Abstract
Background
The Provox Vega High Performance (PVHP) is a newly developed voice prosthesis (VP) with an aim to achieve a longer and more predictable lifetime.
Objectives
This feasibility study aims to assess patient acceptance of the PVHP VP, evaluate adverse events, voice quality, and device lifetime.
Methods
Laryngectomized patients previously using a Provox Vega or ActiValve Light were included. Acceptance and voice outcomes were evaluated at two-time points with a 2-week interval. Baseline measurements were taken with the standard VP, followed by placement of the PVHP for the 2-week assessment.
Results
Fifteen participants completed the study, with thirteen being initial Vega-users. PVHP acceptance was 87% 2 weeks after placement. Median device lifetime for all VPs was 64 d (range 14–370). In the subgroup without periprosthetic leakage, the median device lifetime was 101 d (range 31–370). Acceptance dropped to 40% after device failure. Voice quality did not differ between PVHP and baseline VP. The most reported adverse event was PVHP valve stickiness (46%).
Conclusion and significance
Acceptance of the PVHP is largely dependent on device lifetime, decreasing from 87% to 40% after leakage or replacement. Voice quality remains consistent across different VPs. Developing a long-lasting VP remains a challenge.
Chinese Abstract
背景:Provox Vega 高性能 (PVHP) 是一种新开发的发声假体 (VP), 旨在实现更长、更可预测的使用寿命。
目的:本可行性研究旨在评估患者对 PVHP VP 的接受程度, 评估不良反应事件、语音质量和装置寿命。
方法:纳入先前使用 Provox Vega 或 ActiValve Light 的喉切除患者。在间隔两周的两个时间点评估接受度和声音结果。使用标准 VP 进行基线测量, 然后放置 PVHP 进行为期 2 周的评估。
结果:15 名参与者完成了研究, 其中 13 名是 Vega 的初始用户。 在放置两周后, 对PVHP的接受度为 87%。 所有 VP 的装置中位寿命为 64 天(范围 14-370天)。 在没有假体周围渗漏的亚组, 装置中位寿命为 101 天(范围 31-370天)。装置故障后, 接受度下降至 40%。 PVHP 和基线VP之间的语音质量没有差异。 报道最多的不良事件是 PVHP 阀门粘性 (46%)。
结论和意义:PVHP 的接受度在很大程度上取决于装置的使用寿命。装置泄漏或更换后, 接受度从 87% 下降到 40%。在不同的VP之间, 语音质量保持一致。 开发一种使用寿命长的VP仍然是一个挑战。
Introduction
After total laryngectomy (TL), restoration of voice is an essential goal of rehabilitation. Due to more intelligible speech and better voice quality, trachea-esophageal speech (TES) using voice prostheses (VP) has become the gold standard in the Western World [Citation1,Citation2].
VPs have a limited device lifetime and need to be replaced regularly, on average between 2 and 6 months, depending on the VP type [Citation3,Citation4]. The most common reason for replacement is leakage through the VP due to malfunction of the valve, called transprosthetic leakage [Citation5–8]. A major problem is that the device lifetime is unpredictable and varies enormously [Citation9]. This leads to patients often experiencing issues with voice prostheses for which they need to visit a hospital, which demands a lot of healthcare workers [Citation10]. Most patients use VPs such as the Provox Vega (Atos Medical AB, Hörby, Sweden) or the Blom-Singer Classic (InHealth Technologies, Carpinteria, CA). There are also problem-solving VPs available which can be used for problems such as underpressure, early leakage or fistula widening, causing periprosthetic leakage [Citation11]. An example is the Provox ActiValve which has a valve made of fluoroplastic with a built-in magnet for optimal closure and has proven to have a longer device lifetime (median > 11 months) [Citation9,Citation11]. However, the ActiValve is costly in comparison to other VPs, and therefore, not available for most patients due to reimbursement issues. A more affordable VP with a predictable and prolonged device lifetime would be of added value to the current market. Therefore, Atos Medical AB developed the Provox Vega High Performance (PVHP). The PVHP is made of silicon rubber with a fluoroplastic valve flap and valve seat, a material that resists biofilm destruction similar to ActiValve [Citation12], but without the use of a valve magnet. Fluoroplastic is a sticky material, for which the use of a lubricant is needed to prevent blockage of speech [Citation13,Citation14].
The aim of this study was to investigate patient acceptance of the PVHP. Secondary outcomes were experienced stickiness of the valve, effort to speak, subjective and objective voice quality, and device lifetime.
Material and methods
This is a prospective phase I clinical feasibility study performed at the Netherlands Cancer Institute (NKI-AvL) at the Department Head-and-Neck Oncology and Surgery. The study was approved by the Medical Ethical Committee of the NKI-AvL (NL76694.031.21), and registered in Clinicaltrials.gov (NCT05079386). All participants signed informed consent before participating in this study.
Participants
Seventeen laryngectomized patients, >18 years, and initial baseline users of Provox ActiValve Light or Provox Vega (Atos Medical AB, Hörby, Sweden) (length 4, 6, 8, 10, 12.5, all 22.5 French diameter) were included in this study. Participants with current TEP problems, active recurrent or metastatic disease or unable to give informed consent were excluded.
Procedure and data collection
Between January 2022 and March 2022, patients who met the inclusion criteria were contacted for participation by telephone or during regular hospital visits.
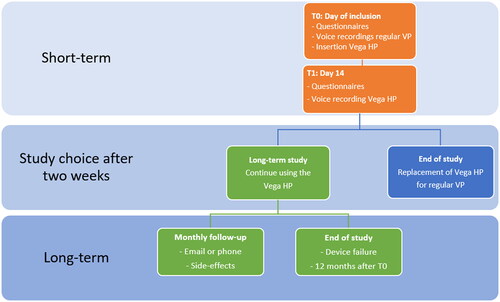
The study consisted of a short- and long-term period assessment (see ). User acceptance was evaluated during an initial 2-week period, with the option to participate in a long-term observation period of up to 12 months. It is expected that 2 weeks is long enough to evaluate the short-term acceptance and short enough to replace the PVHP if the patient is not satisfied. The PVHP was replaced with their regular VP at the end of the study. Acceptance was re-evaluated after ending of the study.
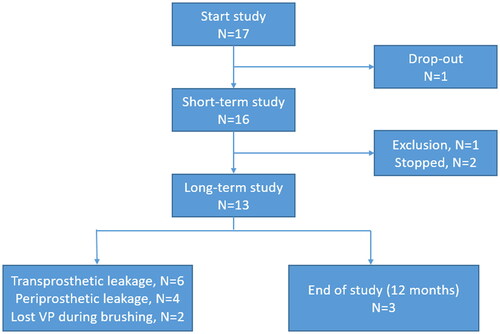
Figure 1. Flowchart of study design. Information regarding the used questionnaires can be found in the sections on primary and secondary outcome measures and Appendix A.
Design of the PVHP
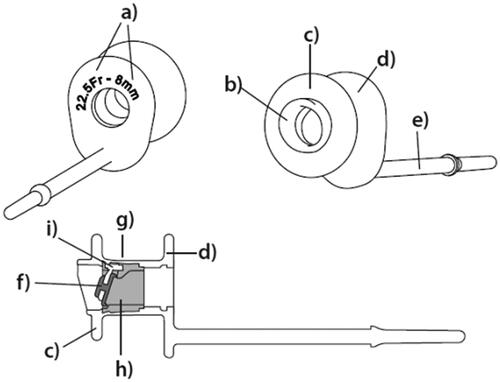
The PVHP is an indwelling VP with an outer diameter of 22.5 French. The housing and valve hinge is molded in transparent silicone rubber, whereas the valve flap and valve seat are made of fluoroplastic, similar to the ActiValve. At the esophageal end of the shaft sits a valve unit consisting of a valve seat, hinge, and valve flap ().
Figure 2. Schematics of the PVHP: (a) size information (shaft diameter and length between flanges) (b) prosthesis hood (c) esophageal Flange (d) tracheal Flange (e) safety strap (f) Radio-opaque fluoroplastic valve flap (g) prosthesis shaft (h) Radio-opaque fluoroplastic valve seat (i) silicon valve hinge.
Use of the PVHP
After cleaning the PVHP in the morning, participants need to apply lubricant by putting a drop on a cleaning brush and rotating the brush in the VP. The lubricant is needed because of the stickiness of the fluoroplastic. The use of lubricant prevents the valve flap from getting stuck, which causes blockage of speech. This is also used by ActiValve-users and has been on the market since 2003 when the ActiValve was released. The lubricant is a medical-grade silicone oil. This one drop should be enough to avoid excessive stickiness but can be reapplied if needed [Citation13,Citation14].
Primary outcome measures
Participant’s acceptance of the PVHP (primary endpoint) is measured with questionnaires, pre and 2-weeks post using the PVHP (see ).
Questionnaires used (details in Appendix A);
Study-specific questionnaires: acceptance of the PVHP, experiences and maintenance of current VP, comparison of VPs
Multiple choice questions about the experiences using the regular versus new VP, maintenance of the VP and possible side-effects (stickiness of the valve, voice quality, and speech), acceptance and preference for a VP. Stickiness of the valve is evaluated in different ways, such as blockage of speech and problems with speech initiation.
Voice handicap index – 10 (VHI-10)
The VHI-10 is a 10-item questionnaire to assess subjective voice quality. It contains ten statements and is used to assess subjective voice quality [Citation15].
Visual analogue scale voice (VAS voice)
A VAS score for effort to speak, where 0 is the most effort to speak they could imagine, and 100 is the least effort to speak.
Voice evaluation
The Roland Edirol (Roland, Osaka, Japan) voice recorder was used for voice recording and assessment [Citation16]. Voice recordings included reading aloud a text, producing a sustained/a/at a normal pitch, and as low, high, soft, and loud as possible. During both meetings questionnaires were filled in by the participant.
Secondary outcome measures
Voice recordings were analyzed and scored for intelligibility through the objective Acoustic Voice Quality Index (AVQI) method [Citation17]. The AVQI score gives a representation of the voice quality and is scored from 0 to 10. A score <2.95 is considered as having a non-pathologic voice. Note: The AVQI is validated, but not for laryngectomized patients [Citation18], but has shown to be useful to evaluate TE-speech [Citation19].
Subjective voice quality and effort to speak ratings were done blinded by two experienced speech-language pathologists (SLP). Voice samples were scored from 0 to 10, where 0–5.5 is rated as not sufficient, 5.5–8 acceptable, and 8–10 good. Maximum phonation time (MPT, in seconds) and loudness (loudest minus softest/a/in dB, 90 and 50 percentile, respectively) are compared for both VPs.
Incidence and severity of reported problems and the comparison of the recorded voice assessments with their regular VP and PVHP are taken as secondary endpoints. Device life and leakage of VPs is noted.
Long-term study follow up
Subjects participating in the long-term study were monthly contacted regarding side effects. This could be through calling, email or during a regular check-up in the hospital. After device failure the PVHPs questionnaire regarding acceptance was filled in and the PVHPs were investigated for reasons of failure.
Statistical analyses
Statistical analysis of the collected data was performed with SPSS 27.0 (SPSS Inc., Chicago, IL). Cross tabulations were used to compare the baseline and follow-up questionnaires. When suitable, the mean, median, standard deviations, range, and variances of the analyzed data were visualized in tables. Because of the descriptive nature of this study, results were not tested for significance. The two-way mixed intra-class correlation coefficients (ICC) with absolute agreement and 95% confidence interval were used to determine the inter-rater reliability of the subjective voice assessments. This was done separately for the voice quality and effort to speak. An ICC of <0 reflects ‘poor’, 0–0.20 ‘slight’, 0.21–0.4 ‘fair’, 0.41–0.60 ‘moderate’, 0.61–0.8 ‘substantial’, and above 0.81 ‘almost perfect’ [Citation20].
Device lifetime of the PVHP was calculated as day from insertion until device failure, or when no failure occurred in the twelve study months, until day of replacement.
Results
Patient characteristics
Of the 17 included patients, we had one immediate drop-out. After placement of the PVHP, he changed his mind and did not feel comfortable with trying a new VP. During the short-term study, one patient was diagnosed with metastatic disease after which he was excluded. This left us with fifteen patients for analysis (). All patient characteristics are shown in . The majority of the participants were male; participants had a mean age of 71 years at the start of the study.
Table 1. Patient, tumor, and treatment characteristics (n = 15).
Regular voice prosthesis
Twelve patients (80%) used a Vega as their regular VP and three (20%) an ActiValve Light. The median device lifetime of the whole group their previous VP was 113 d (range 7–427). For the ActiValve-users this was median 94.5 d (mean 133, range 7–357) and for Vega 117 d (mean 119 d, range 12–427). The main reason for replacement was transprosthetic leakage (N = 12).
Acceptance of the PVHP
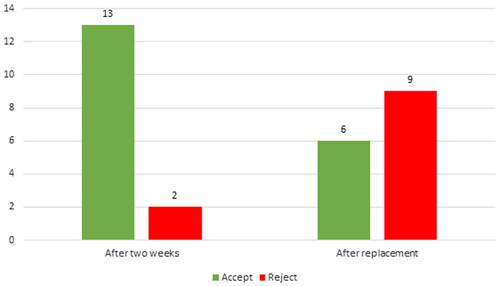
After 2 weeks (short-term follow-up) 13 (86.7%) participants accepted the PVHP, of which nine (60%) preferred the PVHP over their regular VP because of experiencing an improved voice and less effort to speak. Five patients experienced increased stickiness of the valve flap when using the PVHP compared to two patients with their regular VP, regardless of the regular type of VP used. None of the patients reported the daily use of lubricant as a negative aspect.
Short-term follow-up
None of the patients decided to replace the PVHP before the end of the short-term follow-up. Two patients (2/15: 13%) had periprosthetic leakage during these 2 weeks, which was the reason for not participate in the long-term study. One of them is familiar with a short device lifetime (previous device lifetimes were 7 and 15 d).
Subjective voice quality and adverse events (study-specific)
Seven of the fifteen patients (46%) reported a better voice quality with the PVHP, four (27%) with their regular VP and the remaining four (27%) noticed no difference. These seven patients (46%) also reported less effort to speak when using the PVHP, four (27%) when using their regular VP and four (27%) reported no differences. Two (13%) of the patients reported less stickiness of the valve flap with the PVHP (one ActiValve and one Vega User), three (20%) reported no difference between the VPs (one ActiValve and two Vega users), and ten (67%) reported less stickiness with their regular VP. Ten (67%) patients reported disadvantages of the PVHP, which were blocking of speech and stickiness of the valve flap (n = 7, 46%), leakage (n = 4, 27%) and, excessive mucus production (n = 2, 13%).
Net promotor score
A median score of seven was reached (range of 0–9). Ten (67%) participants would recommend the PVHP to other patients, and five (33%) would not.
Voice Handicap Index – 10
Nine (60%) patients had a VHI-10 score of 11 or higher when using their regular VP (a score above 11 is considered limiting in daily life). This number increased to 12 (80%) when using the PVHP. The median VHI-10 score of the regular VPs was 14 (range 4–32 in comparison to 21 (3–35 for the PVHP. When comparing differences within subjects, eight (53%) patients scored higher with the PVHP than with their regular VP, one (7%) had comparable scores for both VPs, and six (40%) scored better with their regular VP (see 4 A).
AVQI score
As shown in , all participants have a higher score (meaning a deviant voice) than the cutoff point (2.95, red line). The median AVQI score of the PVHP was 8.4 (range 7.05–10) and 8.2 (range 6.93–10) for the regular VP.
Figure 4. Overview of subjective and objective voice outcomes. (A) VHI10 scores, range 0–40. A score above 11 points (red line) is considered as having an abnormal voice, which is subjective limiting in daily life. (B) AVQI scores, range 0–10. The lower the score, the better the voice. A score <2.95 (red line) is considered as having a healthy voice. (C) Voice quality as rated by SLPs, range 0–10. A score <5.5 is rated as poor/pathologic. (D) Effort to speak as rated by SLPs, range 0–10. A score <5.5 is rated as too much.
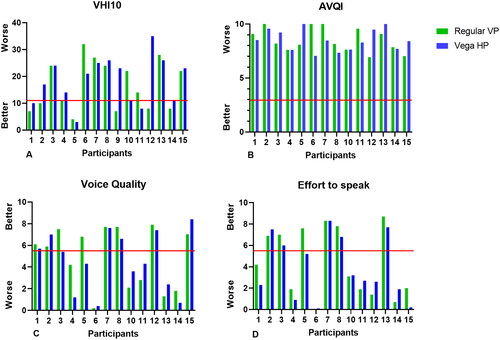
Voice quality and effort to speak (rated by SLPs)
Fourteen voice recordings could be analyzed since one of the participants was not able to speak at the first meeting (participant 9). The inter-rater reliability (ICC) of the subjective voice assessments was ‘almost perfect’ for the effort to speak (0.933, 95% IC 0.856–0.969) and ‘substantial’ to ‘almost perfect’ for the voice quality (0.863, 95% CI 0.707–0.937). The mean effort to speak and voice quality scores were worse for the PVHP in comparison to the regular VP. The voice quality rating decreased from 5.1 (regular VP) to 4.4 (PVHP) and the effort to speak rating decreased from 3.7 (regular VP) to 3.0 (PVHP). Six (43%) patients had better voice quality with the PVHP compared to with their regular VP, and eight (57%) were rated with better voice quality with their regular VP. Seven (50%) needed less effort to speak with the PVHP, and seven (50%) less with their regular VP. See for individual scores.
The MPT with their regular VP was median 4.60 s (range 1.90–27.74), and with the PVHP median 5.37 s (range 0.98–22.79). The loudness with their regular VP was median 4.65 dB (range 2.8–11.0) and for the PVHP median 5.1 dB (range 1.6–10.8).
Long-term follow-up
Device lifetime
To determine device lifetime, data of all fifteen participants were analyzed (see ). Ten subjects (67%) had their PVHP replaced because of leakage (transprosthetic n = 6, periprosthetic n = 4). Three participants (20%) had the PVHP replaced because of reaching the one-year follow-up without device failure/leakage. Noteworthy is that two subjects (13%) brushed the PVHP out of their stoma without the need for medical intervention, except for a replacement. The median device lifetime of the whole group was 64 d (mean 120 d, range 14–370). Upon exclusion of the VPs with periprosthetic leakage and the VPs that were brushed out, the median device lifetime was 101 d for this subgroup (mean 167, range 26–370) (see ).
Figure 5. Kaplan–Meier curve of the device lifetime of the PVHP. (A) The whole group (n = 15), (B) Without periprosthetic leakage and lost VPs (n = 9).
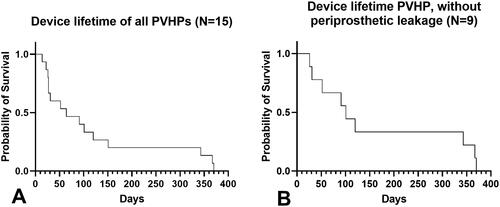
Table 2. Device lifetime of the PVHP.
The trade-off question showed that the minimum desired device life of the PVHP, to outweigh disadvantages, was a median of six months (range 3–12months). During the long-term study period, there were no new adverse events reported.
Acceptance after ending study
Two participants did not accept the PVHP due to early leakage. Of the remaining thirteen participants who continued in the long-term phase, six participants accepted the PVHP but one of them preferred the ActiValve Light because of the experienced longer device lifetime. The remaining seven did not accept the PVHP because of the relatively short device lifetime. In total six out of fifteen participants (40%) accepted the PVHP after ending the study (see and ).
Discussion
This feasibility study found that the short-term acceptance of a newly developed VP called ‘PVHP’ was 87%; however, it dropped to 40% after the replacement of the PVHP due to device failure. Acceptance is a composed outcome measure, in this study, depending on factors related to the patient, but also on other outcomes such as stickiness of the valve, speech and device lifetime. The difference in acceptance rate at these two-time points (short-term versus long-term follow-up) can mainly be explained by the shorter-than-anticipated device life.
The most reported side-effect was the blocking of speech due to the stickiness of the valve, which is caused by the used material and is a well-known side-effect of the ActiValve, made from the same material [Citation11]. All patients were able to solve these problems by coughing, brushing the VP and/or reapplying lubrication. ActiValve-users were common with lubricating and thus reported less side-effects compared to the Vega-users. The use of lubrication was not considered a main issue, comparable with the ActiValve [Citation11,Citation13].
Surprisingly, half of the patients rated their voice quality and effort to speak better with the PVHP compared to their regular VP, which was not found by the blinded perceptual evaluations of the SLPs. This could be the effect of the ‘take-the-best heuristic’, where people assume ‘new is better’, and score new products initially better than they perform [Citation21].
Looking at the results of the VHI-10, only two participants rated their voice as normal and not limiting during daily life, all the others rated their voice as a handicap. This finding is comparable with other publications [Citation2,Citation19]. The AVQI is an objective acoustic outcome measure. The mean AVQI score was the same for the regular and new VP (8.5), which means that all participants had a distorted voice quality (score > 2.5). It is clearly visible that patients rate their voice quality better than the objective scoring, and there are no differences between the types of VP [Citation22]. As none of the objective scorings are validated for TE speech, AVQI scores have to be interpreted carefully [Citation19,Citation23]. When looking at the MPT and loudness, we saw that both were slightly better with the PVHP compared to the regular VP.
Device lifetime varies enormously, both inter- and intra-patient and seems to be very multifactorial [Citation9]. Although this study is not powered to assess a realistic device lifetime, the device lifetime of the PVHP in this pilot was relatively short (median 64 d). This is inferior to the device lifetime of the ActiValve (165 d) [Citation11], but comparable with the Provox2 (63 d), Provox Vega (66 d)[Citation3] and the Blom-Singer Classic (69 d)[Citation4]. The concept of a fluoroplastic valve flap and valve seat was expected to prevent biofilm formation and therefore a longer device lifetime. The main reason for leakage; however, seems to be food residue on the valve or valve seat. However, this needs further study as this is not investigated before in other VPs. This study indirectly confirms that the magnet in the ActiValves is probably the key component in their longer device lifetime [Citation13].
Our cohort included two patients that displaced their PVHP due to brushing. Despite the fact that brushing and lubricant are widely used in VPs such as the ActiValve [Citation13,Citation14], the percentage of such displacements in our clinic, and also in the literature is unknown. One of the two participants has a relatively wide and fragile tracheo-oesophageal fistula, which could potentially be the underlying cause of displacement. For the other participant the cause of the brush-out remains unclear.
Investigating and developing new voice prostheses is quite challenging due to the small number of laryngectomized patients and multifactorial issues determining device lifetime. In vitro research of VPs has shown to be useful for investigating the composition and prevention of biofilm formation [Citation24,Citation25]. But this leaves out all other factors, such as cleaning of the VP, diet, reflux, pressure in neopharynx and stoma problems. To investigate the quality and device lifetime of a VP, a large cohort of patient is needed to give a valid overview of the device lifetime in vivo. The search for a new VP with a longer device lifetime is needed to improve patient acceptance and increase the quality of life for these patients, but this remains a great challenge.
Limitations
This is a feasibility study, investigating the acceptance of a new VP, and only the rating of the voice recordings was blinded. It was not powered in finding differences between VPs. Due to the limited sample size it is hard to draw definitive conclusions on voice quality and device lifetime. Acceptance of a VP is a complex outcome measure associated with many other factors such as stickiness of the valve flap and device lifetime.
Conclusion
This feasibility study of the new VP ‘PVHP’, showed that the patient acceptance was 40% (6/15). Patient acceptance seems to depend on device lifetime rather than side effects such as stickiness of the valve and the use of lubricant. The device lifetime was relatively short (median 64 d) and therefore the main limiting factor in acceptance by patients. Subjective and objective voice ratings did not show differences between the regularly used VP and the PVHP. The search for an affordable new VP with a long device lifetime remains a complicated challenge.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Tang CG, Sinclair CF. Voice restoration after total laryngectomy. Otolaryngol Clin North Am. 2015;48(4):687–702. doi:10.1016/j.otc.2015.04.013.
- van Sluis KE, van der Molen L, van Son RJJH, et al. Objective and subjective voice outcomes after total laryngectomy: a systematic review. Eur Arch Otorhinolaryngol. 2018;275(1):11–26. doi:10.1007/s00405-017-4790-6.
- Petersen JF, Lansaat L, Timmermans AJ, et al. Postlaryngectomy prosthetic voice rehabilitation outcomes in a consecutive cohort of 232 patients over a 13-year period. Head Neck. 2019;41(3):623–631. doi:10.1002/hed.25364.
- Kress P, Schäfer P, Schwerdtfeger FP, et al. Are modern voice prostheses better? A lifetime comparison of 749 voice prostheses. Eur Arch Otorhinolaryngol. 2014;271(1):133–140. doi:10.1007/s00405-013-2611-0.
- Acton LM, Ross DA, Sasaki CT, et al. Investigation of tracheoesophageal voice prosthesis leakage patterns: patient’s self-report versus clinician’s confirmation. Head Neck. 2008;30(5):618–621. doi:10.1002/hed.20764.
- Starmer HM, Agrawal N, Koch W, et al. Does prosthesis diameter matter? The relationship between voice prosthesis diameter and complications. Otolaryngol.-Head Neck Surg. 2011;144(5):740–746. doi:10.1177/0194599810395362.
- Brook I, Goodman JF. Tracheoesophageal voice prosthesis use and maintenance in laryngectomees. Int Arch Otorhinolaryngol. 2020;24(4):e535–e538. doi:10.1055/s-0039-3402497.
- Parrilla C, Longobardi Y, Galli J, et al. Periprosthetic leakage in tracheoesophageal prosthesis: proposal of a standardized therapeutic algorithm. Otolaryngol.-Head Neck Surg. 2021;165(3):446–454. doi:10.1177/0194599820983343.
- Heirman AN, van der Noort V, van Son R, et al. Does prophylactic replacement of voice prosthesis make sense? A study to predict prosthesis lifetime. Otolaryngol Head Neck Surg. 2022;168(3):1945998221116815.
- Parrilla C, Longobardi Y, Paludetti G, et al. A one-year time frame for voice prosthesis management. What should the physician expect? Is it an overrated job? Acta Otorhinolaryngol Ital. 2020;40(4):270–276. doi:10.14639/0392-100X-N0587.
- Soolsma J, van den Brekel MW, Ackerstaff AH, et al. Long-term results of provox ActiValve, solving the problem of frequent candida- and “underpressure”-related voice prosthesis replacements. Laryngoscope. 2008;118(2):252–257. doi:10.1097/MLG.0b013e318159ebde.
- Timmermans AJ, Harmsen HJM, Bus-Spoor C, et al. Biofilm formation on the provox ActiValve: composition and ingrowth analyzed by illumina paired-end RNA sequencing, fluorescence in situ hybridization, and confocal laser scanning microscopy. Head Neck. 2016;38(1):E432–440. doi:10.1002/hed.24014.
- Hilgers FJM, Ackerstaff AH, Balm AJM, et al. A new problem-solving indwelling voice prosthesis, eliminating the need for frequent candida- and ‘underpressure’-related replacements: provox ActiValve. Acta Otolaryngol. 2003;123(8):972–979. doi:10.1080/00016480310015371.
- Provox® ActiValve® Lubricant. 2022. [accessed 19 October 2022]. Available from: https://www.shopatosmedical.com/provox-activalve-lubricant.html
- Arffa RE, Krishna P, Gartner-Schmidt J, et al. Normative values for the voice handicap index-10. J Voice. 2012;26(4):462–465. doi:10.1016/j.jvoice.2011.04.006.
- Corporation R. Roland R-07 | High-resolution audio recorder. Roland. 2022. [accessed 19 October 2022]. Available from: https://www.roland.com/global/products/r-07/
- Maryn Y, De Bodt M, Roy N. The acoustic voice quality index: toward improved treatment outcomes assessment in voice disorders. J Commun Disord. 2010;43(3):161–174. doi:10.1016/j.jcomdis.2009.12.004.
- Batthyany C, Latoszek BBV, Maryn Y. Meta-analysis on the validity of the acoustic voice quality index. J Voice. 2022;S0892-1997(22):00132–00131.
- van Sluis KE, van Son RJJH, van der Molen L, et al. Multidimensional evaluation of voice outcomes following total laryngectomy: a prospective multicenter cohort study. Eur Arch Otorhinolaryngol. 2021;278(4):1209–1222. doi:10.1007/s00405-020-06216-z.
- Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–163. doi:10.1016/j.jcm.2016.02.012.
- Gigerenzer G, Goldstein DG. Reasoning the fast and frugal way: models of bounded rationality. Psychol Rev. 1996;103(4):650–669. doi:10.1037/0033-295x.103.4.650.
- Sluis K v, Brekel M v d, Hilgers FJM, et al. Long-term stability of tracheoesophageal voices. In Interspeech 2016; San Francisco, Sept 9, 2016, ISCA; p. 102–106. doi:10.21437/Interspeech.2016-114.
- Eadie TL, Otero D, Cox S, et al. The relationship between communicative participation and postlaryngectomy speech outcomes. Head Neck. 2016;38(1):E1955–E1961. doi:10.1002/hed.24353.
- Leonhard M, Zatorska B, Tan Y, et al. In vitro biofilm growth on modern voice prostheses. Head Neck. 2018;40(4):763–769. doi:10.1002/hed.25053.
- Free RH, Busscher HJ, Elving GJ, et al. Biofilm formation on voice prostheses: in vitro influence of probiotics. Ann Otol Rhinol Laryngol. 2001;110(10):946–951. doi:10.1177/000348940111001010.
- Krol MW, de Boer D, Delnoij DM, et al. The net promoter score – an asset to patient experience surveys? Health Expect. 2015;18(6):3099–3109. doi:10.1111/hex.12297.
- Rosen CA, Lee AS, Osborne J, et al. Development and validation of the voice handicap index-10. Laryngoscope. 2004;114(9):1549–1556. doi:10.1097/00005537-200409000-00009.
- Langley GB, Sheppeard H. The visual analogue scale: its use in pain measurement. Rheumatol Int. 1985;5(4):145–148. doi:10.1007/BF00541514.
Appendix A
Additional information regarding used questionnaires
Study-specific questionnaires: Acceptance of the PVHP, experiences and maintenance of current VP, comparison of VPs.
Voice Handicap Index – 10 (VHI-10)
The VHI-10 is a 10-item questionnaire to assess subjective voice quality. It contains ten statements and is used to assess subjective voice quality. Each statement has five answer options: never, almost never, sometimes, usually, and always. A score above 11 points is considered as having an abnormal voice, limiting daily life activities (range 0–40 points)[Citation15]. The VHI-10 is often used, but not validated for laryngectomized patients [Citation19,Citation27].
3. Visual Analogue Scale Voice (Vas Voice)
With help of a VAS score, effort to speak is measured. In this score, 0 is the most effort to speak they could imagine, and 100 is the least effort to speak. A VAS is a measurement instrument that tries to measure a characteristic or attitude that is believed to range across a continuum of values and cannot easily be directly measured, most used for pain [Citation28]. This VAS score gives a representation of the effort to speak.