Abstract
Background and Objective
‘Hearing loss’ has been reported as a clinical atypical symptom in some COVID-19 patients. We searched and collated the existing literature for a systematic review and meta-analysis to assess the prevalence of hearing loss during the COVID-19 epidemic.
Methods
An exhaustive search of the PubMed, Embase, Web of Science, China National Knowledge Infrastructure and other sources from the inception of the database until 31st December 2022. The Search terms were set to: ‘COVID-19’, ‘SARS-CoV-2’, ‘2019-nCoV’, ‘hearing impairment’, ‘hearing loss’, ‘auditory dysfunction’. The literature data meeting the inclusion criteria were extracted and analyzed. Prevalence was pooled from individual studies using a randomized effects meta-analysis.
Results
A total of 22 studies were included in the final analysis, involving 14281 patients with COVID-19 infection, of which 482 patients had varying degrees of hearing loss. Our final meta-analysis demonstrated that the prevalence of hearing loss in COVID-19-positive patients was 8.2% (95%CI 5.0–12.1). Subgroup analysis of age showed that the prevalence of middle-aged and older patients aged 50-60 and over 60 years was 20.6% and 14.8%, respectively, which was significantly higher than that of patients aged 30–40 (4.9%) and 40–50 years (6.0%).
Conclusion
Hearing loss is one of the clinical symptoms of COVID-19 infection, compared with other diseases, it is less likely to attract the attention of clinical experts or researchers. Raising awareness of this disease can not only enable early diagnosis and treatment of hearing loss, and improve the quality of life of patients, but also enhance our vigilance against virus transmission, which has important clinical and practical significance.
Chinese Abstract
背景和目的:据报道, “听力损失”是一些 COVID-19 患者的一种临床非典型症状。 我们检索并整理了现有文献, 进行系统回顾和荟萃分析, 以评估 COVID-19 流行期间听力损失的患病率。
方法:详尽检索PubMed、Embase、Web of Science、中国知网和其它来源从数据库创建到 2022 年 12 月 31 日的数据。搜索词设置为:“COVID-19”、“SARS-CoV-2”、“2019-nCoV”、“听力障碍”、“听力
损失”, “听觉功能障碍”。 提取并分析了符合纳入标准的文献资料。 使用随机效应荟萃分析从各个研究项目中汇总了患病率。
结果:共纳入22项研究进行最终分析, 涉及14281例COVID-19感染患者。其中482名患者有不同程度的听力损失。 我们的最终荟萃分析表明, COVID-19 阳性患者听力损失的患病率是8.2% (95%CI 5.0–12.1)。 年龄亚组分析显示, 中年和50-60 岁及 60 岁以上的较老年患者的患病率分别为 20.6% 和 14.8%, 显著高于 30-40 岁(4.9%)和 40-50 岁(6.0%)的患者。
结论:与其他疾病相比, 听力损失是 COVID-19 感染的临床症状之一, 不太可能引起临床专家或研究人员的注意。 提高对本病的认识, 不仅可以使听力损失早诊早治, 提高患者的生活质量, 同时也增强我们对病毒传播的警惕性, 这对临床和实践具有重要意义。
Keywords:
Introduction
The novel coronavirus (COVID-19) was first found to be associated with severe human respiratory disease in Wuhan, Hubei Province, China, on 31 December 2019. On 30 January 2020, the World Health Organization (WHO) officially declared the novel coronavirus pneumonia caused by SARS-CoV-2 as a public health emergency of international concern [Citation1]. Coronaviruses are single-stranded enveloped RNA viruses with a diameter of 60–140 nm, which can cause influenza-like symptoms when they enter the human body [Citation2]. The virus is spread primarily from person to person through droplet transmission and direct contact with the oral and nasal mucosa of 2019-nCoV-positive patients [Citation3]. Studies have shown that SARS-CoV-2 can be transmitted through aerosols as well [Citation4]. Clinically, secretion samples of the upper respiratory tract are mainly collected through nasopharyngeal or oropharyngeal swabs for PCR diagnosis of this disease [Citation1]. 2019-nCoV infection may be asymptomatic or cause different degrees of clinical manifestations. According to clinical reports in Asia, the most common symptoms are cough, fever, sputum, sore throat, headache, joint pain, muscle pain, diarrhea, dyspnoea, etc. [Citation5]. In severe cases, pneumonia and various systemic diseases may be complicated and lead to death.
According to existing research reports, SARS-CoV-2 infection can not only lead to extensive extrapulmonary and various sensory complications, such as reduced sense of smell and taste disorders [Citation6] but also long-term neurological complications, such as tinnitus, hearing loss, vertigo, etc. [Citation7–9]. Studies conducted by Francesco Frenia et al. showed that the appearance of the above symptomatology may be related to the neurotropism of the virus [Citation10].
There are a lot of definitions of hearing loss across different countries and organizations. In this review, hearing loss was defined according to the average pure tone hearing threshold values at 500, 1000, and 2000 Hz, and the classification of hearing loss grades followed the standards of the World Health Organization [Citation11]. Hearing impairment is an atypical symptom of COVID-19 infection. Compared with other accompanying symptoms, hearing loss is not easy to attract the attention of patients or clinicians. However, with the spread of the epidemic and the growth of the sustained cycle, the prevalence of hearing loss in 2019-nCoV-positive patients has been reported in the relevant literature abroad.
Hearing is a significant sensorial function of the body. Studies have shown that viruses can cause hearing loss by directly damaging inner ear structures or by inducing host immune-mediated damage [Citation12]. COVID-19-related hearing loss has a significant impact on individuals and society. We searched the published literature on hearing loss related to COVID-19, conducted a systematic review and meta-analysis to assess the prevalence of hearing loss in COVID-19, and inform the clinical practice of hearing loss related to COVID-19 during the pandemic.
Materials and Methods
This systematic review and meta-analysis were designed, conducted and reported according to the preferred reporting items of the Systematic Review and Meta-Analysis (PRISMA) Guidelines and the Cochrane collaboration handbook. This study is a systematic review and meta-analysis, and does not involve patients’ privacy, so it has not been registered in relevant institutions for ethics.
Search strategy
A systematic search of the PUBMED, Embase, Web of Science, China National Knowledge Infrastructure, and grey literature databases from other sources. The articles included are published from the inception of each database up to 31 December 2022. The author (Tang) created an initial search strategy using the terms: [(COVID-19 or SARS-CoV-2 or 2019-nCoV) and (hearing loss or hearing impairment or auditory dysfunction)]. There is no language restriction, in order to conduct a comprehensive search of the data, references to relevant studies were also reviewed.
Eligibility criteria and study selection
Studies were included if the following criteria were met: (1) Articles that report on the prevalence of hearing loss or the number of people with hearing loss in patients with COVID-19; (2) Patients with mild-to-moderate COVID-19 infection and moderate-to-severe sensorineural hearing loss require outpatient follow-up or hospitalization. The following conditions were excluded: (1) duplicate publications or articles with incomplete data; (2) animal or laboratory studies; (3) protocols, guidelines, editorials, notes, conference papers, comments, surveys, questionnaires, and letters to the editor; (4) articles that out of scope.
Methods for data extraction and quality assessment
Data extraction was divided into three steps: title, abstract and full-text screening. After the first and second screening stages, the full text of the remaining articles was read to exclude articles that did not meet the inclusion criteria. The screening stage was conducted independently by two evaluators (Tang Mi and Wang Jie), and disagreements were resolved through consensus. If they cannot reach the same decision, the third reviewer (Zhang Qinxiu) would intervene in arbitration. In this screening process, there is no disagreement.
The quality of all studies included in this article was independently assessed by two reviewers (Tang Mi and Wang Jie) using the Combie checklist for the cross-sectional study. The evaluation tool consists of seven items, and the answer to each item includes yes, no, and not clear, with a response of ‘yes’ scoring 1 point, ‘no’ scoring 0 points, and ‘not clear’ scoring 0.5 points. The total score of this scale is 7 points, and the quality grade is divided into three levels: A, B, and C, with the score being 6–7 points, 4–5.5 points, and less than 4 points. The extracted content of literature information included the first author, year of publication, region, sample size, sex, age, and reported prevalence of hearing loss. Any disagreements arising from this process were negotiated with the third reviewer (Zhang Qinxiu).
Data analysis
The software Endnote X9 was used to screen qualified literature, Excel 2017 was used to extract relevant data, and stata12.0 was used for data analysis. For the assessment of heterogeneity, we evaluated studies using the I2 statistic. The values of I2 statistics such as 75, 50 and 25%, represented high, medium and low heterogeneity respectively. A random effects model was used when I2 >50% meant greater heterogeneity between studies. Egger’s test was used to evaluate publication bias.
Results
Study characteristics
According to the previously mentioned search strategy, 22 studies were finally included for further analysis, and the flow diagram of the study selection process is shown in . A total of 14281 patients were involved in 22 studies conducted in different countries. Detailed characteristics of the studies are listed in . The included studies were from North Carolina (n = 45), Messina (n = 50), Réunion Island (n = 693), Southern Italy (n = 128), Turkey (n = 3383), Turkey (n = 155), Italy (n = 48), Nigeria (n = 92), Iraq (n = 4850), Finnish (n = 333), Jammu (n = 286), Denmark (n = 225), 10 studies did not report study area.
Table 1. A summary of the characteristics of the studies included in the systematic review and meta-analysis.
Assessment of quality and publication bias
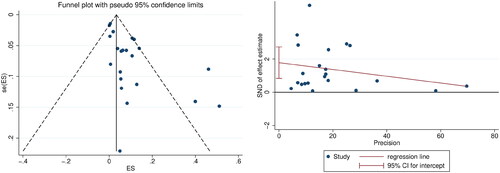
According to the results of the Combie Cross-sectional Research Evaluation Tool, there were 12 studies with Grade A and 10 with Grade B quality, the specific results were shown in . Visual assessment based on funnel plots and Egger’s regression analysis, significant publication bias based on the prevalence of ‘hearing loss’ between studies was noted (p =.001, p<.05) ().
Table 2. Quality of the included studies.
Prevalence of hearing loss in COVID-19 patients
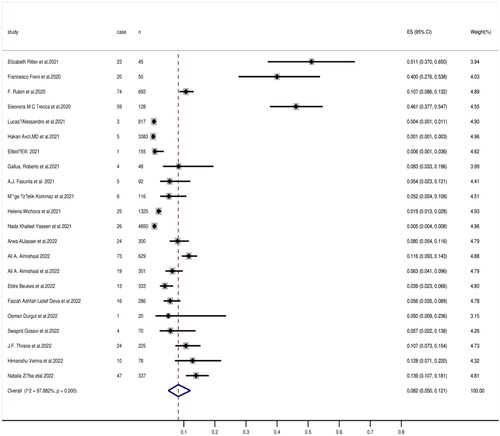
A total of 14281 patients were included for evaluation of hearing loss. Of these, 482 patients reported some degree of hearing dysfunction. The prevalence of hearing loss reported in individual studies ranges from 0.1% to 51.1%. The random effects model was used to conduct a meta-analysis of 22 studies included in this review, and the results showed that the prevalence of hearing loss was 8.2% (95% CI, 5.0–12.1) among 14281 COVID-19 patients. Heterogeneity was detected with I2 being 97.882% (p = .000), confirming the use of a random effects model ().
Subgroup analysis
In this review, the age subgroup analysis of patients with COVID-19 showed that the prevalence of hearing loss in the 30–40, 40–50, 50–60, and greater than or equal to 60- years-old groups was 4.9%, 6.0%, 20.6%, and 14.8%, respectively ().
Discussion
The main objective of this study was to assess the prevalence of hearing loss in COVID-19-positive patients. This review included 22 studies conducted in different countries. In addition to some included studies that did not report observation regions, other studies were conducted in Western countries such as Italy and Nigeria, and there are relatively few domestic studies and reports on this aspect. All included studies reported gender, age, number of patients with the disease, or directly reported prevalence.
Our meta-analysis using a random-effects model showed that the overall prevalence of hearing loss among COVID-19-positive patients from different countries was 8.2% (95% CI, 5.0–12.1). According to long-term clinical observation, the occurrence of hearing impairment is not only caused by various pathological factors, trauma, etc. but also related to many own factors such as the patient’s age. Combined with the physiological condition of the human body and the process of hearing generation and decline, it can be concluded that the general rule, that is, the older the age, the higher the incidence of hearing impairment may be. There is also relevant literature showing that age-related hearing loss is a common disease. Population studies have estimated the prevalence of severe hearing lossin those aged 60–80 years to be 21-27% [Citation13,Citation14]. The results of epidemiological studies conducted by Yueh et al. showed that the prevalence of presbyaculis or age-related sensorineural hearing loss increases with age, from 40-–66% of people aged over 75 years to more than 80% of people over the age of 85 [Citation15]. Therefore, the age of all patients included in the study was analyzed in this review. The results showed that the prevalence of middle-aged and older patients aged 50–60 years and ≥60 years was 20.6% and 14.8%, respectively, which was significantly higher than that of patients aged 30–40 years (4.9%) and 40–50 years (6.0%). Therefore, it can be speculated that age may be one of the influencing factors among the many causes of hearing loss associated with COVID-19 infection. However, so far, there is no scientific evidence to clearly show that the occurrence of hearing loss in patients with COVID-19 infection is related to age. We need to conduct more systematic clinical or basic studies with sufficient sample size to compare the prevalence of hearing loss between 2019-nCoV-positive patients and the general population to observe whether age affects the occurrence of hearing loss.
A growing body of evidence indicates that patients with COVID-19 are at risk of developing hearing loss. However, the pathogenesis of COVID-19-related hearing loss is unclear. According to previous reports, the pathogenesis of hearing loss affected by viruses includes the following: (1) inflammation and oxidative stress mechanisms. Studies have shown that inflammation and oxidative stress can be activated simultaneously under various pathological conditions, including 2019-nCoV-infection [Citation16]. At the same time, it has been pointed out that they can induce acute and chronic inflammation in the occurrence of sudden idiopathic hearing loss, which can be inferred that they may cause indirect damage to the inner ear function of 2019-nCoV-positive patients [Citation17]. (2) the mechanism of brainstem injury. If the SARS-CoV-2 invades and triggers the neuroinflammatory mechanisms, sensory and motor disorders may occur, and even affect advanced human functions [Citation18]. (3) Hematogenous Track. Evidence in humans suggests that SARS-CoV-2 can be transmitted systemically through the circulatory system [Citation19]. The virus can not only destroy the labyrinthine barrier but also invade the inner ear structure, leading to a series of ear symptoms [Citation20].
The results of this systematic review and meta-analysis should be interpreted with caution due to several limitations. Firstly, most of the literature included in this article were retrospective studies, with a high level of bias in the selection of research objects. Secondly, there was significant heterogeneity among included studies. Further limitation includes a small sample size and not comprehensive research data, the studies included in this review were mainly based on data from western countries, and there was a lack of relevant research data in China. However, China is a country with a large population base and high population density, and the lack of clinical data in China may lead to the fact that the prevalence reported in this study cannot represent the current prevalence level of hearing loss in COVID-19-positive patients. Finally, the results of this meta-analysis cannot be extrapolated to the prevalence of hearing loss in the general population. Given the above problems, we can take relevant measures such as increasing the sample size of the included study, expanding the search scope of the database in the later research, in order to obtain more reliable results.
In conclusion, despite the aforementioned limitations, this meta-analysis is warranted based on the COVID-19 pandemic trends and the need for evidence-based information. During the COVID-19 pandemic, hearing loss can be considered as a screening question, not only to help clinical experts identify symptomatic disease but also to identify potentially healthy or asymptomatic disease carriers, which has important implications for reducing the spread of the virus.
Disclosure statement
The authors declare that there are no competing interests.
Data availability statement
The data can be made available upon request to the corresponding author.
Additional information
Funding
References
- Pascarella G, Strumia A, Piliego C, et al. COVID-19 diagnosis and management: a comprehensive review. J Intern Med. 2020;288(2):192–206.
- Lovato A, de Filippis C, Marioni G. Upper airway symptoms in coronavirus disease 2019 (COVID-19). Am J Otolaryngol. 2020;41 4:(3):102474.
- Zhang X, Chen X, Chen L, et al. The evidence of SARS-CoV-2 infection on ocular surface. Ocul Surf. 2020;18(3):360–362.
- Zaki AM, van Boheemen S, Bestebroer TM, et al. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820.
- Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323(15):1488–1494.
- Agyeman AA, Chin KL, Landersdorfer CB, et al. Smell and taste dysfunction in patients with COVID-19: a systematic review and meta-analysis. Mayo Clin Proc. 2020;95(8):1621–1631.
- Saniasiaya J, Kulasegarah J. Auditory cinchonism in COVID era. Ear Nose Throat J. 2020;99(9):597–598.
- Maharaj S, Bello Alvarez M, Mungul S, et al. Otologic dysfunction in patients with COVID-19: a systematic review. Laryngoscope Investig Otolaryngol. 2020;5(6):1192–1196.
- Abboud H, Abboud FZ, Kharbouch H, et al. COVID-19 and SARS-Cov-2 infection: pathophysiology and clinical effects on the nervous system. World Neurosurg. 2020;140:49–53.
- Freni F, Meduri A, Gazia F, et al. Symptomatology in head and neck district in coronavirus disease (COVID-19): a possible neuroinvasive action of SARS-CoV-2. Am J Otolaryngol. 2020;41(5):102612.
- Mathers C, Smith A, Concha M. Global burden of hearing loss in the year 2000. Global Burden ofDisease. 2000;18:1–30.
- Karosi T, Konya J, Petko M, et al. Histologic otosclerosis is associated with the presence of measles virus in the stapes footplate. Otol Neurotol. 2005;26(6):1128–1133.
- Dalton DS, Cruickshanks KJ, Klein BEK, et al. The impact of hearing loss on quality of life in older adults. Gerontologist. 2003;43(5):661–668.
- Hogan A, O’Loughlin K, Miller P, et al. The health impact of a hearing disability on older people in Australia. J Aging Health. 2009;21(8):1098–1111.
- Yueh B, Shapiro N, MacLean C, et al. Screening and management of adult hearing loss in primary care: scientific review. Journal of the American Medical Association. 2003;289(15):1976–1985.
- Delgado-Roche L, Mesta F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch Med Res. 2020;51(5):384–387.
- Li Y, Li H, Fan R, et al. Coronavirus infections in the central nervous system and respiratory tract show distinct features in hospitalized children. Intervirology. 2016;59(3):163–169.
- Benghanem S, Mazeraud A, Azabou E, et al. Brainstem dysfunction in critically ill patients. Critic Care. 2020;24(1):5.
- Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637.
- Baig AM, Khaleeq A, Ali U, et al. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995–998.

![Figure 4. Forest plots of prevalence at different age stages [A: 30–40 y; B: 40–50 y; C: 50–60; D: ≥60(y)].](/cms/asset/47479194-49d4-4964-af73-be955fcd8930/ioto_a_2204909_f0004_c.jpg)