Abstract
Background
To achieve better speech performance following cochlear implantation (CI), measuring the patient’s cochlear duct length (CDL) and determining the appropriate length of the CI array are important.
Objective
To investigate the CDL in CI patients after using the OTOPLAN software preoperatively and compare the results of angular insertion depth (AID) estimation by OTOPLAN and postoperative radiography.
Materials and Methods
The study included 105 Japanese CI patients with normal cochleae. We measured the CDL using OTOPLAN and the position of the tip channel of the electrode for each selected electrode array, and estimated the AID using the software.
Results
The mean CDL was 35.1 ± 1.6 mm. Preoperatively, the mean estimated AID was 580.3 ± 57.8°. Postoperative radiography revealed a mean AID of 583.0 ± 56.7°, demonstrating a strong linear correlation between the two measurements (R2 = 0.635).
Conclusion and Significance
Our findings revealed that CDL varies widely, which is consistent with previous studies. To achieve better speech perception, surgeons should select the appropriate length of CI electrode array based on the individual’s CDL. Preoperative measurement of each CDL by OTOPLAN, which is clinically feasible and comparable to postoperative evaluation, can be used to ensure selection of the appropriate electrode array length.
Chinese Abstract
背景
为了在人工耳蜗植入 (CI) 后获得更好的言语表现, 测量患者的耳蜗管长度 (CDL) 并确定 CI 阵列的适当长度是很重要的。
目的
探讨CI患者在术前使用OTOPLAN软件后的CDL情况, 通过OTOPLAN 和术后射线照相来比较角度插入深度 (AID) 估计的结果。
材料和方法
该研究纳入了 105 名耳蜗正常的日本 CI 患者。 我们使用 OTOPLAN 以及电极尖端通道的位置, 对每个所选电极阵列测量了 CDL, 并使用软件估计 AID。
结果
平均CDL 为35.1 ± 1.6mm。 术前, 平均估计 AID 为 580.3 ± 57.8°。术后X光检查显示平均AID为583.0 ± 56.7°, 表现出两次测量之间有很强的线性相关性(R2 = 0.635)。
结论和意义
我们的研究结果表明, CDL 差异性很大, 这与之前的研究结果一致。 为了获得更好的言语感知, 外科医生应该根据个人 CDL来选择合适的CI 电极阵列长度。 术前通过 OTOPLAN 测量每个 CDL, 以确保选择适当的电极阵列长度, 这在临床上是可行的, 且与术后评估具有可比性。
Introduction
Cochlear implantation (CI) is a standard treatment for restoring hearing in patients with severe-to-profound deafness across all frequencies. Although a variety of CI electrodes have been developed [Citation1], some studies have demonstrated that the insertion of a longer electrode offers better speech perception performance [Citation2–4], which is supported by a study by Landsberger et al. who reported that longer electrode arrays demonstrated significantly smaller deviations from the spiral ganglion map [Citation5]. Similarly, O’Connell et al. and Nassiri et al. reported that a deeper insertion angle resulted in better hearing [Citation6,Citation7]. However, insertion depth is determined by both the length of the electrode and each individual candidate’s cochlear duct as there are large variations in cochlear duct length (CDL) [Citation8]. Therefore, to achieve a greater degree of cochlear coverage by the CI array, surgeons should aim to measure the patient’s CDL and use it to determine the appropriate length of the CI array. Currently, commercially available software (OTOPLAN, MED-EL [Innsbruck, Austria]) can be used to analyze an individual’s CDL using three-dimensional reconstruction based on computed tomography (CT). To date, several papers have described the feasibility of using OTOPLAN to estimate CDL [Citation9–12]. Additionally, OTOPLAN allows surgeons to preoperatively predict cochlear coverage with angular insertion depth (AID) according to the selected electrode. Here, we investigated the CDL in 105 CI patients with normal cochleae and highlighted a strategy to determine the optimal CI length based on the individual’s CDL using the software. Furthermore, we compared the estimated AID using OTOPLAN with the AID measured on postoperative radiographs to evaluate the feasibility of estimating AID prior to implantation.
Material and methods
This study included 105 patients with normal cochleae who underwent cochlear implantation with FLEX SOFT, FLEX 28, FLEX 26, or FLEX 24 electrodes from MED-EL. Complete CI insertion was achieved in all patients. Many patients underwent bilateral CI surgery; however, only the initially implanted ear was included in this study. summarizes the demographic information of the patients. The mean age at implantation was 42.3 years (range 0–90 years). All the patients were treated between April 2018 and March 2023 at the Department of Otorhinolaryngology - Head and Neck Surgery, Shinshu University Hospital.
Table 1. Patient characteristics evaluated in this study (n = 105).
CDL was measured in all patients using the OTOPLAN software developed by CAScination (Bern, Switzerland). The DICOM files from the preoperative CT images (thickness of CT slices: 0.75 mm) were uploaded to the OTOPLAN software. As we have utilized OTOPLAN version 3.0, from August 2021 at Shinshu University Hospital, we investigated 75 cases retrospectively and 30 cases prospectively using OTOPLAN version 3.0 The data were independently analyzed by two otolaryngologists (H.Y. and K.W.) using multiplanar reformation: an image plane parallel to the basal turn of the cochlea was reconstructed, with ‘Diameter’ (the largest distance from the round window to the contralateral wall) and ‘Width’ (the distance between the cochlear walls perpendicular to the Diameter line) measured. Cochlear height was measured in an orthogonal plane. Based on the determined values (diameter, width, and height), the OTOPLAN software calculated the cochlear duct length according to the elliptic-circular approximation method [Citation13]. Results were evaluated in terms of sex, laterality, and implantation age.
In cooperation with MED-EL, OTOPLAN is also used for preoperative planning prior to CI. In our study, OTOPLAN measured the position of the electrode tip channel according to the electrode array (FLEX SOFT, FLEX 28, FLEX 26, or FLEX 24) used, and estimated the AID. Of the 30 cases in which OTOPLAN was used prospectively, we determined the electrode array based on the estimated AID. Stenver’s view radiographs were obtained for all patients to rule out kinking or tip folding of the electrode postoperatively, and the final position of the electrode array was assessed by two experienced otolaryngologists (H.Y. and K.W.). The AID was measured using the method described by Trieger et al. [Citation14]. Additionally, we compared the AID estimated by OTOPLAN preoperatively with that measured using postoperative radiography. This study was approved by the Ethics Committee of the Shinshu University School of Medicine.
Statistical analysis
The sample sizes are noted in the figure legends. Statistical analysis was performed using the Prism 8 software package (GraphPad, San Diego, CA, USA). The two groups were compared using the unpaired t-test. A p-value <.05 was considered significant. In linear regression analysis, R-squared was calculated.
Results
Preoperative estimated CDL by OTOPLAN
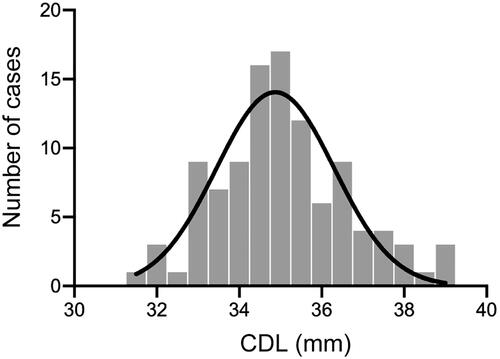
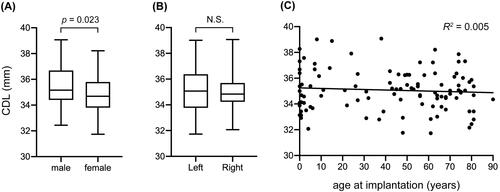
We investigated the CDL in 105 CI patients with normal cochleae using OTOPLAN software ver. 3.0. As a result, the mean CDL, diameter, width and height were 35.1 ± 1.6 mm (range: 31.7–39.1 mm), 9.0 ± 0.4 mm (range: 8.0–9.9 mm), 6.7 ± 0.3 mm (range: 6.0–7.5 mm), and 3.3 ± 0.3 mm (range: 2.7–4.1 mm), respectively (). Results were evaluated in terms of sex, laterality, and implantation age. As a result, males were associated with a longer CDL compared to females (35.5 mm vs. 34.8 mm, p = 0.023), while the other variables demonstrated negative correlations with CDL ().
Comparison of AID by OTOPLAN preoperatively and X-ray postoperatively
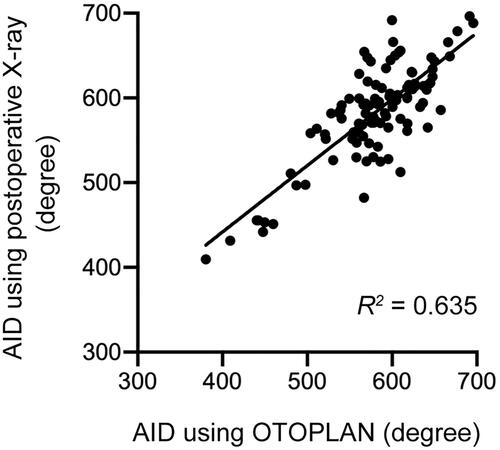
Preoperatively, we used OTOPLAN to estimate AID based on the length of the CI array used. The mean AID was 580.3 ± 57.8° (range: 380.4–695.7°). Postoperatively, we measured AID using radiography. The mean AID was 583.0 ± 56.7°. compares the measurements and demonstrates a strong linear correlation (R2 = 0.635).
Cases determining the length of CI based on the preoperative finding by OTOPLAN
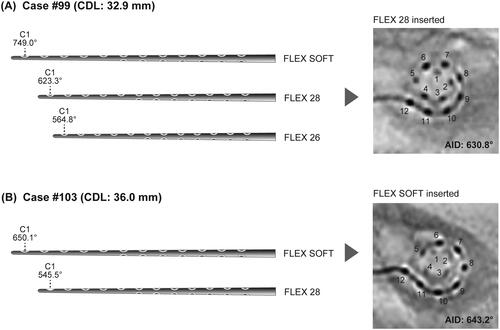
Since August 2021, we have preoperatively analyzed individual CDLs using OTOPLAN version 3.0, and determined the appropriate CI array length based on the results. For example, the CDL of case #99, a 48-year-old female, was measured as 32.9 mm by OTOPLAN ver. 3.0. In addition, when FLEX SOFT, FLEX 28, and FLEX 26 were selected, the software-estimated AIDs were 749.0°, 623.3°, and 564.8°, respectively. Therefore, we used FLEX 28 in this case. Postoperative radiography revealed an AID of 630.8°, which was consistent with the preoperatively estimated AID (). The CDL of case #103, a 76-year-old female, was measured as 36.0 mm by OTOPLAN ver. 3.0. In addition, when FLEX SOFT and FLEX 28 were selected, the software-estimated AIDs were 650.1° and 545.5°, respectively. Therefore, we used FLEX SOFT in this case. Postoperative radiography revealed an AID of 643.2°, which was consistent with the preoperatively estimated AID ().
Figure 4. Image modified from the OTOPLAN results and postoperative radiographs. Each degree means the position of the tip channel (C1) of the electrode according to each selected electrode array. (A) In case #99, FLEX 28 was selected. (B) In case #103, FLEX SOFT was selected. CDL: cochlear duct length; AID: angular insertion depth.
Discussion
To achieve the optimal AID in each CI case, two lengths, CDL and CI array, should be determined. Using OTOPLAN ver. 3.0, our findings indicated that an individual’s CDL can vary widely, which is consistent with the previous report [Citation8]. Therefore, preoperatively measuring the CDL was important, despite the patient’s cochlea being normal. In our study, the mean CDL was 35.1 mm in Japanese patients with normal cochlea, which was comparable to those in other countries (Germany: 36.2 mm [Citation9], United States: 34.0 mm [Citation15], Italy: 32.4 mm [Citation10], Saudi Arabia: 32.9 mm [Citation11]). These results suggest that CDL has not been associated with ethnicity. A longer CDL was observed in males than in females, which is consistent with previous reports [Citation9]. In addition, CDL was not related to age, which is consistent with the fact that human beings are born with a mature inner ear and CDL does not develop after birth.
The appropriate AID in each cochlea following CI is thought to range from 630° to 720°, corresponding to the distribution of the spiral ganglion cell bodies in the cochlea [Citation16–18]. To address this, we utilized OTOPLAN preoperatively and selected the appropriate length of CI array. In case #99 and #103, we selected FLEX28 and FLEX SOFT, respectively, to achieve the appropriate AID (from 630° to 720°). Our findings demonstrated that the average, maximum and minimum CDL was 35.1, 39.1 and 31.7 mm, respectively, meaning that in cases with an average CDL, FLEX soft (31.5 mm) was the optimal CI array (approximate AID: 660°). In cases with a longer CDL, a longer CI array (e.g., FLEX 34) may be acceptable. However, as shown in , in the case of a patient with a shorter cochlear length, we selected a shorter CI array to achieve a sufficient AID.
We applied this strategy not only to patients with severe-to-profound hearing loss across all frequencies, but also to those with high-frequency hearing loss. As in our earlier reports [Citation19,Citation20], most patients with high-frequency hearing loss gradually lose residual acoustic hearing in the implanted ear over time due to the natural course of the underlying disease or other unknown causes, and the hearing preservation scores with longer 28- or 31.5-mm electrodes are comparable to those with shorter electrodes, suggesting that even though residual hearing in the low-frequency region was preserved preoperatively, any length of CI array can be selected based on the individual CDL. In addition, most patients with inner ear malformations have either fewer than two and a half cochlear turns or a cystic apex with a normal external outline of the cochlea [Citation21]. In such cases, a 360° AID, corresponding to the basal turn length, should be sufficient to avoid tip folding. Alshalan et al. reported that the manually measured and estimated basal turn lengths were comparable, allowing us to select a proper CI array based on the length of the basal turns via OTOPLAN in cases with inner ear malformations [Citation22].
Additionally, to validate the preoperative AID measured by OTOPLAN, we compared the software-estimated AID with the AID measured on postoperative radiographs. We are the first to report a strong linear correlation between these findings, suggesting that preoperative simulation was clinically feasible.
In conclusion, to achieve better speech performance following CI, OTOPLAN enabled us to measure the individual CDL and select the appropriate CI array length that led to the optimal AID. The process was validated by demonstrating a correlation with the postoperative AID measurement.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Dhanasingh A, Jolly C. An overview of cochlear implant electrode array designs. Hear Res. 2017;356:93–103. doi:10.1016/j.heares.2017.10.005.
- Canfarotta MW, Dillon MT, Buchman CA, et al. Long-Term influence of electrode array length on speech recognition in cochlear implant users. Laryngoscope. 2021;131(4):892–897. doi:10.1002/lary.28949.
- Buchner A, Illg A, Majdani O, et al. Investigation of the effect of cochlear implant electrode length on speech comprehension in quiet and noise compared with the results with users of electro-acoustic-stimulation, a retrospective analysis. PLoS One. 2017;12(5):e0174900. doi:10.1371/journal.pone.0174900.
- Buchman CA, Dillon MT, King ER, et al. Influence of cochlear implant insertion depth on performance: a prospective randomized trial. Otol Neurotol. 2014;35(10):1773–1779. doi:10.1097/MAO.0000000000000541.
- Landsberger DM, Svrakic M, Roland JT, Jr., et al. The relationship between insertion angles, default frequency allocations, and spiral ganglion place pitch in cochlear implants. Ear Hear. 2015;36(5):e207-13–e213. doi:10.1097/AUD.0000000000000163.
- O’Connell BP, Cakir A, Hunter JB, et al. Electrode location and angular insertion depth are predictors of audiologic outcomes in cochlear implantation. Otol Neurotol. 2016;37(8):1016–1023. doi:10.1097/MAO.0000000000001125.
- Nassiri AM, Yawn RJ, Holder JT, et al. Hearing preservation outcomes using a precurved electrode array inserted with an external sheath. Otol Neurotol. 2020;41(1):33–38. doi:10.1097/MAO.0000000000002426.
- Rask-Andersen H, Liu W, Erixon E, et al. Human cochlea: anatomical characteristics and their relevance for cochlear implantation. Anat Rec. 2012;295(11):1791–1811. doi:10.1002/ar.22599.
- Spiegel JL, Polterauer D, Hempel JM, et al. Variation of the cochlear anatomy and cochlea duct length: analysis with a new tablet-based software. Eur Arch Otorhinolaryngol. 2022;279(4):1851–1861. doi:10.1007/s00405-021-06889-0.
- Lovato A, Marioni G, Gamberini L, et al. OTOPLAN in cochlear implantation for far-advanced otosclerosis. Otol Neurotol. 2020;41(8):e1024–e1028. doi:10.1097/MAO.0000000000002722.
- Khurayzi T, Almuhawas F, Sanosi A. Direct measurement of cochlear parameters for automatic calculation of the cochlear duct length. Ann Saudi Med. 2020;40(3):212–218. doi:10.5144/0256-4947.2020.218.
- Canfarotta MW, Dillon MT, Buss E, et al. Validating a new tablet-based tool in the determination of cochlear implant angular insertion depth. Otol Neurotol. 2019;40(8):1006–1010. doi:10.1097/MAO.0000000000002296.
- Schurzig D, Timm ME, Batsoulis C, et al. A novel method for clinical cochlear duct length estimation toward Patient-Specific cochlear implant selection. OTO Open. 2018;2(4):2473974X18800238. doi:10.1177/2473974X18800238.
- Trieger A, Schulze A, Schneider M, et al. In vivo measurements of the insertion depth of cochlear implant arrays using flat-panel volume computed tomography. Otol Neurotol. 2011;32(1):152–157. doi:10.1097/MAO.0b013e3181fcf04d.
- Canfarotta MW, Dillon MT, Buss E, et al. Frequency-to-Place mismatch: characterizing variability and the influence on speech perception outcomes in cochlear implant recipients. Ear Hear. 2020;41(5):1349–1361. doi:10.1097/AUD.0000000000000864.
- Danielian A, Ishiyama G, Lopez IA, et al. Morphometric linear and angular measurements of the human cochlea in implant patients using 3-dimensional reconstruction. Hear Res. 2020;386:107874. doi:10.1016/j.heares.2019.107874.
- Ariyasu L, Galey FR, Hilsinger R, Jr., et al. Computer-generated three-dimensional reconstruction of the cochlea. Otolaryngol Head Neck Surg. 1989;100(2):87–91. doi:10.1177/019459988910000201.
- Kawano A, Seldon HL, Clark GM. Computer-aided three-dimensional reconstruction in human cochlear maps: measurement of the lengths of organ of corti, outer wall, inner wall, and rosenthal’s canal. Ann Otol Rhinol Laryngol. 1996;105(9):701–709. doi:10.1177/000348949610500906.
- Yoshimura H, Moteki H, Nishio SY, et al. Electric-acoustic stimulation with longer electrodes for potential deterioration in low-frequency hearing. Acta Otolaryngol. 2020;140(8):632–638.
- Van de Heyning PH, Dazert S, Gavilan J, et al. Systematic literature review of hearing preservation rates in cochlear implantation associated with medium- and Longer-Length flexible lateral wall electrode arrays. Front Surg. 2022;9:893839. doi:10.3389/fsurg.2022.893839.
- Sennaroğlu L, Bajin MD. Classification and current management of inner ear malformations. Balkan Med J. 2017;34(5):397–411. doi:10.4274/balkanmedj.2017.0367.
- Alshalan A, Almuhawas F, Alhabib S, et al. Method to estimate the basal turn length in inner ear malformation types. Sci Rep. 2023;13(1):66. doi:10.1038/s41598-022-23911-5.