Abstract
Background
Trismus is a complication post-radiotherapy for head and neck cancer (HNC), which causes pain, eating limitations and reduced quality-of-life. However, little is known about the condition long-term or how trismus fluctuates within an irradiated population.
Aim/Objective
To prospectively map trismus prevalence in irradiated HNC patients up to 5-years following treatment completion including describing intra-group fluctuation of maximum interincisal opening (MIO).
Materials and Methods
211 patients receiving curatively intended radiotherapy for HNC were included in this prospective study. Patients were followed pre-radiotherapy (baseline), 3-, 6-, 12-, 24-, 36- and 60-months post-radiotherapy completion using MIO.
Results
Mean MIO at baseline, 12-months and 5-years following radiotherapy were 51.5 mm, 41.7 mm and 41.3 mm respectively. A total of 28% (n = 36) fulfilled the trismus criterion at 5-years post-radiotherapy. Eighty percent of patients (n = 24) with trismus at 5 years post-radiotherapy also suffered from trismus at 12 months post-radiotherapy, whilst 88–92% of all patients reported reduced MIO at any given timepoint compared to baseline. 15% of patients never exceeded an MIO of >35 mm at any time-point.
Conclusion
Trismus is a prevalent long-term complication of HNC and its treatment, which does not appear to heal spontaneously. The majority of fluctuations in MIO occur during the first 12 months post-radiotherapy completion.
Chinese Abstract
背景:牙关紧闭是头颈癌 (HNC) 放疗后的一种并发症, 导致疼痛、饮食限制和生活质量下降。然而, 人们对这种病况或牙关紧闭在受辐射人群中的波动情况知之甚少。
目的:前瞻性地绘制接受放疗的 HNC 患者在治疗完成后长达 5 年的牙关紧闭患病率, 包括描述组间最大切面间开口(MIO)的波动。
材料和方法:211 名接受 HNC 治疗性放疗的患者被纳入本前瞻性研究。患者在放疗前(基线)、完成放疗后3、6、12、24、 36 个月和 60 个月接受关于 MIO的随访。
结果:基线时、放疗后 12 个月和 5 年的平均 MIO 分别为 51.5 mm、41.7 mm和 41.3 毫米。放疗后 5 年, 共有 28% (n = 36) 的患者满足牙关紧闭标准。 80% 的患者 (n = 24) 在放疗后 5 年出现牙关紧闭症, 放疗后 12 个月出现牙关紧闭, 而 88-92% 的患者报告, 在与基线相比的任何给定时间点 MIO 降低。 15% 患者的MIO在任何时候都没有超过 >35 mm 。
结论:牙关紧闭是 HNC 及其治疗常见的长期并发症, 似乎不会自然愈合。 MIO 的大部分波动发生在放疗完成后的前 12 个月。
Introduction
Head and neck cancer (HNC) accounts for 4–5% of new cancer cases worldwide and causes nearly half a million deaths, annually [Citation1]. Limited mouth opening ability (trismus) is a complication that develops both due to initial tumor burden but also as a result of the radiotherapeutic oncologic treatment, where it is considered to result from a combination of cell death in normal tissue and excessive accumulation of fibrotic tissue causing damage by abnormal wound healing [Citation2]. The most widely employed criterion for trismus is that of Dijkstra et al. [Citation3], which defines trismus as a maximum interincisal opening (MIO) ≤ 35 mm.
Prevalence of trismus amongst irradiated HNC patients has been reported as high as 37% up to three years post treatment completion in the largest to date prospective study [Citation4], but its prevalence further long-term is lacking as is knowledge of the intra-group fluctuations in MIO that occur post-radiotherapy (post-RT). Patients with trismus compared to those without have been shown to suffer from more pain, eating limitations and reduced health related quality of life (HRQL) [Citation5,Citation6].
Hence, this study aims to prospectively map the prevalence of trismus in irradiated HNC patients up to 5-years following treatment completion including describing fluctuations of MIO within the HNC population over time.
Materials and methods
Study population and study design
All patients with a primary diagnosis of HNC in the region of Western Sweden attend a weekly multidisciplinary tumor conference at Sahlgrenska University Hospital in Gothenburg, Sweden. Between the years 2007 − 2012 patients were consecutively asked for study participation if they fulfilled the inclusion criteria. These included being at least 18 years of age, receiving curatively intended radiotherapy with or without chemotherapy/surgery and a tumour site in the oral cavity (tongue, gingiva and floor of mouth), oropharynx (tonsil or base of tongue), nasopharynx (including nasal cavity and paranasal sinuses), salivary glands or cervical carcinoma of unknown primary (CCUP). Exclusion criteria encompassed recurrent disease, a tumor site not expected to be associated with the development of post-radiation trismus (larynx, oesophagus or hypopharynx), surgical treatment alone, a performance status or mental capacity too poor to partake in examinations.
Patients were followed prior to radiotherapy (baseline), 3-, 6-, 12-, 24-, 36- and 60-months post-completion of radiotherapy. At each time-point, MIO was measured. Comorbidity according to the Adult Comorbidity Evaluation 27 (ACE27) was recorded at baseline.
Oncologic treatment
Tumors were classified according to global standard, the TNM-staging system by the International Union Against Cancer (UICC) involving tumor size (T), regional lymph nodes involvement (N) and distant metastasis (M). Curatively intended radiotherapy was administered according to regional guidelines. During 2007–2009 external radiation therapy was generally administered through accelerated hyperfractionated therapy as 1.7 Gray (Gy) per fraction, 2 fractions daily, 5 days per week, totaling 64.6 Gy. During 2010–2012, the external radiation therapy was administered through accelerated fractioning 2 Gy per fraction 1–2 times per day 5 days per week with a total of 6 treatments per week, totaling 68 Gy. Induction (cisplatin and 5-fluorouracil) or concomitant (weekly cisplatin) chemotherapy was added to the radiation therapy mainly for patients with stage III to IV disease in accordance with local treatment standards at our department (n = 141).
Surgical treatment was used in combination with radio- and chemotherapy for some tumor sites according to established regional guidelines for surgical management of HNC tumors [Citation7]. For tumors in the oral cavity, smaller tumors were only surgically resected (and excluded from the study), whereas combined surgery and radiotherapy was employed for stage III-IV disease or chemoradiotherapy only if the tumor was deemed unresectable. Non-surgical treatment was clinical practice for oropharyngeal and nasopharyngeal tumors, whereas advanced stage nasal tumors received combined treatment. Neck dissections and primary site tumors were removed surgically if located in the salivary glands with post-operative radiotherapy. The former with additional chemoradiotherapy was administered to all CCUP unless patients had bilateral metastases or an inoperable tumor, in which case only curatively intended chemoradiotherapy was administered.
Maximum interincisal opening (MIO)
The criterion for trismus used was that defined by Dijkstra et al. [Citation3], namely MIO ≤ 35 mm. Maximum interincisal opening was measured in millimeters with a ruler as the maximum distance between the upper and lower incisors. MIO in edentulous patients was measured with the dental prostheses in place.
Statistical analysis
Number and percentages are presented for categorical variables, whilst continuous variables are presented using mean and 95% confidence interval (CI). In addition, for MIO, median and range are presented. For comparisons between groups, Fischer’s Exact test was used for dichotomous variables, Mantel-Haenszel Chi Square test for ordered categorical variables, Chi Square test for non-ordered categorical variables and Mann-Whitney U-test for continuous variables. The Sign test was used for paired intra-group comparisons over time for ordered categorical variables. Wilcoxon signed rank test was used for intra-group comparison over time for continuous variables. In order to find variables influencing trismus development at each study time-point, univariable logistic regression analysis was performed and odds ratio calculated. Factors analyzed were age, gender, living alone, number of school years, employment status, smoking status, Karnofsky performance status, BMI, dental status, tumor stage, tumor site, cancer treatment and ACE27. All tests were two-sided and conducted at a 5% significance level. The SAS version 9.4 for PC was used for analyses.
Ethical considerations
The study was conducted according to the Declaration of Helsinki and was approved by the Regional Ethic Review Board in Gothenburg, Sweden (Dnr 287-04, 278-13, T721-07). All participants gave their written informed consent before study inclusion. A minor part of the study population (n = 50) was also included in another previously published study [Citation8].
Results
Patient characteristics
A total of 225 patients fulfilled the inclusion criteria. Of these, 14 patients were excluded from the analyses due to having trismus as baseline (pre-radiotherapy). The sociodemographic and clinical data for the included 211 patients are presented in . At the 12-month follow-up, 24 patients had ended their participation in the study of which 17 had tumor recurrence, one had reduced performance status, four gave no reason and two died of unknown cause. An additional seven patients were still enrolled in the study but failed to attend the 12-month follow-up. The corresponding figure at the 5-year follow-up was 82, of which 39 had tumor recurrence, four had reduced performance status, 13 died of unknown cause and 26 gave no reason. The total number of patients deceased at the 12- and 60- month follow up were 11 and 38 respectively. A total of 53 patients reported that they had performed jaw exercise training at some point during the study period, albeit not structured and at varying time-points.
Table 1. Sociodemographic and clinical characteristics for study participants n = 211.
The 129 patients still included in the study at the 5-year follow-up were, when compared to those not completing the study (n = 82), at baseline statistically significantly younger (59.5, SD 9.7 vs 63.4, SD 10.5, p = .009), had higher (better) Karnofsky scores (98.7, SD 5.1 vs 95.6, SD 8.2, p < .001) and lower comorbidity, i.e. more patients scoring no comorbidity on ACE27 (n = 50% vs 33%, p = .006). There were no other statistically significant differences between those completing the study and the drop-out group at baseline regarding gender, tumor stage or site, employment status, smoking status, BMI or type of cancer treatment. Regarding MIO, there was only a statistically significant difference in MIO between the two groups at one time-point, occurring at 24 months post-RT in favor of those completing the study (38.0 vs 42.3 mm, p = .008). A total of 48% in the drop-out group (n = 39) developed tumor recurrence during the study-period and were excluded from continued participation. None of the 129 patients completing the study developed a locoregional failure during the study period (p < .0001).
All patients received curatively intended radiotherapy and 35 patients (17%) also underwent a surgical procedure. Of these 35, 10 patients had tumors in the oral cavity, two were oropharyngeal, seven in the salivary glands, four in the nose/paranasal sinuses/nasopharynx and 12 were CCUP. The main surgical treatment was neck dissection (n = 28), but also local tumor resection in the tongue, salivary gland and nose (n = 19).
Five-year overall survival and locoregional control
Eleven patients (5%) were deceased and 17 (8%) had suffered a tumor recurrence by the 12-month follow-up. At 5-years following radiotherapy, 38 patients (18%) were deceased and 39 (18%) had recurrence. The patients were excluded from continued study participation once a loco-regional recurrence was diagnosed.
Mio
Mean MIO at baseline, 12-months and 5-years following radiotherapy were 51.5 mm, 41.7 mm and 41.3 mm respectively. At 12 months post-radiotherapy, 27% (n = 49) fulfilled the criterion for trismus (MIO ≤ 35 mm), whereas 73% (n = 131) did not. Corresponding figures for 5-years following radiotherapy were 28% (n = 36) and 72% (n = 93) respectively (). The mean change in MIO in mm and percent from each time-point compared to baseline is summarized in , which ranges from mean decreases of 9.1 − 10.6 mm throughout the study period. All decreases in MIO were statistically significant when compared to baseline but only deteriorations at 3- and 6 months were significant when comparing change from preceding time-point.
Table 2. MIO and trismus in the study population up to 5 years post-radiotherapy.
Table 3. Mean (95% CI) change in MIO in millimetres and percent at each time-point compared to baseline.
Sociodemographic and clinical variables affecting MIO
Statistical analysis of differences in all sociodemographic and clinical variables presented in was conducted between the two groups (trismus and no trismus) at each follow-up visit. There were no statistically significant differences found between the groups at any time-point apart from the trismus group having a higher percentage of smokers compared to the non-trismus group at 3- and 6 months post-RT. A univariable logistic regression model, including all sociodemographic and clinical variables listed in , was used to calculate the odds ratio for having trismus at each time-point. The only significant results included being a smoker, which increased the risk of having trismus at 3- and 6 months post-RT by 2.21 and 2.28 times respectively (p = .024 and p = .018). This was not a significant factor at any other time-point. Having a tumor site in the oral cavity increased risk of having trismus at 36 months post-RT by 3.03 times compared to other tumor sites (p = .048). This was not a significant factor affecting trismus at any other time-point.
Change in MIO over time
At 3 months post-RT, 92% (n = 183) of patients had reduced MIO compared to baseline, whereas 3.5% (n = 7) improved. The corresponding figures for 12 months were 91% (n = 162) and 6% (n = 10), whilst at 60 months post-RT 88% (n = 113) reported reduced MIO and 7% (n = 9) had increased MIO when compared to baseline. MIO also changed between time-points where shows the percentages of patients that experience an improvement, deterioration or remain unchanged in MIO between each time-point. At 3- and 6 months post-RT 92% and 44% of patients respectively reported a deterioration compared to the previous time-point, which represented a statistically significant change. However, at 12 months post-RT, significantly more patients had improved MIO rather than deteriorated compared to the 6-month follow-up (45% vs 27%, p = .007). There were no statistically significant differences from preceding time-point after 12 months post-RT.
Figure 1. Percentage of patients with a change in MIO compared to the previous time-point. *a statistically significant change (p < 0.05) compared to the preceding time-point.
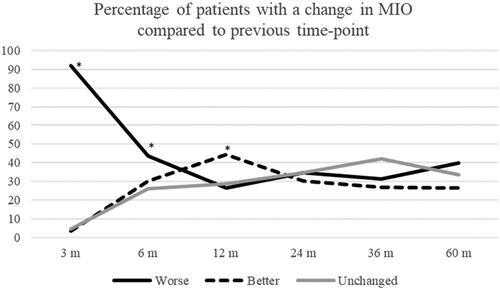
A total of 179 patients (85%) recorded a MIO of > 35 mm on at least one of the study time-points with the baseline time-point excluded. The remaining 15% of patients never exceeded an MIO of > 35 mm at any time-point. A total of 27% (n = 48) patients deteriorated during the 5-year study period and once again fulfilled the trismus criterion after having been free of trismus.
At the 12-month follow-up, 49 patients reported trismus. Of the 42 patients reporting trismus at 24 months post-RT, 74% (n = 31) also had trismus at the 12-month follow-up. Corresponding figures for 36 months post-RT and 60 months post-RT were 71% (n = 25) and 80% (n = 24) respectively.
Change in MIO over time when divided into having trismus or no trismus
When patients were divided into those having MIO ≤ 35 mm (trismus) and those with an MIO > 35 mm (no trismus), it was noted that the change (deterioration) in MIO was always significantly larger in the subgroup of patients with trismus compared to those without ().
Table 4. Change from baseline when divided into MIO ≤ 35 mm and > 35 mm.
Discussion
This study is the largest prospective 5-year study investigating the prevalence of trismus in an irradiated HNC population and the first to describe the development and changes of mouth opening ability in a long-term perspective.
Trismus is both a prevalent and long-term complication following treatment for HNC, which was seen as 27% of HNC patients suffered from trismus at 12 months – a figure that remained essentially unchanged at the study endpoint, i.e. 5-years post-RTs completion. Of those suffering from trismus at 24-, 36- and 60 months post-RT, 71 to 80% had also reported trismus at the 12-month follow-up. Hence, trismus was not a condition that resolved or healed spontaneously, probably due to underlying radiation fibrosis [Citation9]. This is further emphasized as 15% of the study population never reached an MIO > 35 mm post-RT during the entire study period. A recent systematic review and meta-analysis by Watters et al. [Citation10], encompassing 15 studies and 2786 patients, established a 32% prevalence of trismus both at 12 months and 10 years following radiotherapy. However, only 5 studies were included in the 3–10-year follow-up and these were severely hampered by flaws in study design. Kraaijenga, Oskam et al. [Citation11] reported that 55% of their 22 patients had trismus 10 years post-radiotherapy from cross-sectional data specifically regarding mouth opening in their otherwise prospective, longitudinal study with a small study population. At 6 years, in the prospective study by Kraaijenga, van der Molen et al. [Citation12], only 5% of patients reported trismus, yet again with a very small study sample (n = 22). In contrast, Kamstra et al. [Citation4] prospectively followed 641 HNC patients. However, due to a large dropout with only 59 patients remaining at 48 months, the authors decided to report trismus (37%) in the 105 patients that remained at 36 months. Finally, Karlsson et al. [Citation13] prospectively followed 100 HNC patients with trismus in an intervention study up to 3 years post-radiotherapy completion. The control group comprised half of the cohort and consisted of patients with post radiation trismus of which 47% still suffered from trismus at the study endpoint. Hence, this prospective study with a large remaining cohort at study endpoint now cements the fact that trismus remains a frequent complication in the irradiated HNC population.
Albeit results in this study indicate that trismus is a prevalent long-term complication, fluctuations in MIO were highlighted that have not in detail been described before, emphasizing that mouth opening ability is to some extent a dynamic process. The largest deterioration appeared to take place during the first six months following radiotherapy completion with MIO reductions of 9–10 mm equaling up to a 19% MIO reduction. Whilst the deteriorations from baseline to 3- and 6 months post-RT were statistically significant, no other fluctuations in MIO between time-points were. This early window of deterioration was also supported by Pauli et al, who concluded that the highest incidence of trismus at 38% was found at 6 months post-RT [Citation6]. When comparing fluctuations in MIO and the number of patients whose MIO changed between time-points, it was observed that approximately one third of patients improved, deteriorated or remained unchanged between each follow-up from baseline to 12 months – changes that were statistically significant. However, no significant fluctuations in MIO occurred after 12 months post-RT, emphasizing that the first year following RT-completion is the most dynamic. Additionally, a total of 27% of patients who were free of trismus deteriorated to again fulfil the trismus criterion during the 5-year period further emphasizing a certain degree of fluctuation in the trismus condition. What caused this fluctuation is unknown but may be attributed to the fact that some patients have performed jaw exercises albeit in an unstructured manner.
Additionally, it appears that patients with trismus suffered a significantly larger deterioration in MIO at each time-point when compared to baseline compared to those who did not have trismus. It could be hypothesized that once fibrosis is established, it progresses more rapidly. However, a subgroup of patients may also be genetically predisposed to developing fibrosis and trismus. Tumor growth factor beta (TGF-β) is one such gene which has been associated with increased risk of fibrosis in patients with HNC [Citation14,Citation15]. Further studies in the research group regarding genetic predisposition for trismus are ongoing.
Several factors in the literature have been associated with an increased risk of developing trismus, namely having a tumor site near masticatory muscles [Citation16–Citation19], increasing doses to the masseter and pterygoids [Citation18,Citation19] and receiving combined treatment in terms of chemoradiotherapy when compared to surgery alone [Citation17,Citation20]. The systematic review by Watters et al. found a 10-times increased risk of developing trismus if receiving chemoradiotherapy versus RT alone, albeit only based on two studies [Citation10]. In this study, 62% and 21% of patients received chemoradiotherapy and RT respectively and treatment modality was not found to be a factor influencing trismus, possibly due to the small sizes when subgrouped. Additional factors such as age, gender and smoking status have been discussed but conflicting evidence exist and no consensus has been reached [Citation17]. Although tumor size also falls into the latter category as some studies fail to find an association with trismus [Citation19,Citation20], larger systematic reviews do conclude that increasing T-size may in fact be a risk factor for trismus [Citation17]. Very few clinical and sociodemographic factors were found to influence trismus development in this study. Only at 36 months post-RT did a tumor site in the oral cavity show an increased risk (OR 3.03) of developing trismus, with significant findings lacking for other time-points or variables, such as tumor size or treatment type. This may be explained by the tumor sites in the study being homogenous in the sense that they all have an increased risk of causing trismus, whereas other studies also included HNC-sites less likely to contribute to trismus development including laryngeal and hypopharyngeal tumors.
The strengths in this study lie in its long-term prospective design, with the largest number of patients still enrolled in a trismus study of HNC patients at 5 years following radiotherapy. It is however still limited by the number of drop-out at the 5-year follow-up as we do not have data for those alive but not enrolled in the study.
Clinical implications
Trismus is now an established frequent and long-term complication following radiotherapy for HNC that does not heal spontaneously and can vary over time, with most fluctuation seen in the first 6 months post-RT. It is therefore of outmost importance to offer rehabilitation in the form of jaw exercise training early and in a structured manner, which has been shown to aid in increasing MIO [Citation13] and should be offered to all patients suffering from trismus. An on-going study in the authors’ research group is now investigating if exercise training should in fact be offered prophylactically, i.e. before trismus develops.
Conclusions
Trismus is a prevalent long-term complication of HNC and its treatment, which does not appear to heal spontaneously. Most fluctuations in MIO, both deteriorations and improvements, occur during the first 12 months following radiotherapy completion.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Yarnold J, Brotons MC. Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol. 2010;97(1):149–161.
- Dijkstra PU, Huisman PM, Roodenburg JL. Criteria for trismus in head and neck oncology. Int J Oral Maxillofac Surg. 2006;35(4):337–342.
- Kamstra JI, Dijkstra PU, van Leeuwen M, et al. Mouth opening in patients irradiated for head and neck cancer: a prospective repeated measures study. Oral Oncol. 2015;51(5):548–555.
- Johnson J, Johansson M, Rydén A, et al. Impact of trismus on health-related quality of life and mental health. Head Neck. 2015;37(11):1672–1679.
- Pauli N, Johnson J, Finizia C, et al. The incidence of trismus and long-term impact on health-related quality of life in patients with head and neck cancer. Acta Oncol. 2013;52(6):1137–1145.
- Väst RC. Tillämpning av Nationellt vårdprogram för Huvud- och halscancer. 2019.
- Pauli N, Fagerberg-Mohlin B, Andréll P, et al. Exercise intervention for the treatment of trismus in head and neck cancer. Acta Oncol. 2014;53(4):502–509.
- Bhatia KS, King AD, Paunipagar BK, et al. MRI findings in patients with severe trismus following radiotherapy for nasopharyngeal carcinoma. Eur Radiol. 2009;19(11):2586–2593.
- Watters AL, Cope S, Keller MN, et al. Prevalence of trismus in patients with head and neck cancer: a systematic review with Meta-analysis. Head Neck. 2019;41(9):3408–3421.
- Kraaijenga SA, Oskam IM, van der Molen L, et al. Evaluation of long term (10-years+) dysphagia and trismus in patients treated with concurrent chemo-radiotherapy for advanced head and neck cancer. Oral Oncol. 2015;51(8):787–794.
- Kraaijenga SA, van der Molen L, Jacobi I, et al. Prospective clinical study on long-term swallowing function and voice quality in advanced head and neck cancer patients treated with concurrent chemoradiotherapy and preventive swallowing exercises. Eur Arch Otorhinolaryngol. 2015;272(11):3521–3531.
- Karlsson O, Karlsson T, Pauli N, et al. Jaw exercise therapy for the treatment of trismus in head and neck cancer: a prospective three-year follow-up study. Support Care Cancer. 2021;29(7):3793–3800.
- Ghazali N, Shaw RJ, Rogers SN, et al. Genomic determinants of normal tissue toxicity after radiotherapy for head and neck malignancy: a systematic review. Oral Oncol. 2012;48(11):1090–1100.
- Lyons AJ, Crichton S, Pezier T. Trismus following radiotherapy to the head and neck is likely to have distinct genotype dependent cause. Oral Oncol. 2013;49(9):932–936.
- van der Geer SJ, van Rijn PV, Kamstra JI, et al. Prevalence and prediction of trismus in patients with head and neck cancer: a cross-sectional study. Head Neck. 2019;41(1):64–71.
- van der Geer SJ, van Rijn PV, Roodenburg JLN, et al. Prognostic factors associated with a restricted mouth opening (trismus) in patients with head and neck cancer: Systematic review. Head Neck. 2020;42(9):2696–2721.
- Strojan P, Hutcheson KA, Eisbruch A, et al. Treatment of late sequelae after radiotherapy for head and neck cancer. Cancer Treat Rev. 2017;59:79–92.
- Lindblom U, Gärskog O, Kjellén E, et al. Radiation-induced trismus in the ARTSCAN head and neck trial. Acta Oncol. 2014;53(5):620–627.
- Steiner F, Evans J, Marsh R, et al. Mouth opening and trismus in patients undergoing curative treatment for head and neck cancer. Int J Oral Maxillofac Surg. 2015;44(3):292–296.
