Abstract
Background
Many studies have discussed the factors influencing hearing outcomes after cochlear implantation, but few have addressed improvements in speech perception for these patients over time.
Objective
To investigate the relationship between preoperative factors and the pattern of longitudinal improvement in speech perception following cochlear implantation (CI).
Materials and methods
This study enrolled 83 patients (96 ears) who underwent CI at Shinshu University Hospital. The patients were assessed up to 12 months after CI by a monosyllable test, and showed either delayed improvement (DI), early improvement (EI), or stable improvement (SI) when compared with their preoperative score. Eight preoperative variables were also examined for their effects on speech perception over time.
Results
The DI, EI, SI groups comprised 35.4%, 43.8%, and 20.8% of all patients, respectively. Patients in the DI group were older at surgery than those in the EI and SI groups, and their onset age were also older than that in the SI group. No other preoperative variables showed significant differences across the three groups.
Conclusions and significance
Our findings revealed that age at implantation and age at onset of hearing loss significantly affected the improvement pattern of speech perception. Age may be useful in predicting recovery of speech perception after CI.
Chinese Abstract
背景:多项研究讨论了影响人工耳蜗植入术后听力结果的因素, 但很少有人解决过这些患者言语感知的改善。
目的:探讨术前因素与人工耳蜗植入 (CI) 后言语感知的纵向改善模式之间的关系。
材料和方法:本研究纳入了在信州大学医院接受 CI 的 83 名患者(96只耳)。 患者在 CI 后 12 个月内通过单音节测试得到评估, 与他们的术前评分相比, 表现出延迟改善 (DI)、早期改善 (EI) 或稳定改善 (SI)。 还检查了八个术前变量对言语感知的影响。
结果:DI组、EI组、SI 组分别占所有患者的 35.4%、43.8% 和 20.8%。在手术时, DI 组患者比 EI组 和 SI 组年龄大, 并且他们的发病年龄也比 SI 组大。 三组之间没有其它术前变量表现出显著差异。
结论和意义:我们的研究结果表明, 植入年龄和听力损失发病年龄显著影响言语感知的改善模式。 年龄可能用于预测 CI 后言语感知的恢复。
Introduction
Cochlear implantation (CI) is a standard treatment for improving the speech perception of patients with congenital and late-onset hearing loss. CI effectiveness for congenital hearing loss is well established, and the earlier the surgery is performed, the better the postoperative outcome [Citation1]. However, the postoperative outcome of late-onset hearing loss varies highly from case to case, and recovery is often partial because the background factors vary greatly from patient to patient [Citation2]. Factors influencing postoperative hearing outcome in CI patients with late-onset hearing loss have been reported to include age at surgery [Citation3], duration of hearing loss [Citation2], residual hearing [Citation4], and cause of hearing loss [Citation5].
Many studies have discussed the factors influencing hearing outcomes after CI, but few have discussed how patients’ speech perception improves after surgery over time. Both physicians and patients could benefit from a method of predicting the likelihood of either significant improvement in speech perception in the early postoperative period or a gradual improvement over a longer period of nearly 1 year. Therefore, we aimed to investigate the relationship between patients’ preoperative factors and the longitudinal improvement pattern of speech perception following CI. In this study, we followed the postoperative results of CI patients over time (3, 6, and 12 months after surgery) and compared three groups of patients based on their postoperative speech perception improvement. Additionally, we investigated the preoperative patient factors responsible for the differences in postoperative speech perception.
Materials and methods
This study was approved by the Shinshu University School of Medicine ethics committee (Approval number: 5572). The study included 96 ears from 83 patients who underwent CI at Shinshu University Hospital between May 2011 and September 2020. Inclusion criteria involved patients: (1) with late-onset hearing loss at ≥ 6 years, (2) ≥ 20 years at the time of surgery, and (3) who were evaluated for speech perception before and after surgery for up to 12 months (). The causes of hearing loss in these patients were genetic mutation in 7%, otosclerosis in 3%, syndrome in 2%, bilateral sudden hearing loss in 2%, chronic otitis media, meningitis, and auditory nerve tumor in 1% each, and the remaining 83% were of unknown cause. In all cases, the CI electrode array was inserted using the round window approach.
Table 1. The preoperative factors for participants in this study.
The speech perception performance was evaluated using the monosyllable test, which employs 60 Japanese alphabetical sounds. In quiet, the sound pressure was calibrated to a 65-decibel level at 1 m in front of the centre of the speaker. The percent of correct responses was calculated.
Patients were classified into three groups based on their postoperative monosyllable test scores over time as described below:
Delayed improvement (DI): the improvement in the monosyllable score at 12 months postoperatively was less than 15% from the 3-months score, and the score at 12 months was under 60%
Early improvement (EI): the improvement in the monosyllable score at 12 months postoperatively was less than 15% from the 3-months score, and the score at 12 months was over 60%
Stable improvement (SI): the improvement in the monosyllable score was more than 15% from the 3-months score to the 12-months score
The following preoperative patient factors were considered: (1) sex, (2) side of implantation, (3) age at surgery, (4) age at onset of hearing loss, (5) duration from when the patient became aware of hearing loss until surgery, (6) preoperative pure-tone audiometric thresholds (average of 0.5, 1, 2 and 4 kHz), (7) preoperative aided thresholds (average of 0.5, 1, 2 and 4 kHz), and (8) preoperative monosyllable test score. We compared each factor among the three groups for significant differences. The Chi-square test was used for (1) and (2); and analysis of variance was used for (3) to (8). Statistical significance was set at p < .05.
In addition, the average monosyllable scores at 3, 6, 12, and 24 months were obtained postoperatively because 59 of the 96 patients had monosyllable results at 24 months postoperative.
Results
Monosyllable score at 3, 6, 12, and 24 months after CI
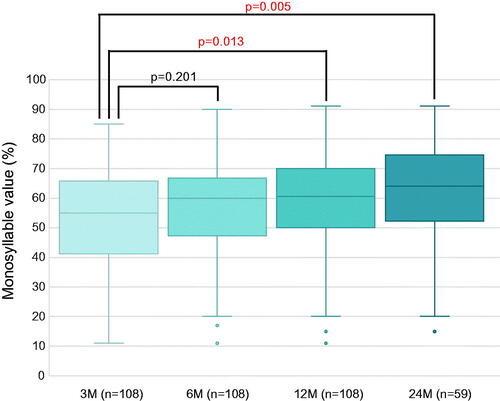
The postoperative monosyllable test score average was 55% correct (41.75–65.25) (median [first quartile–third quartile]) at 3 months, and 60% correct (47.75–66.25), 60.5% (50–70), and 64% (52.75–73.5) at 6, 12, and 24 months, respectively. Significant differences were observed between scores at 3 and 12 months and at 3 and 24 months postoperatively ().
Figure 1. Monosyllable score at 3, 6, 12, and 24 months after cochlear implantation for all patients. The median monosyllable score increased gradually at 3, 6, 12, and 24 months postoperatively. However, significant differences were observed only between scores at 3 and 12 months and between 3 and 24 months, suggesting a gradual improvement in speech perception over time.
Relationship between postoperative monosyllable score improvement and preoperative patient factors
The relationship between the monosyllable score at 12 months postoperatively and each preoperative patient factor except sex and side of implantation was investigated, but no correlations were observed for these factors (Supplementary Figure S1). Subsequently, we divided the patients into three groups based on their postoperative monosyllable score. The DI, EI, and SI groups accounted for 35.4% (34/96), 43.8% (42/96), and 20.8% (20/96), respectively. shows the score for each group preoperatively and at 3, 6, and 12 months postoperatively. The median score at 12 months postoperatively for each group (DI, EI, and SI) was 49%, 68%, and 64.5%, respectively. When postoperative monosyllable scores were compared by preoperative factors, no significant differences were observed among groups for implantation side, duration of hearing loss, preoperative pure-tone audiometric thresholds, or preoperative aided thresholds ().
Figure 2. Monosyllable score at preoperative, 3, 6, and 12 months postoperatively for each group. Bold lines indicate the mean score for each group (delayed improvement, early improvement, and stable improvement).
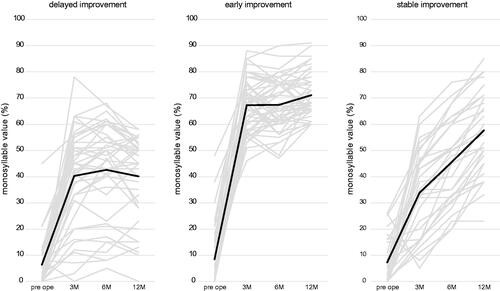
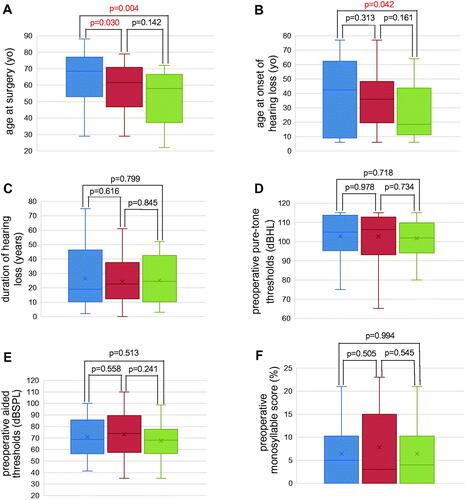
Figure 3. Relationship between postoperative monosyllable score improvement and preoperative patient factors. (A) Age at surgery for each group. The delayed improvement group was the oldest among the three groups, and the stable improvement group was the youngest. The delayed improvement group was significantly older than the early and the stable improvement groups. There was no statistically significant difference between the early and the stable improvement group, but the early improvement group tended to be older (Blue bar indicates DI group, red bar indicates EI group, and green bar indicates SI group). (B) Age of onset of hearing loss for each group. The stable improvement group was the youngest among the three groups, with the median age of onset of hearing loss of stable improvement in the teens, which is very young compared to the other two groups (delayed improvement and early improvement). (C) Duration of hearing loss for each group. (D) Preoperative pure-tone audiometric thresholds for each group. (E) Preoperative aided thresholds for each group. (F) Preoperative monosyllable score for each group. No significant differences were observed among groups for duration of hearing loss, preoperative pure-tone audiometric thresholds, or preoperative aided thresholds.
Sex
Twelve of the 34 (35.3%) patients were males and 22 (64.7%) were females in the DI group. Of the 42 patients in the EI group, 22 (52.4%) were male and 20 (47.6%) were female. Of the 20 patients in the SI group, 5 (25.0%) were male and 15 (75.0%) were female. The SI group had a significantly higher percentage of females than did the EI group (p = .042), but there was no significant difference by sex between the DI and EI groups or the DI and SI groups (p = .136 and p = .432, respectively).
Age at surgery
The median age at surgery for all patients in this study was 62.5 years (50–73.25); median ages at surgery for the DI, EI, and SI groups were 68.5 years (53.5–76.75), 61.5 years (47.5–69.5), and 58 years (41.75–65.5), respectively. Patients in the DI group were significantly older than those in the EI and SI groups (p = .030, p = .004, respectively). There was no significant difference between age at surgery for the EI and SI groups, but the EI group tended to be older (p = .142, ).
Age at onset of hearing loss
The median age at onset of hearing loss for all patients in this study was 35.5 years (14.5–53.25). The median ages at onset of hearing loss for the DI, EI, and SI groups were 42.5 years (11.75–62), 36 years (20–47.25), and 18.5 years (11.75–41.25), respectively. Patients in the DI group were significantly older at the onset of hearing loss than those in the SI group (p = .042, ), but no significant differences existed between the DI and EI groups or between the EI and SI groups (p = .313, p = .161).
Discussion
CI is effective in improving speech recognition in patients with severe to profound hearing loss. However, in general, it takes several months after CI surgery for the patient to gradually come to recognize speech. Several factors (including age at surgery, duration of hearing loss, residual hearing and cause of hearing loss) influencing postoperative hearing outcome in CI patients have been reported [Citation2–5]. However, details of the effects of these factors on speech perception are still unclear. Therefore, predicting the degree of postoperative improvement in speech perception based on preoperative factors would be extremely useful for patients and physicians. We investigated the longitudinal changes in postoperative speech perception in 96 ears of 83 patients who had undergone CI, and the preoperative patient factors involved in the improvement pattern.
This study revealed three patterns of improvement in speech perception after cochlear implantation. First, patients whose speech perception ability reached a plateau 3 months after surgery were divided into two groups: those with relatively minor improvement (DI group) and those with major improvement (EI group). However, the third group improved throughout the first postoperative year. This pattern of SI in speech perception after CI has not yet been reported. Many previous reports have stated that postoperative speech perception reaches a plateau at 3 to 6 months [Citation6–8]. Significantly, approximately 20% of the patients in the present study were in this SI group.
The median monosyllable score in the EI group was 68% at 12 months postoperatively. Previous linguistic studies have reported scores after 12 months from CI of approximately 60% [Citation9–10]. This score probably represents the technical limit of CI. The monosyllable score of approximately 60% in the EI group in this study is consistent with previous reports. It is not surprising that this group, which was in the middle of the age range compared to the other two groups, had similar results to previous studies.
We found that the median monosyllable score increased gradually at 3, 6, 12, and 24 months postoperatively, from 55% correct to 60%, 60.5%, and 64% correct, respectively. Significant differences were observed only between scores at 3 and 12 months and between 3 and 24 months, suggesting a gradual improvement in speech perception over time. Many studies have focused on the outcome of surgery at 1 year postoperatively [Citation11,Citation12], yet few articles describe how speech-language performance changes at other long-term follow-up points, such as 3 months, 6 months, or 2 years postoperatively. Chatelin et al. [Citation6] reported that the maximum improvement was achieved 3 months after CI in young and elderly patients. Other reports have demonstrated that in post-lingual patients undergoing CI, significant improvement in patients’ speech perception occurred during the first 6 months postoperatively [Citation7,Citation8]. However, our findings indicated that speech perception gradually improves at least until 24 months postoperatively. This result is consistent with a report by Dillon et al. [Citation13], who followed CI patients for a decade and noted that speech perception improved significantly between 1 and 5 years postoperatively.
Why do patients’ improvement in speech perception differ after CI surgery? Previous studies have investigated patient factors influencing speech-language performance after CI [Citation2–5], but none have reported a pattern of longitudinal improvement in postoperative speech-language performance. We offer several explanations for this variation in results. First, the age at surgery was significantly higher in the DI group than in the other two groups. This suggests that improved speech perception occurs early in the postoperative period but not significantly in elderly patients. Previous reports have indicated that the results of CI in elderly patients are inferior to those in younger patients, and this is consistent with our findings. This may be due to a decrease in spiral ganglion cells [Citation14], age-related impairment of central auditory and integrative pathways (central presbycusis) [Citation15], and cognitive decline [Citation13], which are consistent with our findings. However, our results do not contraindicate CI in the elderly. The preoperative monosyllable score was nearly zero, but the median monosyllable score at 12 months postoperatively was 49%, which represents a marked improvement. We believe that CI is worthwhile even in elderly patients. Second, we focus on the SI group. This group was significantly younger than the DI group and tended to be younger than the EI group. This means that patients who underwent CI at a young age did not improve significantly in their speech perception in the early postoperative period, but gradually improved over time, up to 1 year after the surgery. We hypothesized that this minor improvement in the early postoperative period could be due to compensatory habituation to normal speech. In this study, the age of onset of hearing loss tended to be younger in the SI group than in the other two groups, with a median age in the teens. In contrast, the median age of onset of hearing loss in the other two groups was above 30 years. Children with bilateral mild hearing loss and their parents are usually unaware of their hearing loss, leading to delays in detection and a distorted perception of learned speech and language. The SI group experienced normal hearing for a shorter time, and therefore had less time to learn normal sounds than the other two groups, so the speech they originally perceived as correct may have been incorrectly pronounced or otherwise distorted. Therefore, the monosyllable score was poor because the normal speech they heard in the early postoperative period after CI differed from the speech they remembered from before the onset of hearing loss. However, they were able to recognize the correct speech after long-term training. Furthermore, we speculated that ‘cross-modal plasticity,’ the extent to which other sensory processing sites replaced the auditory cortex in patients who underwent CI, was associated with improvement in speech perception in the young patients. The degree to which the auditory cortex is replaced by other sensory information processing is important for predicting CI effectiveness. This form of reorganization may adversely affect auditory rehabilitation after CI [Citation16]. In this study, the speech perception of young patients did not improve significantly in the early postoperative period, but it improved gradually up to 1 year postoperatively. CI could reverse this cross-modal deprivation of the auditory cortex by other senses. Previously, it was thought that cross-modal plasticity in the brain cortex could not be reversed. However, recent reports indicate that this is inaccurate. For example, patients with age-related hearing loss who used a hearing aid for 6 months showed improved visual cross-modal reorganization in the auditory cortex [Citation17]. It has also been reported that preoperative visual cross-modal reorganization improved with CI in cases of single-sided deafness [Citation18]. In this study, it is possible that younger patients showed less improvement in speech perception in the early postoperative period due to the persistent effects of cross-modal plasticity. When these were gradually reversed, speech perception improved. Early in the postoperative period, the older patients reached a plateau and their speech perception did not improve significantly, likely because of insufficient reversal of cross-modal plasticity. To our best knowledge, no current studies have examined the likelihood of cross-modal plasticity or its reversal in young and elderly participants, so we can only speculate on this connection.
In conclusion, we found that the age at implantation and that at the onset of hearing loss significantly affect the longitudinal improvement pattern of speech perception. Our findings can serve as feasible information for follow-up of CI patients. Future studies are required to determine the links between CI and cross-modal plasticity across age groups.
Supplemental Material
Download PDF (158.9 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Black J, Hickson L, Black B, et al. Prognostic indicators in paediatric cochlear implant surgery: a systematic literature review. Cochlear Implants Int. 2011;12(2):67–93.
- Blamey P, Artieres F, Başkent D, et al. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants: an update with 2251 patients. Audiol Neurootol. 2013;18(1):36–47.
- Lazard DS, Vincent C, Venail F, et al. Pre-, per- and postoperative factors affecting performance of postlinguistically deaf adults using cochlear implants: a new conceptual model over time. PLoS One. 2012;7(11):e48739.
- Li J, Ji F, Chen W, et al. Analysis of the performance of post-lingually deafened patients with nurotron® VenusTM cochlear implants. Acta Otolaryngol. 2014;134(6):609–614.
- Miyagawa M, Nishio SY, Usami SI. A comprehensive study on the etiology of patients receiving cochlear implantation with special emphasis on genetic epidemiology. Otol Neurotol. 2016;37(2):e126-134–e134.
- Chatelin V, Kim EJ, Driscoll C, et al. Cochlear implant outcomes in the elderly. Otol Neurotol. 2004;25(3):298–301.
- Ahmad FI, Demason CE, Teagle HFB, et al. Cochlear implantation in children with postlingual hearing loss. Laryngoscope. 2012;122(8):1852–1857.
- Fryauf-Bertschy H, Tyler RS, Kelsay DM, et al. Performance over time of congenitally deaf and postlingually deafened children using a multichannel cochlear implant. J Speech Hear Res. 1992;35(4):913–920.
- Sladen DP, Gifford RH, Haynes D, et al. Evaluation of a revised indication for determining adult cochlear implant candidacy. Laryngoscope. 2017;127(10):2368–2374.
- Krueger B, Joseph G, Rost U, et al. Performance groups in adult cochlear implant users: speech perception results from 1984 until today. Otol Neurotol. 2008;29(4):509–512.
- Ramakers GGJ, Smulders YE, van Zon A, et al. Correlation between subjective and objective hearing tests after unilateral and bilateral cochlear implantation. BMC Ear Nose Throat Disord. 2017;17:10.
- Pulsifer MB, Salorio CF, Niparko JK. Developmental, audiological, and speech perception functioning in children after cochlear implant surgery. Arch Pediatr Adolesc Med. 2003;157(6):552–558.
- Dillon MT, Buss E, Adunka MC, et al. Long-term speech perception in elderly cochlear implant users. JAMA Otolaryngol Head Neck Surg. 2013;139(3):279–283.
- Nadol JB.Jr. Electron microscopic findings in presbycusic degeneration of the basal turn of the human cochlea. Otolaryngol Head Neck Surg (1979). 1979;87(6):818–836.
- Welsh LW, Welsh JJ, Healy MP. Central presbycusis. Laryngoscope. 1985;95(2):128–136.
- Sandmann P, Dillier N, Eichele T, et al. Visual activation of auditory cortex reflects maladaptive plasticity in cochlear implant users. Brain. 2012;135(Pt 2):555–568.
- Glick HA, Sharma A. Cortical neuroplasticity and cognitive function in early-stage, mild-moderate hearing loss: evidence of neurocognitive benefit from hearing aid use. Front Neurosci. 2020;14:93.
- Sharma A, Glick H. Cross-modal re-organization in clinical populations with hearing loss. Brain Sci. 2016;6(1):4.
