?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Manières disease (MD) is a chronic inner ear disease characterized by recurrent vertigo and fluctuation in auditory symptoms. Vertigo spells have a sudden onset and are difficult for patients to handle. Therefore, treating a patient with MD is still a challenge for clinicians.
Aims
This study aims to analyse the short-term effects of intratympanic dexamethasone (ITD) on the various symptoms of unilateral MD.
Materials and methods
The study comprised 27 patients with unilateral MD and severe vertigo who failed medication therapy. Treatment was with ITD as an alternative to destructive therapy. Treatment is evaluated after four months.
Results
Significant improvements were measured with Dizziness Handicap Inventory (DHI), Tinnitus Handicap Inventory (THI), frequency of vertigo attacks longer than 20 min, Functional Level Scale (FLS), and tinnitus sensation measured by the Analog Visual Scale (AVS). Patients with severe symptoms grading with DHI and THI experienced the most improvement. Patients have achieved substantial vertigo control in 73%.
Conclusion
ITD application shows improvement in controlling vertigo and tinnitus in patients under exacerbation in MD.
Significance
It is a promising non-destructive addition to the ‘stepwise treatment concept’ in MD and can be used as a first-line treatment in vertigo control.
Chinese Abstract
背景
玛尼诶病(MD)是一种慢性内耳疾病, 其特征是反复性眩晕和听觉症状的波动。 眩晕发作突然, 患者很难应付。 因此, 治疗MD患者仍然是临床医生面临的挑战。
目的
本研究旨在分析鼓室内注射地塞米松 (ITD) 对单侧MD患者的不同症状的短期影响。
材料和方法
该研究包括 27 名患有单侧 MD 和严重眩晕的患者, 他们的药物治疗失败。 采用 ITD 进行治疗, 作为破坏性治疗的替代方法。 治疗四个月后进行评估。
结果
通过头晕障碍量表 (DHI)、耳鸣障碍量表 (THI)、眩晕发作频率超过 20 分钟、功能水平量表 (FLS)、以及通过模拟视觉量表(AVS)测量的耳鸣感觉, 测量到显著改善。 使用 DHI 和 THI 进行分级的症状严重的患者改进最大。 患者取得了实质性的 眩晕控制率73%。
结论
ITD 的应用显示出对MD 加重的患者的眩晕和耳鸣的控制有所改善。
意义
它是对MD “逐步治疗概念”的一种有前景的非破坏性补充, 可用作控制眩晕的一线治疗方法。
Introduction
MD is a chronic idiopathic inner ear syndrome characterized by vertigo, tinnitus, and hearing problems. The vertigo spells have a sudden onset and often come with severe nausea and vomiting, making it difficult for affected patients to handle [Citation1]. MD is a rare disease with global variation in incidence. Although the prevalence is low, due to the chronic nature of the disease, the patient group is well-known by many health professionals. MD commonly debuts in middle-aged patients and has a female-to-male ratio of 1.9:1 [Citation2]. Studying the efficacy of different treatments for MD is difficult due to the disease’s tendency to fluctuate, the subjective nature of the symptoms, and the possibility of spontaneous regression. Because of this, most treatment modalities lack high-level evidence.
The treatment of MD currently focuses on symptom management due to the unclear understanding of its underlying pathophysiological mechanism. The current approach to treating MD follows a stepwise concept, typically commencing with medication as an initial step. Betahistine and lifestyle modifications like a low-sodium diet, with or without diuretics, make up the first line of treatment for MD. Patients whose MD is uncontrolled on this medication are considered to have intractable MD. In this situation, the clinician must decide whether to provide the patient with destructive or non-destructive treatment. Among the destructive methods are gentamicin, labyrinthectomy, and vestibular nerve section. The non-destructive techniques include endolymphatic shunts and intratympanic steroid instillation. In recent years, this intratympanic non-destructive treatment modality has increased in popularity. The method is considered low-risk and can easily be performed in an outpatient clinic using solely local anesthesia [Citation3].
Intratympanic steroids
Mechanism of action
The rationale behind steroid medication partly stems from the idea that MD is an inflammatory disease. Many examples support this claim, including elevated immune complexes and IgG deposits. Additionally, glucocorticoid receptors have been identified in the inner ear structures, suggesting that steroids directly alter the function of vestibulocochlear tissues, rather than having a systemic anti-inflammatory effect [Citation4]. Contrarily, a study by MacArthur et al. found that intratympanic steroids may lead to the upregulation of cytokines and cause an inflammatory reaction, and instead suggests that the therapeutic effects may come from improvement in ion homeostasis [Citation5]. The effect of steroids on ion homeostasis is attributed to aquaporins. The arginine vasopressin-aquaporin2 system is important in the water homeostasis of the inner ear, and it has been suggested that MD is the result of a dysfunction in this system. Intratympanic steroids have been shown to upregulate AQP1 mRNA in the cochlea, possibly leading to the regulation of the endolymphatic fluids [Citation6]. Since AQP1 is expressed in other parts of the inner ear, including the stria vascularis which is important in the regulation of inner ear fluids, it could be assumed that upregulation of AQP1 mRNA occurs in these organs also. In summary, although there are many theories, the mechanism of action for intratympanic steroids is not yet clarified.
Benefits of local steroid administration
The administration of steroids directly to the inner ear comes with several benefits. Firstly, intratympanic administration of steroids produces higher perilymph concentrations when compared to systemic administration. A study by Bird et al. showed intratympanic concentrations that were 126-fold higher after intratympanic administration, compared to 1 mg/kg intravenous administration, of the same drug [Citation7]. Another study using fluorescent dexamethasone in rats revealed higher-level accumulation of the drug in the perilymph after intratympanic administration compared to systemic, and the concentrations were sustained for longer [Citation8]. Secondly, the systemic doses of glucocorticoids needed to reach detectable levels in the perilymph are high, whereas intratympanic administration leads to low systemic concentrations, thus avoiding the adverse effects associated with high doses of systemic steroid medication. Intratympanic steroid injections have shown to be safe and have a low rate of adverse events. While the intervention can be experienced as unpleasant, the pain is short-lived. Unlike gentamicin, intratympanic applicated steroids do not appear to deteriorate hearing, and no ototoxic properties have been detected [Citation9].
Treatment efficacy
Unfortunately, the efficacy of intratympanic steroids against MD is contested. A mini-review done by Patel in 2017 looked at 12 studies meeting a 2-year follow-up. The included studies showed a wide range of results, with a percentage of class A vertigo control ranging from 15%–91% at 2 years [Citation10]. It was presumed that the wide range of effects was due to the short-lived effect of steroids [Citation11]. Overall, the results from the review support the use of intratympanic steroids in MD, especially when delivered as needed. These as-needed injections seemed to be equally effective as gentamicin for vertigo control. The authors state that ITS should be considered as a first choice instead of gentamicin for these patients due to its non-destructive effect [Citation12]. The placebo-controlled, double-blind randomized trial, conducted by Garduño-Anaya et al. did show positive results with a significant decrease in vertigo attacks following intratympanic treatment with dexamethasone. Unfortunately, the placebo group in this study also showed improvement in their vertigo [Citation13]. In 2016, Lavigne et al. reviewed all prospective randomized clinical trials for ITS in MD. Out of the 6 studies found, 3 found no significant benefit of ITS over placebo [Citation14]. In a review by Webster et al. the number of vertigo episodes were evaluated in ten randomized clinical trials with ITD. It was found that ITD might reduce the number of episodes but only by a small amount [Citation15]. Despite the unclear evidence, due to its safety, ITD is recommended as a second line of treatment. More importantly, ITS should be used before destructive techniques are considered, especially in patients with serviceable hearing or bilateral MD. Today, ITS is frequently used by physicians worldwide, largely thanks to positive clinical experience.
Material and method
A retrospective single-case clinical study is conducted at the Hearing and Balance Department at the Karolinska University Hospital in Stockholm between 2018 and 2022. The study was approved by the Ethical Review Board, and the diary number for this study is 2015/741-31 with amendment 2021-06481-02. The patients were diagnosed with definite MD according to the American Academy’s 1995 diagnostic criteria (AAO-HNS) [Citation1]. The inclusion criteria for ITD were patients diagnosed with MD who failed to respond to conventional medical therapy for vertigo control and had at least one vertigo attack lasting shorter or longer than 20 min in the last month prior to inclusion. Written consent was collected from all participants to undergo intervention and for analyzing the collected data.
Method
Patients received four injections of dexamethasone 4 mg/ml over 10 days and they were injected through the whole eardrum. If the patients had a grommet placed in the eardrum, it was extracted, and the decision was grounded on the authors’ clinical experience. The air pressure in the middle ear expels the added fluid, and the liquid runs out if it is injected through the grommet. The method of injecting liquid with a thin spinal needle with two openings on the membrane proved optimal conditions for the middle ear to keep the liquid. One-hole acts as an air vent in the upper part, and another hole just below it is for injection. The reason for choosing 4 injections over 10 days is also based on the authors’ clinical experience and the available concentration of dexamethasone on the market. Higher concentration has shown a better effect on MD [Citation16] but nationally, dexamethson injektions of 4 mg/ml is solely preparation in clinical use. The volume of fluid coming into the middle ear varies from 0.2 to 0.7 ml. Speaking and swallowing displace liquid through the auditory tube after 30 min to one hour.
Intratympanic injection technique
The patient is placed in a supine position, and an ear microscope is used to visualize the tympanic membrane. The ear canal and tympanic membrane are anaesthetized with Xylocaine spray. Once the anesthesia has achieved its planned effect, the excess fluid is removed. Two small incisions are made in the tympanic membrane, one in the anterior superior quadrant to release air and the other just below it for injection. A 2 ml syringe with a spinal needle No.12 is used. The needle is bent at the midpoint to create a 30-degree angle and 1 ml of dexamethasone 4 mg/ml is injected into the middle ear until the middle ear is full. The amount needed to fill the middle ear can range from 0.2 to 1 ml. The patient is then instructed to remain on their back with their head turned toward the healthy ear for 30 min. During this time, the patient should refrain from swallowing.
Outcomes were measured at baseline and four months after the intervention. The Wilcoxon Signed-Rank test was used to observe whether the difference between baseline and 4 months after treatment was significant. The P-value of ≤.05 was used to define statistical significance. All patients graded their own level of disability in various ways in this survey. Patients could select the level of influence on daily activities on a FLS, answering the questionnaire: DHI and THI, Vertigo rapport (VR), own designed questionnaire, was used for grading other symptoms. The VR consists of a part aimed at counting the frequency of vertigo attacks shorter than 20 min and longer than 20 min during the month prior to inclusion/four months after and another part consisting of AVS for four symptoms: overall dizziness, tinnitus, subjective hearing level, and aural fullness.
The participants were divided into groups corresponding to the severity level of DHI and THI. For DHI, the groups were as follows: mild (16–34), moderate (36–52), or severe (54+) handicaps. For THI, the groups were: no handicap (0–16), mild (18–36), moderate (38–56), severe (58–76), and finally catastrophic (78–100).
The number of attacks per month was used to calculate vertigo control according to the AAO-HNS guidelines [Citation1]. The formula used to calculate vertigo control requires values for six months, but for this study, only results at 4 months were used. The calculation produces a value that translates to a vertigo class. A low score indicates that the number of attacks is lower after treatment than before, implying good vertigo control. A high score indicates inadequate control as it entails that the frequency of attacks has increased following treatment. Specifically, the cut-off values are: 0 = complete control Class A, from 1 to 40 is substantial control and it is Class B. Class A and B are considered sufficient symptom control. Class C is ranged from 41 to 80 and represents limited control of vertigo attacks with treatment.
If participants have insignificant (81–120 = Class D) and (>120 = Class E) worse control of vertigo then it classifies treatment without effect. It seems that the cut for the treatment efficacy is about 80 points and all classes (A–C) with lower points represent improvement in vertigo control.
Results
The study initially included 39 patients, but 12 were subsequently excluded from the data analysis. Two subjects discontinued after the second injection, and three withdrew after the third injection. Four patients were excluded due to incomplete form filling, and three were excluded because they had complicated MD, involving dizziness from another source. Three patients who received a second treatment and did not properly fill out or complete the form were excluded from the analysis of the second round of therapy and associated variables.
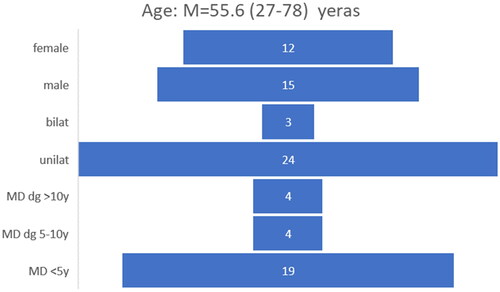
Demographic data from 27 patients, representing the study, are presented in , indicating that most patients experienced symptoms on one side. Among the 27 patients, 19 had MD for duration of five years or less.
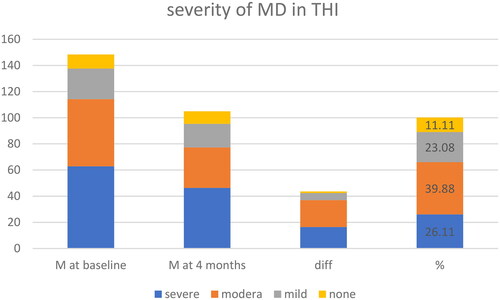
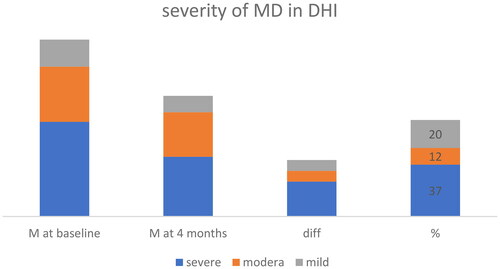
Regarding the DHI and THI, despite all patients having intractable MD, there was a significant variation in baseline outcomes. The baseline DHI scores ranged from 10 to 92, with a mean value of 44.8 (). After four months, the mean value decreased to 30.7, representing a 31.41% reduction. The Wilcoxon signed-rank test demonstrated a significant difference (p-value:.0002), indicating a decrease in dizziness-related disability. A reduction in handicap caused by dizziness experienced 70% of patients. At baseline, 10 of participants had no tinnitus handicap according to THI scores. The mean THI value was 34.27 at baseline and 24.93 at four months, resulting in a 27% reduction in tinnitus-related problems. The Wilcoxon signed-rank test showed a significant decrease in tinnitus handicap (p-value:.0005). Patients were categorized based on the severity of their DHI and THI scores at baseline ( and ).
Figure 2. Most significant improvement in DHI score is for patients with hanzdicap score >54 points.
The most evident treatment benefits were observed in the severe DHI category, with a mean difference of 24.9% or 37% and moderate THI category. Subjective tinnitus and aural fullness, as measured by the AVS, decreased by 23.06% and 18.4%, respectively. The confidence intervals for both variables were relatively small, and the Wilcoxon signed-rank test showed a significant decrease in tinnitus (p-value: significant) but not for aural fullness ().
Table 1. Results before and four months after treatment with ITD including 4 injections in 10 days.
The number of vertigo attacks lasting longer than 20 min decreased based on VR results from 27 participants. The mean number of such long attacks decreased from 3.96 per month at baseline to 1.43 at four months, representing a 69% reduction. The Wilcoxon signed-rank test showed a significant decrease (p-value:.0034), indicating high statistical significance. Vertigo control classes were calculated using the formula:
According to the AAO-HNS classification, 67% of participants achieved sufficient vertigo control (Class A and B), and including Class C (limited control), approximately 73.4% of patients experienced some degree of treatment benefit. Results are shown in .
Table 2. Classes A, B, and C represent limited vertigo control.
The number of shorter attacks lasting less than 20 min did not yield promising results. The mean score decreased from 4.85 at baseline to 2.04 at four months, with a mean difference of 2.43% or 50.20%. However, most participants reported either increased symptoms or no change (17% or 61%). The Wilcoxon signed-rank test did not show significant results for the shorter attacks suggesting an uncertain effect.
On the FLS, the mean decrease was 0.73 at four months, which, although not substantial, was statistically significant (p-value:.0042). According to AAO-HNS recommendations, treatment outcomes were categorized as improved (13/27), unchanged (12/27), or worse (2/27). Using this classification, 48% of the group reported an improvement in the level of disability.
General dizziness and subjective hearing loss, measured on the AVS scale ranging from 0 to 10, decreased overall following ITD treatment, although not all reached statistical significance. Eight patients reported increased symptoms, four reported no change, and no significant results were observed for ITD treatment on general dizziness. Subjective hearing loss exhibited similar results, with a decrease of 9.74% at four months.
Discussion
As represented in the results, this group comprises mostly unilateral MD, where the majority of patients presented symptoms for no longer than five years. In the demographic analysis, Gurkov et al. [Citation17] reported that the median combined audiovestibular disease duration (i.e. the time since both vertigo AND hearing loss appeared) was 2.5 years. Patients in the present study were treated differently, mostly with drugs, until the decision for intratympanic intervention was taken. It is important to notice that all these patients are, at the time of intervention, qualified for chemical (gentamicin) destruction based on clinical criteria. The referenced study shows that patients with MD for less than 7 years mostly suffer from paroxysmal vertigo attacks and less from hearing problems. Vertigo attacks are more frequent in the first few years of the disease. Therefore, disabling vertigo attacks during the last month were inclusion criteria for our studied population.
Since a high frequency of vertigo spells can be debilitating to a patient suffering from MD, drugs are generally geared towards reducing the number of vertigo spells. The results of this study suggest that ITD could be useful in achieving this goal. The studied population achieved complete vertigo control (Class A) in 60% and were thus entirely free from vertigo spells after four months. Only three out of the 27 patients were candidates for retreatment. A total of 66% of participants achieved sufficient vertigo control (Class A or B). Comparing these results to other trials, the proportion achieving complete vertigo control at 2 years ranges from 15.1% to 82%. This follow-up period of 2 years has been proposed by the AAO-HNS as a means of properly evaluating the long-term efficacy of treatments. Unfortunately, this study did not meet this standard. The values for vertigo control were instead reported at 4 months, which do not provide representative values as recommended. Although the participants were not followed up for an adequate period, the results still demonstrate good short-term vertigo control. This finding supports the idea that ITD could be a useful means for achieving vertigo control, especially during periods of increasing inner ear activity. The group with the highest frequency of vertigo spells showed the best vertigo control, with all six patients experiencing 7 or more vertigo spells in one month achieving complete vertigo control. The mean change in the number of attacks was smaller for the groups with fewer attacks, and they also had worse vertigo control.
Three participants received ITD treatment twice. They were not classified as failures since it has been proposed that ITD be given ‘as-needed’ rather than as a one-time treatment [Citation18]. Among the participants who received treatment twice, two out of three achieved complete vertigo control (Class A) after the first treatment, and all three achieved complete control after the second treatment.
We conducted measurements of vertigo-related disability using three distinct methods, all of which demonstrated notable improvement. The application of these findings in clinical practice suggests the advantages of utilizing ITD as an effective initial treatment option prior to medication, or in conjunction with medication. However, additional clinical trials are required to further assess and evaluate its efficacy.
In the placebo-controlled study from 2005, Garduño-Anaya [Citation12] found that 82% of patients in the ITD group achieved Class A vertigo control compared to 57% in the control group at 2 years. While the results for ITD were positive, the placebo group also showed significant improvement. This highlights the potential for MD to go into spontaneous regression, making it difficult to evaluate the efficacy of treatments. In this study, it is challenging to determine whether the results from this small sample are caused by the treatment or a consequence of time and the natural course of the disease.
Based on this information, two arguments can be raised in favor of this short-term study. Firstly, in the natural course of the disease, periods with frequent vertigo do not last longer than 4 months, and the effects of treatments need to be evaluated during the active period of MD. Silverstein et al. reported a statement that ITD should be the first-line therapy for patients with MD at any stage of the disease [Citation13]. Secondly, showing no change in hearing level in the present study indicates that the patients were treated during the active phase and not during spontaneous regression.
Methodological considerations/limitations
There are several limitations to the study design. To achieve high levels of evidence, treatments should be evaluated using Randomized Control Trial (RCT). Therefore, it is difficult to generalize the treatment outcomes shown in the results. At the same time, many studies have been conducted with similar inclusion criteria and treatment outcomes, thus increasing external validity. The trial does not meet the standard follow-up time of 2 years recommended by the AAO-HNS. The population used for the study was relatively small, with 39 participants, of which 27 completed the study.
This problem is not uncommon in studies of MD due to its low prevalence, spontaneous regression, and sudden symptom manifestation. Creating larger studies without broadening the inclusion criteria is difficult, and including patients in different stages and subgroups makes the results uncertain. The small sample size weakens the power of the study. While many statistically significant results were obtained in this trial, the small sample size decreases the likelihood that these results represent a true effect.
A limitation in designing trials with MD patients is the overall low motivation for participation in placebo trials. Patients have experienced disabling vertigo attacks and have a very low tolerance for partially effective treatments. Total vertigo control is highly valued, which is why three participants in the present study received treatment twice, in addition to experiencing positive effects after the first treatment.
There are several problems associated with self-report forms. Patients can exaggerate their symptom burden as a means of being taken more seriously and receiving better care, or perhaps to obtain sick leave. The results filled out on the forms are also affected by an individual’s daily condition, i.e. a good day may produce positive results, whereas a bad day may produce negative results. It is difficult for a patient to recall exactly how the previous month has been. However, both the DHI and THI are standardized inventories that have shown high test-retest reliability, implying that the difference between baseline and after treatment is probably attributable to the treatment rather than the lack of reliability of the instrument.
Silverstein et al. in an RCT on patients with MD in stage four, which is characterized by severe hearing loss and subsidence of dizziness, showed no benefit on symptom outcomes with ITS [Citation13]. How to evaluate and what to expect in results when most of the disabling symptoms with vertigo are not current, besides deterioration in hearing? To simplify, the present study comprises patients in stages 2 and 3, so the outcomes of these two studies cannot be compared.
MD is a chronic disease that seriously affects a patient’s quality of life. Many factors influence a patient’s quality of life, including the severity of symptoms. There are a lot of limitations in studying this patient population. Further improvement in Meniere’s classification and characterization of subgroups can provide a better evaluation of different treatments. Therefore, the stage of the disease, long or short-term outcomes, injection technique, and number of injections need to be well-defined for studies’ comparability.
Yardley showed that perceived support from a health professional was strongly associated with better quality of life in patients with MD [Citation19]. This factor could contribute to the improvement seen in handicap and FLS scores in this trial, meaning that the improvement was not only due to the treatment but also partly due to the increased perceived support from healthcare professionals. Uncertainty remains about the effects of many interventions, including intervention with ITD. There are many different potential pathways through which MD patients could be targeted with ITS to improve outcomes. We have presented one of them in this trial with promising results, so further studies can elucidate different treatments and their outcomes.
Conclusion
This study suggests that ITD can be useful in the treatment of MD. It appeared to have the best short-term effect on patients with severe dizziness handicaps and moderate tinnitus handicaps. It provides limited vertigo control in 73% of patients with attacks longer than 20 min. Participants suffering from a higher frequency of vertigo spells seemed to achieve greater vertigo control. The treatment′s positive effects last at least four months. However, further research is needed to truly underscore ITS as a treatment for MD.
Acknowledgements
The author thanks Dr Gabriella Josefsson for her assistance in writing the manuscript. Thanks to my colleague Dr Luca Verreccia and Dr Niki Karpeta for helping in conducting the study. Many thanks to Tessa Lauronen for the data collection.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Menière’s disease. American Academy of otolaryngology-head and Neck Foundation, Inc. Otolaryngol Head Neck Surg. 1995;113(3):181–185.
- Harris JP, Alexander TH. Current-day prevalence of Meniere’s syndrome. Audiol Neurootol. 2010;15(5):318–322. doi: 10.1159/000286213.
- Nevoux J, Barbara M, Dornhoffer J, et al. International consensus (ICON) on treatment of Ménière’s disease. Eur Ann Otorhinolaryngol Head Neck Dis. 2018;135(1S):S29–S32. doi: 10.1016/j.anorl.2017.12.006.
- Rarey KE, Curtis LM. Receptors for glucocorticoids in the human inner ear. Otolaryngol Head Neck Surg. 1996;115(1):38–41. doi: 10.1016/S0194-5998(96)70133-X.
- MacArthur C, Hausman F, Kempton B, et al. Intratympanic steroid treatments may improve hearing via ion homeostasis alterations and not immune suppression. Otol Neurotol. 2015;36(6):1089–1095. doi: 10.1097/MAO.0000000000000725.
- Fukushima M, Kitahara T, Uno Y, et al. Effects of intratympanic injection of steroids on changes in rat inner ear aquaporin expression. Acta Otolaryngol. 2002;122(6):600–606. doi: 10.1080/000164802320396268.
- Bird PA, Begg EJ, Zhang M, et al. Intratympanic versus intravenous delivery of methylprednisolone to cochlear perilymph. Otol Neurotol. 2007;28(8):1124–1130. doi: 10.1097/MAO.0b013e31815aee21.
- Lee JJ, Jang JH, Choo OS, et al. Steroid intracochlear distribution differs by administration method: systemic versus intratympanic injection. Laryngoscope. 2018;128(1):189–194. doi: 10.1002/lary.26562.
- Casani AP, Piaggi P, Cerchiai N, et al. Intratympanic treatment of intractable unilateral Meniere disease: gentamicin or dexamethasone? A randomized controlled trial. Otolaryngol Head Neck Surg. 2012;146(3):430–437. doi: 10.1177/0194599811429432.
- Patel M. Intratympanic corticosteroids in Ménière’s disease: a mini-review. J Otol. 2017;12(3):117–124. doi: 10.1016/j.joto.2017.06.002.
- Atrache Al Attrache N, Krstulovic C, Pérez Guillen V, et al. Response over time of vertigo spells to intratympanic dexamethasone treatment in Meniere’s disease patients. J Int Adv Otol. 2016;12(1):92–97. doi: 10.5152/iao.2016.2177.
- Garduno-Anaya MA, Couthino De Toledo H, Hinojosa-Gonzalez R, et al. Dexamethasone inner ear perfusion by intratympanic injection in unilateral Meniere’s disease: a two-year prospective, placebo-controlled, double-blind, randomized trial. Otolaryngol Head Neck Surg. 2005;133(2):285–294. doi: 10.1016/j.otohns.2005.05.010.
- Silverstein H, Isaacson JE, Olds MJ, et al. Dexamethasone inner ear perfusion for the treatment of Meniere’s disease: a prospective, randomized, double-blind, crossover trial. Am J Otol. 1998;19(2):196–201.
- Lambert PR, Nguyen S, Maxwell KS, et al. A randomized, double-blind, placebo-controlled clinical study to assess safety and clinical activity of OTO-104 given as a single intratympanic injection in patients with unilateral Ménière’s disease. Otol Neurotol. 2012;33(7):1257–1265. doi: 10.1097/MAO.0b013e318263d35d.
- Webster KE, Lee A, Galbraith K, et al. Intratympanic corticosteroids for Ménière’s disease. Cochrane Database Syst Rev. 2023;2(2):CD015245.
- Boleas-Aguirre MS, Lin FR, Della Santina CC, et al. Longitudinal results with intratympanic dexamethasone in the treatment of Ménière’s disease. Otol Neurotol. 2008;29(1):33–38. doi: 10.1097/mao.0b013e31815dbafc.
- Gürkov R, Jerin C, Flatz W, et al. Clinical manifestations of hydropic ear disease (Menière’s). Eur Arch Otorhinolaryngol. 2019;276(1):27–40. doi: 10.1007/s00405-018-5157-3.
- Leng Y, Liu B, Zhou R, et al. Repeated courses of intratympanic dexamethasone injection are effective for intractable Meniere’s disease. Acta Otolaryngol. 2017;137(2):154–160. doi: 10.1080/00016489.2016.1224920.
- Yardley L, Kirby S. Evaluation of booklet-based self-management of symptoms in Ménière disease: a randomized controlled trial. Psychosom Med. 2006;68(5):762–769. doi: 10.1097/01.psy.0000232269.17906.92.