) in Europe and worldwide to be 0.3% and 0.7%, respectively [Citation11]. In the US, Goman et al. defined bilateral severe and profound HL as 60–79 dB and >80 dB, respectively, with a prevalence of 0.8% in patients aged ≥12 years (sample size, n = 9,648) [Citation12].
Since STPHL causes disability, it requires hearing rehabilitation [Citation5]. Technical rehabilitation interventions for STPHL include conventional hearing aids and cochlear implants (CI). CIs are cost-effective and can efficiently improve the quality of life in patients with STPHL [Citation13]. There are national differences in the cochlear implantation rate, which is lower in Eastern Europe than in Western Europe [Citation14]. De Reave et al. reported that 6.6% of the Belgian adult population with STPHL had received a CI [Citation15]. In the USA, Sorkin estimated that 5% of all individuals eligible for CIs had received at least one CI [Citation16]. In the Swedish registry for adult patients with STPHL, HL ≥ 70 dB in the better ear is an inclusion criterion, which corresponds to the hearing level criterion for CI in Sweden. In this Swedish registry, 10% of the included patients have received a CI [Citation10]. In Sweden, cochlear implantation is exclusively performed by public healthcare providers. A registry on the number of surgeries performed in Sweden is publicly available at the Swedish National Board of Health and Welfare. However, regional differences within countries remain unexplored.
Most cases of congenital profound HL have a genetic non-syndromic aetiology, with >110 genes having being currently identified [Citation17]. The genetic causes of HL vary widely and are unequally distributed throughout regions worldwide. Mutations in connexins, especially connexin 26, are the most common cause of HL with genetic non-syndromic aetiology. The frequency of connexin 26 mutations varies across Europe [Citation18]; however, it does not differ across Swedish regions [Citation19].
It is important to obtain reliable figures regarding the prevalence of conditions with extensive rehabilitation needs (including STPHL) in order to inform the distribution of medical resources. Studies on the prevalence of STPHL in Sweden have the advantage that patients are rehabilitated through the public health care system; therefore, they are more accessible to epidemiological studies. Currently, only an estimated prevalence of STPHL has been reported in Sweden [Citation10], which is yet to be validated.
This cross-sectional study aimed to establish the prevalence of STPHL in Sweden and to compare it with the rate of cochlear implantation in different Swedish regions.
Methods
Study population
Patients with STPHL were identified using Auditbase (Auditdata, Copenhagen, Denmark), which is an audiometry software application. Auditbase is used by the public healthcare system in 20 out of 21 regions in Sweden, covering >99% of the entire population. All recorded audiograms until January 31, 2020, were extracted from Auditbase and transferred to an Excel file (Microsoft, Redmond, WA, USA), which was used to establish a national audiogram database in Sweden.
By applying specific search criteria, we only included adult patients (≥19 years) with PTA4 ≥ 70 dB in the better ear on the last performed audiogram. This study was conducted within the framework of the Swedish registry for adult patients with STPHL [Citation10]; accordingly, the age criterion (≥19 years) was based on this registry. Audiograms for patients with hearing threshold measurements for only one ear were manually validated in three regions. Accordingly, we determined that the missing measurements were due to unattained hearing thresholds or deafness established in previous audiograms; therefore, we included these patients in the analysis.
The search criteria ensured that only living patients registered as Swedish residents by January 31, 2020, were included. Further, age groups, sex and current address were registered. Supplement 1 presents a detailed description of the selection procedure.
Hearing measurements
We defined STPHL as a bilateral pure tone average at frequencies of 0.5, 1, 2, and 4 kHz (PTA4) of 70 dB hearing level or worse [Citation3]. The hearing level was determined by a qualified audiologist in public hearing service based on international audiology methods (ISO 8253–1, 1989) through pure tone audiometry performed in a soundproof booth using a clinical audiometer. Hearing thresholds were measured at octave frequencies of 0.125–8, 3, and 6 kHz. If masking was performed, we only used the masked value for analysis. The air conduction hearing thresholds were present an analysed in all last performed audiograms. Numerous patients showed missing bone conduction hearing thresholds and speech audiometry; accordingly, they were not analysed.
Cochlear implants
In Sweden, the national hearing threshold criterion for implanting CIs is PTA4 ≥ 70 dB, which is consistent with our definition for STPHL. Since 1998, the National Board of Health and Welfare in Sweden has collected information on all surgeries performed in the country, including CI surgery. From the agency’s website, the public can access information regarding the annual number of surgeries as well as sex, age, and region of residence at the time of surgery. We extracted the average annual rates of CI surgeries per 100,000 inhabitants for each region in 2015–2019 from the register and compared it with the prevalence of STPHL in that region.
Statistical analysis
The prevalence is expressed as numbers and percentages. Given the non-normal distribution of the cochlear implantation rate, we used Kendall’s tau-b rank correlation to investigate the correlations between the prevalence of STPHL and the annual rate of cochlear implantation in different regions. We used the Swedish version of Excel 2016 (Microsoft, Redmond, WA, USA) to manage the extracted audiograms from Auditbase. Statistical analyses were performed using SPSS version 26 (IBM, Chicago, IL, USA). Statistical significance was set to p < .05.
Ethical considerations
This study was approved by the Regional Ethical Review Board in Umeå (2018/283-31).
Results
Prevalence
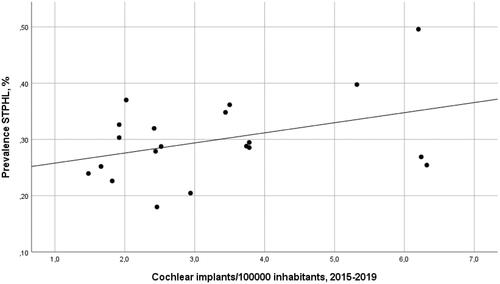
The adult population (≥19 years) in Sweden amounted to 8,142,219 on January 31, 2020. After excluding inhabitants from the only Swedish region not using Auditbase, 8,093,654 individuals remained. Approximately 15 million audiograms were collected from hearing clinics across Sweden. Based on the selection criteria, there were 22,298 (0.28%) patients exhibiting STPHL in all regions (). The prevalence ranged from 0.18 to 0.50% across the regions.
shows the distribution of male and female patients with STPHL. Men comprised the majority of individuals aged 70–79 and 80–89 years, while women comprised the majority of the oldest age group (≥90 years). As expected, the prevalence increased with age. The prevalence rates were 0.06% and 3.64% in the youngest and oldest age groups, respectively (). The between-sex prevalence ratio was approximately 1:1 in all age groups until the age of 60–69 years. In the older age groups, there was a greater increase in the prevalence in men than in women. The male to female (M:F) ratios among older patients were 1.26:1, 1.51:1, and 1.61:1 in the 70–79, 80–89, and 90+ age groups, respectively. The overall M:F ratio was 1.04:1.
Figure 1. Female, male, and total number of patients with severe-to-profound hearing loss divided into 10-year age bands.
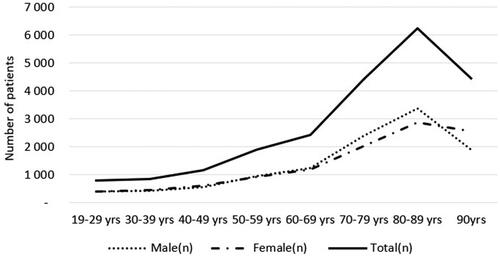
Figure 2. Prevalence of severe-to-profound hearing loss among females and males and total divided into 10-year age bands.
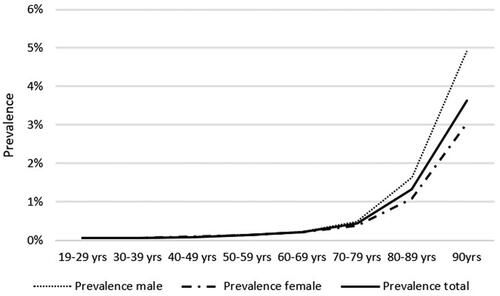
Table 1. Prevalence of severe-to-profound hearing loss for women, men, and total in Sweden.
Cochlear implants
According to the Swedish Board of Health and Welfare, the average annual cochlear implantation rate from 2015 to 2019 ranged from 1.5/100,000 to 6.3/100,000 in the regions (). The average annual implantation rate across the regions was 2.8/100,000. Kendall’s rank correlation revealed no correlation between the prevalence of STPHL and cochlear implantation rate (tau-b = 0.16, p = .31) ().
Discussion
In this study, we identified 22,298 patients with STPHL (≥70 dB) in Sweden, resulting in a prevalence of 0.28%. We believe that this number is a reliable estimation since it was retrieved from all regions except one, covering >99% of the Swedish population.
To our knowledge, no study has used accumulated audiogram data from a whole country. However, Im et al. used a South Korean national registry of patients with HL and reported a prevalence (0.27%) similar to that in our study [Citation8]. It is difficult to compare our findings with those of other studies since no other studies have performed similar sampling at a nationwide level. Turton et al. similarly examined 310,000 inhabitants in the UK and reported a clinical prevalence of 0.7% [Citation7]; however, they did not exclusively assess the last-performed audiogram. Assessment of the latest audiogram in a subset of patients (302/2199) revealed that 19% of patients had moderate HL in at least one ear, which could have led to an overestimation of the prevalence. In 1995, Davis estimated a prevalence of 0.7% (PTA4 ≥ 75 dB) based on a population study (n = 2663) performed in the early 1980s [Citation6]. However, since the prevalence is a statistical estimate only based on 43 individuals with hearing levels ≥75 dB, it is difficult to assess the reliability. Some prevalence variations are expected across European countries. For example, connexin 26-mutations are more common in southern Europe (2.48%) than in northern Europe (1.53%) [Citation20]. There were no particular differences in the mutation carrier frequency between the UK and Sweden [Citation18,Citation19]. Differences in the prevalence of STPHL could be attributed to differences in the mutation frequency in other genes [Citation17], noise exposure, infections, and access to health care. Large nationwide studies are warranted to minimize the influence of these factors on the estimated prevalence.
Differences exist in the rates of cochlear implantation across countries [Citation14,Citation16]. We observed varying cochlear implantation rates across Swedish regions, which were not correlated with the prevalence of STPHL. There are no reimbursement issues in Sweden since the costs for surgery, implants, and rehabilitation are free for all Swedish citizens. There is a need to increase CI awareness, especially with the evolving criteria for its use, which now may include residual low-frequency hearing or single-sided deafness. As aforementioned, there have been inconsistent reports regarding the prevalence of patients with STPHL. Moreover, to our knowledge, there has been no report of a correlation between the prevalence of STPHL and the cochlear implantation rate. Although the reasons underlying this lack of correlation are unclear, a lack of awareness of CI benefits and ignorance of referral pathways could contribute to the low cochlear implantation rate [Citation16].
Since the criteria for STPHL differs across studies, we performed a subset analysis of one Swedish region. The WHO criterion of PTA4 ≥ 65 dB HL was applied to audiograms in this region. In this region, the adult population was 227,227 on January 31, 2020, with 1036 patients meeting the WHO criterion, which resulted in a prevalence of 0.46% compared with 0.23% when using the 70 dB criterion.
The major strength of this study is that it covered almost the entire nation and included a large number of audiograms. Our findings could be considered reliable since we used a national audiogram database with high population coverage. However, this study has several limitations. First, since this study was based on clinical patients who had visited hearing clinics for an audiogram, we did not include patients with STPHL without an audiogram, which could have led to the underestimation of the prevalence. However, most patients with STPHL in Sweden could have likely visited a hearing clinic and undergone a hearing test. Further, the underestimation could result from some patients only accessing private hearing services rather than public hearing services. However, this number is likely to be small, as indicated by the Registry for hearing rehabilitation in Sweden, which includes both public and private hearing services [Citation10]. Accordingly, there is a low possibility of underestimation of the prevalence. Finally, an additional limitation is that speech audiometry could not be analysed for reasons described in the methods section.
In conclusion, we estimated the prevalence of STPHL (≥70 dB) in Sweden to be 0.28%. Although CI is very valuable for the rehabilitation of patients with STPHL, we observed no correlation between the prevalence of STPHL and the cochlear implantation rate across Swedish regions.
Supplemental Material
Download MS Word (16.6 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1204–1222.
- World Health Organization. Addressing the rising prevalence of hearing loss [Internet]. 2018; [cited 2022 Feb 15]. Available from: https://apps.who.int/iris/handle/10665/260336.
- British Society of Audiology. Recommended procedure. Bone-conduction threshold audiometry with and without masking [Internet]. British Society of Audiology. 2012; [cited 2020 Sep 15]. Available from: http://www.thebsa.org.uk/wp-content/uploads/2014/04/BSA_RP_PTA_FINAL_24Sept11_MinorAmend06Feb12.pdf.
- World Health Organization. World report on hearing [Internet]. 2021; [cited 2022 Feb 23]. Available from: https://www.who.int/publications/i/item/world-report-on-hearing.
- Ciorba A, Bianchini C, Pelucchi S, et al. The impact of hearing loss on the quality of life of elderly adults. Clin Interv Aging. 2012;7:159–163.
- Davis A. Hearing in adults. London (UK): Whurr Publishers Ltd.; 1995.
- Turton L, Smith P. Prevalence & characteristics of severe and profound hearing loss in adults in a UK national health service clinic. Int J Audiol. 2013;52(2):92–97.
- Im GJ, Ahn JH, Lee JH, et al. Prevalence of severe-profound hearing loss in South Korea: a nationwide population-based study to analyse a 10-year trend (2006–2015). Sci Rep. 2018;8(1):9940.
- Hannula S, Mäki-Torkko E, Majamaa K, et al. Hearing in a 54- to 66-year-old population in northern Finland. Int J Audiol. 2010;49(12):920–927.
- Swedish quality registry of otorhinolaryngology [Internet]; [cited 2021 Dec 27]. Available from: www.entqualitysweden.se.
- Haile LM, Kamenov K, Briant PS, et al. Hearing loss prevalence and years lived with disability, 1990–2019: findings from the global burden of disease study 2019. Lancet. 2021;397(10278):996–1009.
- Goman AM, Lin FR. Prevalence of hearing loss by severity in the United States. Am J Public Health. 2016;106(10):1820–1822.
- Contrera KJ, Betz J, Li L, et al. Quality of life after intervention with a cochlear implant or hearing aid. Laryngoscope. 2016;126(9):2110–2115.
- Raeve L De HRv. Prevalence of cochlear implants in Europe: What do we know and what can we expect? J Hear Sci. 2020;3:9–16.
- De Raeve L. Cochlear implants in Belgium: prevalence in paediatric and adult cochlear implantation. Eur Ann.Otorhinolaryngol. Head Neck Dis. Elsevier Masson SAS. 2016;133:S57–S60.
- Sorkin DL. Cochlear implantation in the world’s largest medical device market: utilization and awareness of cochlear implants in the United States. Cochlear Implants Int. 2013;14(Suppl 1): S4–S12.
- Shearer AE, Hildebrand MS, Smith RJ. Hereditary hearing loss and deafness overview. GeneReviews® [Internet]. 2017; [cited 2021 Dec 1]; Available from: https://www.ncbi.nlm.nih.gov/books/NBK1434/.
- Adadey SM, Wonkam-Tingang E, Aboagye ET, et al. Connexin genes variants associated with non-syndromic hearing impairment: a systematic review of the global burden. Lifeand. 2020;10(11):258–230.
- Hederstierna C, Möller C, Åhlman H, et al. The prevalence of Connexin 26 mutations in the Swedish population. Audiol Med. 2005;3(3):154–157.
- Mahdieh N, Rabbani B. Statistical study of 35delG mutation of GJB2 gene: a meta-analysis of carrier frequency. Int J Audiol. 2009;48(6):363–370.