Abstract
Background
Infants and young children with vestibulocochlear nerve (VCN) hypoplasia/aplasia present with severe hearing loss and are candidates for cochlear implantation (CI). It is unknown whether vestibular function is related to CI outcome and if vestibular tests can guide the operation decision.
Aims/Objectives
Our aim was to describe the vestibular function in patients with VCN hypoplasia/aplasia before a possible CI.
Materials and Methods
Forty-two ears in 23 patients were tested between 2019 and 2022 with bone-conducted cervical vestibular evoked myogenic potentials (BCcVEMP), video head impulse test (vHIT) and miniice-water caloric test (mIWC).
Results
All ears could be tested with at least one vestibular test and 83% could be tested with more than one method. Twenty-nine ears (61%) showed normal function with at least one method. The presence of a normal response to any test doubled the likelihood of a measured hearing threshold after CI, the best predictors being the BCcVEMP and vHIT (p < 0.05).
Conclusion
Canal function may represent a predictor of auditive pathway integrity with a possible favourable audiological outcome after CI operation
Significance
Our results demonstrate high vestibular response rates suggesting a functioning pathway despite the radiological diagnosis.
Chinese Abstract
背景: 前庭耳蜗神经 (VCN) 发育次全/发育不全的婴儿和幼儿患有严重听力损失, 是人工耳蜗植入 (CI) 的人选。 尚不清楚前庭功能是否与 CI 结果相关, 前庭测试是否可以引导手术决定。
目的: 我们的目的是在可能进行CI 之前, 描述 VCN 发育次全/发育不全患者的前庭功能。
材料和方法: 2019 年至 2022 年间, 对 23 名患者的 42 只耳朵进行了测试骨导颈前庭诱发肌源电位 (BCcVEMP)测试、视频头脉冲测试(vHIT) 和小冰-水热量测试 (mIWC)。
结果: 所有耳朵都可进行至少一项前庭测试, 83% 的耳朵可进行一种以上的测试。 29 只耳朵 (61%) 通过至少一种测试法显示出正常功能。 对任何测试的正常反应都会使测得的CI后听力阈值的可能性加倍, 最好的预测因子是 BCcVEMP 和 vHIT (p < 0.05)。
结论: 耳道功能可能代表听觉通路完整性、CI 手术后良好的听力学结果的预测因子。
意义: 我们的结果表现出较高的前庭反应率, 说明尽管有放射学诊断, 还是有一条功能通路的。
Introduction
Cochlear implantation is indicated in infants and young children with severe hearing loss. In a subgroup of these cases, hearing loss is associated with a malformation of the vestibulocochlear nerve (VCN) in the form of nerve hypoplasia or aplasia. It is calculated that 2% of severe hearing loss at birth is attributed to cochlear nerve hypoplasias/aplasias [Citation1]. The diagnosis is established with magnetic resonance imaging (MRI) and it refers to the cochlear nerve calibre in comparison to the facial nerve [Citation2]. The radiological references to the vestibular nerve are often unclear. The resolution of the vestibular nerve bundles is either absent or very limited. Moreover, it is often difficult to distinguish between a hypoplasia versus a total VCN aplasia, as the nerve axons might not maintain the expected calibre throughout the nerve pathway.
Cochlear nerve hypoplasia or aplasia is a major diagnostic consideration when hearing loss is detected during universal newborn hearing screening programmes, because of the urgency of cochlear implantation surgery (CI) or in special cases auditory brainstem implantation (ABI) [Citation3–5]. Less attention has been given to the vestibular nerve function in these conditions. Until recently vestibular testing was not always available in very young children. However, technological progress has made it possible to assess vestibular function in infants and toddlers [Citation6–9]. This advancement is of major importance for early clinical management since up to 70% of children with severe hearing loss also present with a vestibular dysfunction. This leads to a poorer postural control and delay in motor development when compared with healthy peers [Citation10–12]. VCN hypoplasia/aplasia can be accompanied by inner ear malformations but has also been described as a sole finding in MRI [Citation13, Citation14]. Among the few available classification systems for VCN hypo/aplasia, the one proposed by Casselman et al. [Citation15] is most commonly applied in clinical practice and focuses on the identification of the cochlear nerve with or without inner ear malformations.
In this study we investigated the vestibular function in cases of childhood deafness related to VCN malformations. We explored whether the vestibular function is related to auditory function before and after CI and if a vestibular test can be used as an indicator for a surgical decision.
Material and methods
In this retrospective cohort study, we collected data from patient files between 2019 and 2022. Patients were referred to the Audiology and Neurotology department for vestibular testing as a part of the candidacy assessment for CI or ABI. All patients had a radiological diagnosis of vestibulocochlear unilateral or bilateral nerve aplasia/hypoplasia established by MRI scan. All cases were reviewed by an expert radiologist and classified according to Casselman’s grading scale: Grade 1 = no identifiable VCN (), 2 A = VCN with aplasia/hypoplasia of its cochlear branch in the presence of labyrinthine malformations (), and 2B = VCN with aplasia/hypoplasia of the cochlear branch with normal labyrinth (). In two ears initially classified as VCN aplasia/hypoplasia, the review of the MRI results identified normal nerve anatomy with isolated inner ear malformation and were excluded from the analysis. In two ears the nerve anatomy was normal and were also excluded from the study. One patient was excluded because we could not retrieve MRI scans for review and grading. Twenty- three patients (42 ears) were included in the study.
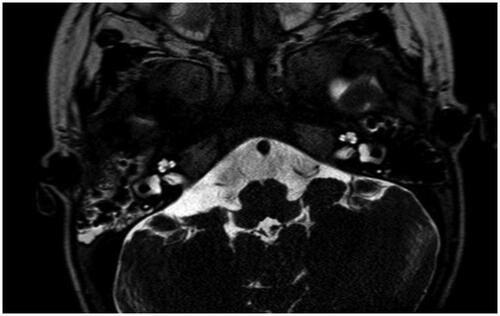
Figure 1. Magnetic resonance imaging of a case with vestibulocochlear nerve aplasia, Casselman grade 1. Arrow points to the facial nerve.
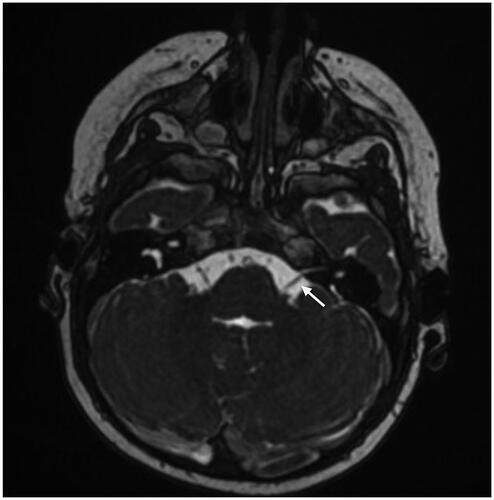
Figure 2. Magnetic resonance imaging of a case with vestibulocochlear nerve hypoplasia on the right side and bilateral inner ear malformation, Casselman grade 2 A. Arrow points to the left vestibulocochlear nerve (normal nerves on left side).
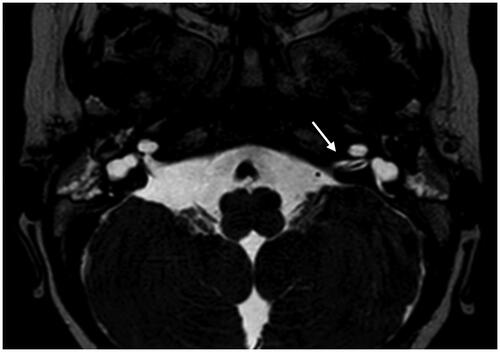
Figure 3. Magnetic resonance imaging of a case with bilateral vestibulocochlear nerve aplasia and normal inner ear anatomy, Casselman grade 2B.
To evaluate the vestibular function, we used a standard test protocol for children. The methodology is described in detail elsewhere [Citation8] and consisted of:
Bone conducted cervical vestibular myogenic evoked potentials (BC c-VEMP)
Video head impulse test (vHIT)
Mini ice-water caloric test (mIWC)
A short description of the three test modalities follows.
BC c-VEMP was used to study the sacculo-collic reflex and the inferior vestibular nerve function. Infants were tested in supine position in the parent’s lap with the investigator suspending the child´s head to achieve sternocleidomastoid muscle (SCM) activation. Older children sat in an upright position with their head rotated watching a film on a screen in order to achieve SCM contraction on the contralateral side. BC c-VEMP was recorded with an evoked potentials device (Interacoustics Eclipse EP 25, Interacoustics, Middelfart, Denmark). Skin Electrodes were placed on the upper part of each sternocleidomastoid muscle, on the patient’s forehead (ground) and on the patient’s suprasternal notch (Vertex-reference electrode). Bone stimulation was attained with the Radio Ear B71 transducer (Radio Ear Corp, New Eagle, PA, USA) on the mastoid, one ear at a time. Two trials of at least 100 sweeps were recorded on each side. The device permitted an electromyographical (EMG) driven stimulus delivery with an EMG window stated at 50 μV − 250 μV. The response was defined as a positive-negative (P1-N1) EMG deflection at short latencies (between 12 and 30 ms). The device has an algorithm for the calculation of wave reproducibility in percentage, which we considered acceptable when equal or higher than 70%. If the P1/N1 (rectified) wave was not identified after two trials, this was defined as a pathological response, indicative of vestibular loss.
The vHIT is a video-assisted method to quantify the vestibulo-ocular reflex (VOR) and superior vestibular nerve function based on the clinical head impulse test. It measures the ratio (gain) between the eye and head velocities at peak of head acceleration during jerk horizontal rotations. We used the Synapsys vHIT Ulmer Device, version 2 (Synapsys, Marseille, France) which incorporates a remote video camera. Children were tested sitting in their parents’ lap about 90 cm away from a fixed point or a screen. Three to five head impulses were performed on the lateral plane. A vestibular loss was defined as a gain value lower than 0.7 in infants (<12 months) and 0.8 for 1 year old or older subjects. The presence of repetitive pathological saccades was also taken into account in cases of uncertainty i.e. when not enough head shake repetitions could be obtained. The recorded data of the head impulses were reviewed for this purpose.
mIWC is another method to study the VOR by thermal stimulation. It was used to complement the canal/superior vestibular nerve examination or in cases where vHIT was impossible to perform due to the subjects’ young age. The test was performed with 6–10 °C ice water filling the subject’s outer ear canal for 10 s and looking for a caloric nystagmus between 30 to 90 sec after irrigation with an adapted videonystagmoscopy system. One side was tested at a time. The absence of a clear caloric nystagmus was defined as vestibular loss.
A parameter named ‘’Vestibular Function’’ was introduced to describe normal responses in at least one test modality (BC c-VEMP, vHIT or mIWC), representing vestibular function per ear.
Furthermore, we reviewed the relation between the vestibular responses and the pre-operative Auditory Brainstem Response (ABR), and intraoperative recordings of electrically evoked stapedial reflexes (ESRT) and the electrically-evoked ABR (eABR). The last two tests were performed as a standard procedure during CI implantation to assess responses from the auditory system elicited by electrical stimulation using the CI. Finally, the preoperative vestibular responses were compared to the postoperative aided hearing threshold (average across 0.5, 1, 2, 4 kHz, measured by presenting frequency-modulated tones in a double-walled sound booth with a cochlear implant sound processor active) here called ‘response to sound’ (RS). RS was considered absent when no behavioural response at any frequency up to 90 dB HL could be documented. Aided hearing thresholds were assessed by an experienced paediatric audiologist using age-appropriate methodology such as visual reinforcement or play audiometry.
Statistical analysis
Descriptive statistics were used to present demographics and the different parameters related to subject (Casselman´s grading, age at testing and implantation), vestibular testing (normal response =1, vestibular loss = 0, non-applicable = n/a) and audiological tests (response present = 1, absent = 0, non-applicable = n/a). Responses referred to ears and not to patients, as VCN malformations can occur differently between the sides or unilaterally. Non-parametric tests (Fisher exact test) were used to study the association between the different parameters obtained at vestibular tests and hearing testing. Level of statistical significance was stated at α < 0.05. In case of significant associations, we have calculated also the effect size with Cramer´s V-test.
Ethics
The study was approved by the Swedish national ethics committee (application number 2022-03381-01).
Results
Six of the 23 patients were female. Age at vestibular testing ranged between 5.8 months and 19 years with a median age of 13 months (IQR 22) and a mean age of 37.4 months (SD 58.5). The demographics and radiological characteristics of all 23 patients are shown in .
Table 1. Demographics and radiological characteristics.
Compliance with testing
Patients’ compliance varied for each vestibular test, as it is age sensitive. All 42 ears could however be tested with at least one vestibular test and 40 ears (95%) could be tested with more than one method.
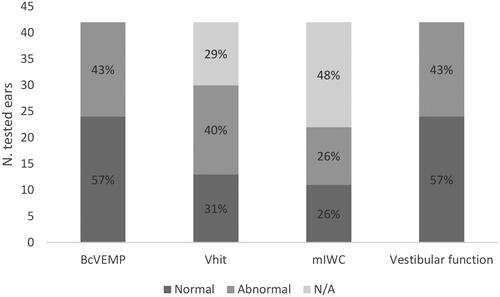
The proportion of normal and abnormal responses to BC c-VEMP, vHIT and mIWC are shown in . The majority of tested ears (57%) were deemed as having vestibular function (c.f. rightmost bar in ), showing normal responses in at least one vestibular test.
Figure 4. Response rates assessed by BcVEMP, vHIT, mIWC, and vestibular function module. N/A: Not available.
According to Casselman’s grading, five ears were classified as Casselman 1 (). We recorded a vestibular function in 3 of these 5 ears (60%) combining the responses from the different vestibular tests.
Casselman 2 A was the most common classification (23 ears) (). A vestibular function could be recorded in 44% of the tested ears. We detected reproducible responses in 44% and 35% respectively in BC c-VEMP and vHIT. mIWC, when applicable, was consistent with vHIT responses.
The 2B subgroup consisted of 14 ears. We detected vestibular responses in 11/14 (78%) of the tested ears. BC c-VEMP showed the highest response rate (92%) among all testing methods followed by vHIT (66%) and mIWC (58%).
Relation between vestibular function and preoperative ABR
We could retrieve information of preoperative ABR in 38 ears. (). Depending on the vestibular test, a valid preoperative ABR was obtained in 24–55% of ears with a normal response, whereas ears with vestibular loss showed a valid ABR in 9–18% of the cases. The mIWC test was related to a three-fold higher prevalence of a valid preoperative ABR. Generally, the odds of obtaining a preoperative ABR were double in the presence of a measured vestibular responses, in particular when it was possible to obtain a vestibular response with mIWC (Fisher’s exact test, p = .05).
Table 2. Relation between preoperative ABR and vestibular responses.
Relation between vestibular function and intraoperative measurements in CI operated ears
Twenty two of the 42 ears (52%) were implanted with CI. Electrical auditory brainstem responses (eABR) were tested intraoperatively in 20 ears, electrically evoked stapedial reflexes (eSRT) in 17 ears. All 22 ears could be tested with at least one method of vestibular testing preoperatively, BC c-VEMP was possible to perform in 22 ears, vHIT in 16 ears and mIWC in 13 ears.
eABR and eSRT showed no significant association with vestibular test responses. However, eSRT could only be evoked in ears with a valid BC c-VEMP or vHIT response and eABR was only seen in ears with BC c-VEMP responses.
Relation between vestibular function, preoperative ABR and post-operative acoustic tests
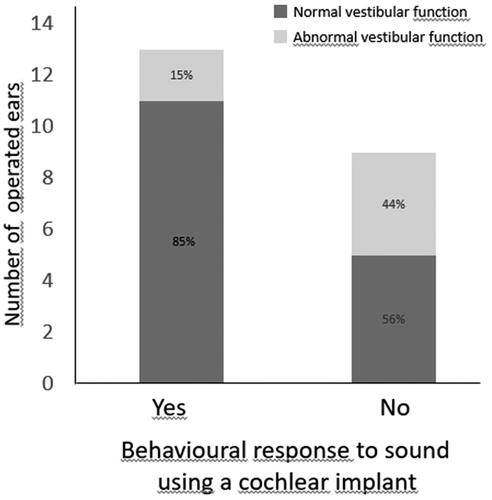
Behavioural response to sound (RS) was tested in all 22 ears postoperatively. The relationship between the Vestibular function module and the postoperative aided hearing threshold tests is shown in . The mean age at the aided hearing threshold measurement was 2.6 years. Aided hearing thresholds (i.e. an average threshold across 0.5, 1, 2, and 4 kHz) were obtained in 76% and 86% of ears with normal responses at BC c-VEMP and vHIT, respectively, whereas thresholds were only recorded in 16% (BC c-VEMP) and 22% (vHIT) of ears with vestibular loss (p = .02 with effect size = 0.52 and p = .04 with effect size = 0.63 respectively). For ears with normal responses for either vHIT or BC c-VEMP, the mean average hearing threshold was 43 dB HL (33–51 dB HL).
Group 2B was of particular interest as it presented with isolated cochlear nerve hypoplasia/aplasia with no inner ear anatomical malformations. Six of the 8 operated ears in this category demonstrated measurable aided hearing thresholds post-operatively. Vestibular function had been tested in all eight ears. An acoustic response was always present when BC c-VEMP or vHIT was present preoperatively: a complete dichotomization was the case of vHIT test, with four ears with normal vHIT showing also measurable hearing thresholds and two ears with vestibular loss showing no response to acoustic stimulation (Fisher´s exact test, p = .067).
We could obtain an ABR test in 21 of the 22 operated ears. Five of the six ears with a reproducible ABR response (86%) demonstrated also a present post-operative RS. Seven (47%) of the remaining 15 ears with no consistent preoperative ABR showed a present post-operative RS but this was not statistically significant ((Fisher’s extact test p = .178).
Discussion
Our findings demonstrate vestibular response in more than half of the cases with VCN malformation tested in our department. We found that otolith function (BC c-VEMP) showed the highest score in terms of vestibular response and test feasibility (57% normal responses and 81% tested ears) followed by vHIT (normal in 31% and 71% tested). mIWC could be measured in only 52% of ears, either confirming the vHIT results or providing canal function information in cases where vHIT testing was unsuccessful or unclear. Whereas the combination of mIWC and BC c-VEMP is more effective in determining the canal and otolith function in very young children (< 6 months), the combination of vHIT and BC c-VEMP seems to be more feasible for testing children. The feasibility of the vestibular test battery in this age group has been shown in previous studies [Citation7, Citation8]. In this study we could confirm that it is an adequate clinical protocol with the possibility to alternate between three different vestibular test modalities.
There are a few radiological grading systems [Citation2, Citation15] for VCN malformations, none of which are universally accepted. Limitations of MRI resolution make it difficult to distinguish between hypoplasia and aplasia of the VCN and even more challenging to distinguish between the involvement of the vestibular versus the cochlear branch. Moreover the VCN can vary in volume throughout its pathway to the brainstem [Citation16] something that can also affect both the audiological and the vestibular results. Previous studies focus mainly on the hypoplasia/aplasia of the cochlear nerve and how this anatomical issue can affect auditory and speech outcomes after implantation [Citation3, Citation17]. In this series of patients we have only included cases of aplasia and severe hypoplasia.
VCN hypoplasias/aplasias are usually studied together with other aetiological factors of profound hearing loss[Citation4] and little is known about the vestibular function in cases with VCN hypoplasia/aplasia. Furthermore, the majority of the studies focus on hearing results after a CI or an ABI [Citation4]. Our findings demonstrate that the presence of a vestibular response in cases of VCN hypoplasia, but more interestingly in cases of VCN aplasia, can be attributed to the presence of a functioning nerve pathway that is not identifiable, or underestimated, with MRI. The high vestibular response rate in our findings supports this. As in the referenced publication of Verrecchia et al. 2020 the main outcome of vestibular testing in so young children is to ascertain the presence or absence of a vestibular response in each ear. The definition of vestibular response in our study is described in the method section. A unilateral or bilateral vestibular absence, is of clinical interest for early and correct intervention and treatment as supported by other studies [Citation18]. These previous studies have shown that the motor development of toddlers is affected in cases of complete vestibular failure whereas a residual vestibular function is related to a better or near normal motor development [Citation9]. While a unilateral residual function can still be associated with normal motor development, a bilateral vestibular failure leads to motor proficiency developmental delays [Citation18]. Early and correct diagnosis together with dedicated rehabilitation protocols seem to improve the motor development of children with vestibular impairments [Citation19].
We examined the association between vestibular responses and auditory brainstem responses. We found that canal function (measured with mIWC) was significantly related to an auditory response at preoperative ABR. This result, if confirmed by larger studies, emphasizes the utility of vestibular testing in assessment of candidacy for Cochlear/Brainstem implants in children with VCN malformations.
We have also determined the relationship between the preoperative vestibular responses and the audiological responses in CI operated children. Although the relationship between the preoperative vestibular tests and the intraoperative CI tests was inconclusive due to heterogeneity in the dataset, we observed a robust trend between preoperative vestibular responses and postoperative aided behavioural responses to frequency-modulated tones. A valid response with BC c-VEMP or vHIT was significantly associated with a postoperative RS. This was also indicated by our results in subgroup 2B (isolated VCN malformations), where vHIT was a good indicator of RS in this particular group. A study by Ozdoğmuş et al. [Citation20] showed that both the superior and inferior vestibular nerve branches may receive fibres from the facial and/or cochlear nerve within the internal auditory meatus towards the brainstem end. These connections might explain why a present vestibular function is positively related to an RS postoperatively or a pre-op ABR with possible identifiable action potentials for 80–90 db nHL intensity levels.
In conclusion, in our study, a vestibular response was seen in more than half of the tested ears, where these responses, and in particular normal canal function, were positively associated with audiological responses, both pre- and postoperatively. This study is limited by its retrospective design and the heterogeneity of the dataset with some missing data. We suggest that future prospective studies aim to confirm the findings presented here, thereby increasing our knowledge on whether vestibular testing can represent an important add-on in the clinical assessment of VCN malformations.
Disclosure statement
The authors report there are no competing interests to declare.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Freeman SR, Sennaroglu L. Management of cochlear nerve hypoplasia and aplasia. Adv Otorhinolaryngol. 2018;81:81–92. doi:10.1159/000485542.
- Glastonbury CM, Davidson HC, Harnsberger HR, et al. Imaging findings of cochlear nerve deficiency. AJNR Am J Neuroradiol. 2002;23(4):635–643.
- Chao X, Luo J, Wang R, et al. Long-Term auditory and speech outcomes of cochlear implantation in children With cochlear nerve aplasia. Ear Hear. 2023;44(3):558–565. doi:10.1097/AUD.0000000000001299.
- Cushing SL, Gordon KA, Rutka JA, et al. Vestibular end-organ dysfunction in children with sensorineural hearing loss and cochlear implants: an expanded cohort and etiologic assessment. Otol Neurotol. 2013;34(3):422–428. doi:10.1097/MAO.0b013e31827b4ba0.
- Ehrmann-Müller D, Kühn H, Matthies C, et al. Outcomes after cochlear implant provision in children with cochlear nerve hypoplasia or aplasia. (1872-8464 (Electronic)).
- Martens S, Dhooge I, Dhondt C, et al. Vestibular infant screening (VIS)-flanders: results after 1.5 years of vestibular screening in hearing-impaired children. Sci Rep. 2020;10(1):21011–20201203. doi:10.1038/s41598-020-78049-z.
- Verrecchia L, Karpeta N, Westin M, et al. Methodological aspects of testing vestibular evoked myogenic potentials in infants at universal hearing screening program. Sci Rep. 2019;9(1):17225–20191121. doi:10.1038/s41598-019-53143-z.
- Verrecchia L, Galle Barrett K, Karltorp E. The feasibility, validity and reliability of a child friendly vestibular assessment in infants and children candidates to cochlear implant. Int J Pediatr Otorhinolaryngol. 2020;135:110093. doi:10.1016/j.ijporl.2020.110093.
- Janky KL, Rodriguez AI. Quantitative vestibular function testing in the pediatric population. Semin Hear. 2018;39(3):257–274. doi:10.1055/s-0038-1666817.
- Cushing SL, Papsin BC, Rutka JA, et al. Evidence of vestibular and balance dysfunction in children with profound sensorineural hearing loss using cochlear implants. Laryngoscope. 2008;118(10):1814–1823. doi:10.1097/MLG.0b013e31817fadfa.
- Verbecque E, Marijnissen T, De Belder N, et al. Vestibular (dys)function in children with sensorineural hearing loss: a systematic review. Int J Audiol. 2017;56(6):361–381. doi:10.1080/14992027.2017.1281444.
- Tribukait A, Brantberg K, Bergenius J. Function of semicircular canals, utricles and saccules in deaf children. Acta Otolaryngol. 2004;124(1):41–48. doi:10.1080/00016480310002113.
- Sennaroglu L, Saatci I. A new classification for cochleovestibular malformations. Laryngoscope. 2002;112(12):2230–2241. doi:10.1097/00005537-200212000-00019.
- Sennaroglu L. Cochlear implantation in inner ear malformations–a review article. Cochlear Implants Int. 2010;11(1):4–41. doi:10.1002/cii.416.
- Casselman JW, Offeciers FE, Govaerts PJ, et al. Aplasia and hypoplasia of the vestibulocochlear nerve: diagnosis with MR imaging. Radiology. 1997;202(3):773–781. doi:10.1148/radiology.202.3.9051033.
- De Foer B, Kenis C, Van Melkebeke D, et al. Pathology of the vestibulocochlear nerve. Eur J Radiol. 2010;74(2):349–358. doi:10.1016/j.ejrad.2009.06.033.
- Birman CS, Powell HR, Gibson WP, et al. Cochlear implant outcomes in cochlea nerve aplasia and hypoplasia. Otol Neurotol. 2016;37(5):438–445. doi:10.1097/MAO.0000000000000997.
- Rine RM, Wiener-Vacher S. Evaluation and treatment of vestibular dysfunction in children. NeuroRehabilitation. 2013;32(3):507–518. doi:10.3233/NRE-130873.
- Rine RM, Braswell J, Fisher D, et al. Improvement of motor development and postural control following intervention in children with sensorineural hearing loss and vestibular impairment. Int J Pediatr Otorhinolaryngol. 2004;68(9):1141–1148. doi:10.1016/j.ijporl.2004.04.007.
- Ozdoğmuş O, Sezen O, Kubilay U, et al. Connections between the facial, vestibular and cochlear nerve bundles within the internal auditory canal. J Anat. 2004;205(1):65–75. doi:10.1111/j.0021-8782.2004.00313.x.