Abstract
Background
Age-related hearing loss (ARHL) is a major cause of chronic disability among the elderly. Individuals with ARHL not only have trouble hearing sounds, but also with speech perception. As the perception of auditory information is reliant on integration between widespread brain networks to interpret auditory stimuli, both auditory and extra-auditory systems which mainly include visual, motor and attention systems, play an important role in compensating for ARHL.
Objectives
To better understand the compensatory mechanism of ARHL and inspire better interventions that may alleviate ARHL.
Methods
We mainly focus on the existing information on ARHL-related central compensation. The compensatory effects of hearing aids (HAs) and cochlear implants (CIs) on ARHL were also discussed.
Results
Studies have shown that ARHL can induce cochlear hair cell damage or loss and cochlear synaptopathy, which could induce central compensation including compensation of auditory and extra-auditory neural networks. The use of HAs and CIs can improve bottom-up processing by enabling ‘better’ input to the auditory pathways and then to the cortex by enhancing the diminished auditory signal.
Conclusions
The central compensation of ARHL and its possible correlation with HAs and CIs are current hotspots in the field and should be given focus in future research.
Chinese abstract
背景
年龄相关性听力损失(ARHL)是老年人慢性障碍的主要原因。患有 ARHL 的人不仅在听觉上有困难, 而且在言语感知上也有困难。 由于对听觉信息的感知依赖于广布的大脑网络之间的整合来解释听觉刺激, 包括听觉系统和听觉外系统, 主要包括视觉、动作和注意力系统, 在 ARHL 的补偿中发挥着重要作用。
目的
更好地了解 ARHL 的补偿机制并激发更好的可能会缓解 ARHL的干预措施。
方法
我们主要关注ARHL相关的中心补偿的现有信息。 还讨论了助听器 (HA) 和人工耳蜗 (CI) 对 ARHL 的补偿作用。
结果
研究表明, ARHL可诱发耳蜗毛细胞损伤或丧失以及耳蜗突触病, 可能引起中枢补偿, 包括听觉和听觉外神经网络的补偿。 HA 和 CI 的使用可以通过“更好”的输入至听觉通路, 然后输入到皮层, 通过增强减弱的听觉信号来改进自下而上的处理。
结论
ARHL 的中心补偿及其与 HA 和 CI 的可能相关性是当前该领域的热点, 应该在未来研究中给予关注。
Introduction
Hearing loss, with especial reference to age-related hearing loss (ARHL), is one of the world’s most prevalent sensory deficits. According to the WHO, 32.8% of the world’s population over 65 years old has been diagnosed with hearing loss, with this prevalence continuing to grow(Hearing loss in persons 65 and older based on WHO global estimates on prevalence of hearing loss. 2012. http://www.who.int/pbd/deafness/news/ GE_65years). ARHL is now the fourth leading contributor to years lived with disability globally [Citation1]. Typically, those who have ARHL not only have problems hearing sounds, but also with speech perception. The effects of age on hearing can manifest at multiple levels of the auditory pathway. Given these obstacles, one might quite reasonably conclude that speech comprehension by older adults would be, if not impossible, at least severely compromised. However, the comprehension of natural speech is usually well maintained throughout older age. More central auditory regions may exhibit compensatory plasticity due to this reduced peripheral drive. A deeper understanding of these compensatory changes, as well as of the contributions of top-down (regulation from the central to the periphery) auditory, and non-auditory processes, is very important to improve all aspects of speech recognition ability among the elderly.
The auditory changes in age-related hearing loss
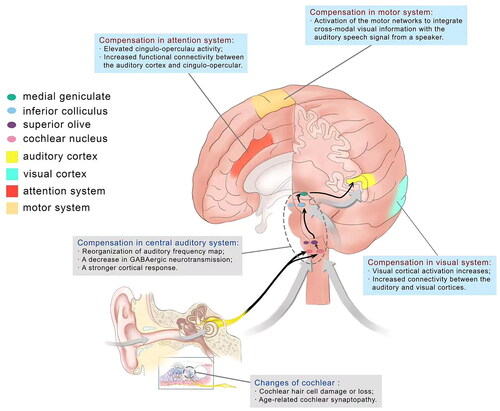
In the auditory periphery, cochlear hair cell damage or loss has long been considered a hallmark of age-related sensorineural hearing loss, which is often associated with poorer hearing thresholds. In aging populations, outer hair cell loss is preferentially observed at the basal end of the basilar membrane responsible for encoding high frequency information [Citation2]. In addition to hair cell loss, animal models have revealed a more subtle reduction in processing efficiency that stems from synaptic dysfunction and degeneration of cochlear nerve axons. Synapses between inner hair cells (IHCs) and auditory nerve fibers (ANFs) are highly vulnerable to aging [Citation3]. Normal aging results in a progressive decline in the number of these synapses. Synaptic loss precedes the loss of hair cells and changes in hearing thresholds. Loss of ANFs and their cell bodies in the spiral ganglion follows with a delay, but the functional connections/communication between affected fibers and their target IHCs are lost with the synapses. Age-related cochlear synaptopathy, which means the early events include diffuse loss of synapses between inner hair cells (IHCs) and cochlear nerve fibers throughout the cochlea with aging, can be exacerbated by early exposure to noise that causes synaptopathy but no permanent threshold shifts [Citation4]. Since subtotal synaptopathy goes largely undetected by threshold audiogram, it has been termed a ‘hidden’ form of hearing loss.
Beyond the cochlea, animal and human data demonstrate age-related changes in the function of spiral ganglion neurons [Citation5], cochlear nuclei, the superior olivary complex, and other midbrain structures up through the inferior colliculus [Citation6]. Age-related changes in the auditory brainstem can result in altered temporal processing ability, which may be reflected as a reduced ability in tasks such as detecting a brief temporal gap in a continuous tone. Furthermore, age-related differences in temporal processing have been associated with poorer speech perception [Citation7]. The auditory brainstem response (ABR; see Glossary) is commonly used for evaluating the integrity of the auditory system, as it reflects the time-locked firing of subcortical auditory nuclei in response to an acoustic stimulus.
Compensation of age-related hearing loss in central auditory system
The compensatory mechanism for peripheral hearing loss in the auditory system of the ageing listener remains unclear. Older adults often experience difficulties in speech perception in noise, which likely have neurophysiological contributors from various levels of auditory processing. Within the cochlear nuclei, auditory nerve projections terminate onto a variety of excitatory and inhibitory cell types that each process their inputs differently and have different spectro-temporal profiles, and all of which change with age [Citation8]. Cochlear synaptopathy and the silencing of auditory neurons may affect suprathreshold coding by degrading the representation of temporal cues being sent to the rest of the auditory pathway [Citation9], which are seen before losses in neural thresholds. This would occur across cochlear nucleus neurons tuned to a wide range of frequencies due to the contribution of the low-frequency tails of higher-frequency neurons [Citation10].
Different parts of the central system may all show compensatory plasticity in an attempt to reestablish the homeostatic balance between excitation and inhibition. These compensatory changes are mediated in part by cortical inhibitory circuits, which are thought to decline with a decrease in peripheral inputs. The inhibitory neurotransmitters GABA and glycine serve to shape responses to temporally complex sounds in the central auditory pathway, including in the cochlear nucleus, inferior colliculus, auditory thalamus, and auditory cortex. A decrease in GABAergic neurotransmission with age was observed at different levels of the auditory system, including in the cochlear nucleus, inferior colliculus, auditory forebrain, auditory thalamus, and auditory cortex [Citation11]. This change in the balance between inhibitory and excitatory transmissions, such as decreased inhibitory neurotransmission occurring with age across various central auditory nuclei, may lead to a modulation frequency selective increase in the representation of envelope cues at the level of the auditory midbrain and cortex. Furthermore, researchers have demonstrated that age-related temporal speech-processing deficits arising from the midbrain may be compensated by a stronger cortical response [Citation12].
Extra-auditory systems supporting degraded speech comprehension in age-related hearing loss
The perception, and particularly comprehension, of auditory information is reliant on integration between widespread brain networks to interpret auditory stimuli. Studies of age-related functional connectivity changes report that older adults have less distinct brain networks. It was found that elderly showed a decrease in connectivity between areas belonging to the same functional network, and increased connectivity between areas within these networks and areas belonging to different functional networks [Citation13]. Studies have found that even mild forms of hearing loss alter how the brain routes information within the auditory–motor loop [Citation14]. Meanwhile, increased connectivity between visual and auditory sensory cortices [Citation15] as well as in the attention networks [Citation16] were reported in cases of ARHL. Currently the visual, attention, and motor cortices are thought to be the three main systems that support speech perception in addition to the auditory cortex in cases of ARHL.
Older adults with hearing loss demonstrate a reduced ability to suppress activity in other sensory brain areas during auditory processing when compared to those without hearing loss. For example, visual cortical activation increases during auditory word recognition tasks when intelligibility decreases due to increased background noise [Citation17]. Furthermore, resting-state fMRI revealed increased connectivity between the auditory and visual cortices in cases of ARHL [Citation18]. It is deemed that increased visual activation may help support the auditory system during interpretation of degraded auditory information. In turn, individuals with ARHL also show increased activation in auditory areas during the presentation of visual stimuli [Citation19]. The relative integrity of the visual subnetwork is essential, not only for processing visual information about static and moving objects, but also for providing spatial awareness, guidance of actions, and object recognition. Within the larger context of multisensory integration for perceptual accuracy, the augmentation of visual performances has been ascribed to an adaptive mechanism compensating for the lack of auditory input.
The motor network is also thought to play an important role in compensation for ARHL. Accumulating evidence suggests the articulatory motor cortex is involved in speech perception, particularly when speech perception is required under challenging listening conditions. It is thought that, when listening becomes more challenging, the individual relies on integration across numerous brain areas to understand the auditory message (for example, by recruiting the motor cortices to provide motor representations of speech). As for how motor networks are utilized for speech perception in older adults with hearing loss, the motor compensation hypothesis suggests that activation of the motor networks compensates for impaired auditory processing in ARHL [Citation20]. This hypothesis assumes that the articulatory motor cortex is upregulated during speech perception in persons with auditory deficits, and that this process compensates for impaired auditory function to aid speech perception. These sensory and sensorimotor functional connections may underlie the ability to integrate cross-modal visual information (e.g. articulatory movements and facial expressions) with the auditory speech signal from a speaker, which is known to greatly improve comprehension of auditory signals [Citation21].
As for attention system, currently thought to be mainly related to cingulo-opercular, which involves several brain areas including the anterior insula, anterior cingulate cortices, and thalamus, is also believed to be of importance to speech processing in both normal-hearing and hearing-impaired individuals [Citation22]. This view is supported by neuroimaging evidence that older adults with elevated cingulo-opercular activity perform better in challenging speech recognition tasks [Citation23]. These findings are important because they indicate that cingulo-opercular activity is a normal response to improve performance and increased cingulo-opercular activity has been associated with increased effort. The cingulo-opercular network is involved in goal oriented behaviors through the initiation and top-down (central to peripheral) maintenance of task sets, adjusting performance in response to errors which means incorrect response due to a lapse in attention or accident to optimize performance [Citation18], so the cingulo-opercular network is advantageous for speech perception especially in challenging listening conditions. In according with these, greater atrophy of the anterior cingulate cortex was observed in individuals with ARHL, where this atrophy was related to greater memory impairment [Citation24]. Evidence also suggests that cases of ARHL show increased functional connectivity between the auditory cortex and cingulo-opercular network in resting-state fMRI [Citation25], which provides insight into potential compensatory neural activation associated with ARHL. It has been suggested that impaired auditory processing in ARHL leads to more effortful listening, which depletes the limited resource capacity available for both listening and nonauditory cognitive functions [Citation26]. Researchers have proposed that the activation of neural networks involved in effortful listening could contribute to the observed neural degeneration of these areas in ARHL, including for instance degeneration due to glutamate excitotoxicity of cingulate neurons [Citation24].
Compensation related to hearing aids (HAs) and cochlear implants (CIs) among individuals with ARHL
The compensatory mechanisms of auditory and external auditory system have been reviewed before, then if there are some interventions which we can use to regulate the ARHL-related neural plasticity to promote better compensation. The ability of neurons in the central auditory system to accurately encode important temporal features of speech may be limited by impaired neural synchrony, delayed neural recovery, reduced phase locking [Citation27], or other mechanisms associated with aging. This effects are especially salient when the target speech stream is masked by background noise. Hearing aids amplification as the main clinical interventions for ARHL can result in stimulation of the auditory pathways that have been altered by processes associated with aging. In addition, hearing aids significantly modify the physical characteristics of sound, which may explain why adjusting to new hearing aids requires time [Citation28]. At present, HAs and CIs are the main clinical interventions for ARHL. The reintroduction of auditory stimuli with HAs and CIs may stimulate neuroplasticity induced by hearing exposure, which modifies or reverses the plasticity caused by hearing loss that has already occurred (i.e. secondary remodeling) [Citation29]. A series of clinical studies have been conducted about the impact of HAs and CIs on hearing loss induced (HLI) plasticity. Researches have shown that the use of HAs and CIs not only improves communication, but also significantly improves cognition, psychosocial function, family relations, and quality of life [Citation30]. As mentioned earlier, presbycusis, which is dominated by high-frequency hearing loss, may lead to HLI plasticity changes in the central auditory system (CAS), and this process can be promoted by enhanced acoustic stimulation. The sensory devices are used as a compensatory methodology to enhance the diminished auditory signal, and improve bottom-up processing by enabling ‘better’ input to the auditory pathways and then to the cortex. Karawani’s finding in 2018 suggested that HAs may alter subcortical processing and offset neural timing delay [Citation31].
There is no unified conclusion as to whether unilateral or bilateral hearing aids are more beneficial in treating hearing loss. Most studies suggest that bilateral HAs can more effectively reduce listening effort and improve auditory perception [Citation32], while other studies concluded that bilateral hearing aids are no better than their unilateral counterparts [Citation33]. As for CIs, most current studies have shown that bilateral CIs can effectively improve both the patient’s auditory perception function and localization ability, take advantage of the head shadow, and improve speech perception in noise [Citation32]. Few studies have shown that patients with bilateral CIs do not experience any substantial benefits in localization [Citation33]. In addition to bilateral HAs and bilateral CIs, previous studies have also indicated that binaural advantages can be obtained by using both a hearing aid and cochlear implant in opposite ears as a form of bimodal listening. It is recommended that bimodal stimulation be standard practice for the rehabilitation of adults who wear unilateral cochlear implants [Citation34]. Overall, between HAs and CIs, there is no final conclusion as to which kind of intervention offers more advantages in improving speech perception [Citation35].
One reasonable explanation for why bilateral hearing compensation is better than unilateral hearing compensation is ‘auditory deprivation effects’. Sillman et al. [Citation36] found that the use of monaural HAs in adults with bilateral sensorineural hearing loss caused a decrease in over-threshold speech perception by the naked ear, which was attributed to ‘delayed hearing deprivation’. In subsequent retrospective and prospective studies, similar results were obtained, further indicating that continuous asymmetric auditory stimulation can induce significant deprivation effects [Citation37]. These deprivation effects can be partially or completely reversed by using binaural magnification in some, but not all cases [Citation29]. The changes in the physiological and/or anatomical properties of the CAS (i.e. neuroplasticity) may be caused by unilateral magnification. Many neurons in the central auditory system respond to binaural input – therefore, input from ‘deprived’ ears will not effectively activate these neurons, whereas input from amplified ears will greatly activate these neurons. As neurons ‘compete’ with synapses, the input activated by the amplified ear will overwhelm the deprived ear, thereby reducing the effectiveness of the sound entering that ear. Studies have shown that with the increase of age, the central nervous system of the elderly retains a strong ability for plasticity [Citation38]. Therefore, understanding how to use central plasticity to improve the effectiveness of HAs and CIs is of a great importance to treat presbycusis.
Conclusion
Studies have shown that ARHL can induce cochlear hair cell damage or loss and cochlear synaptopathy, which could induce central compensation including compensation of auditory and extra-auditory neural networks (), to help the elderly maintain relatively decent communication functioning. Additionally, the use of HAs and CIs can improve bottom-up processing by enabling ‘better’ input to the auditory pathways and then to the cortex by enhancing the diminished auditory signal. The central compensation of auditory and extra-auditory neural network in case of ARHL and its possible correlation with HAs and CIs are current hotspots in the field and should be given focus in future research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Wilson BS, Tucci DL, Merson MH, et al. Global hearing health care: new findings and perspectives. Lancet. 2017; Dec 2390(10111):2503–2515. doi:10.1016/S0140-6736(17)31073-5.
- Flood LM. SCHUKNECHT’S PATHOLOGY of the EAR, 3rd edn. S merchant, J nadol. McGraw-Hill education (UK), 2010 ISBN 978 1 60795 030 1 pp 960 price 202.39. J Laryngol Otol. 2013;127(3):329–329. doi:10.1017/S0022215112003064.
- Kujawa SG, Liberman MC. Synaptopathy in the noise-exposed and aging cochlea: primary neural degeneration in acquired sensorineural hearing loss. Hear Res. 2015; Dec330(Pt B):191–199. doi:10.1016/j.heares.2015.02.009.
- Fernandez KA, Jeffers P, Lall K, et al. Aging after noise exposure: acceleration of cochlear synaptopathy in "recovered" ears. J Neurosci. 2015;35(19):7509–7520. doi:10.1523/JNEUROSCI.5138-14.2015.
- Bao J, Ohlemiller KK. Age-related loss of spiral ganglion neurons. Hear Res. 2010; Jun 1264(1-2):93–97. doi:10.1016/j.heares.2009.10.009.
- Caspary DM, Ling L, Turner JG, et al. Inhibitory neurotransmission, plasticity and aging in the mammalian Central auditory system. J Exp Biol. 2008; Jun211(Pt 11):1781–1791. doi:10.1242/jeb.013581.
- Walton JP. Timing is everything: temporal processing deficits in the aged auditory brainstem. Hear Res. 2010; Jun 1264(1–2):63–69. doi:10.1016/j.heares.2010.03.002.
- Schatteman TA, Hughes LF, Caspary DM. Aged-related loss of temporal processing: altered responses to amplitude modulated tones in rat dorsal cochlear nucleus. Neuroscience. 2008; Jun 12154(1):329–337. doi:10.1016/j.neuroscience.2008.02.025.
- Aravindakshan P, Kujawa SG. Synaptopathy in the aging cochlea: characterizing early-neural deficits in auditory temporal envelope processing. J Neurosci. 2018;38(32):7108–7119. doi:10.1523/JNEUROSCI.3240-17.2018.
- Lai J, Bartlett EL. Masking differentially affects envelope-following responses in young and aged animals. Neuroscience. 2018;386:150–165. S0306452218304159-. doi:10.1016/j.neuroscience.2018.06.004.
- Stebbings KA, Choi HW, Ravindra A, et al. Ageing‐related changes in GABAergic inhibition in mouse auditory cortex, measured using in vitro flavoprotein autofluorescence imaging. J Physiol. 2016;594(1):207–21. doi:10.1113/JP271221.
- Presacco A, Simon JZ, Anderson S. Speech-in-noise representation in the aging midbrain and cortex: effects of hearing loss. PLoS One. 2019;14(3):e0213899. doi:10.1371/journal.pone.0213899.
- Tomasi D, Volkow ND. Aging and functional brain networks. Mol Psychiatry. 2012; May17(5):471–471. 549-58. doi:10.1038/mp.2012.27.
- Bidelman GM, Mahmud MS, Yeasin M, et al. Age-related hearing loss increases full-brain connectivity while reversing directed signaling within the dorsal-ventral pathway for speech. Brain Struct Funct. 2019; Nov224(8):2661–2676. doi:10.1007/s00429-019-01922-9.
- Puschmann S, Thiel CM. Changed crossmodal functional connectivity in older adults with hearing loss. Cortex. 2017;86:109–122. doi:10.1016/j.cortex.2016.10.014.
- Husain FT, Schmidt SA. Using resting state functional connectivity to unravel networks of tinnitus. Hear Res. 2014;307(1):153–162. doi:10.1016/j.heares.2013.07.010.
- Vaden KI, Kuchinsky SE, Ahlstrom JB, et al. Cingulo-Opercular function during word recognition in noise for older adults with hearing loss. Exp Aging Res. 2016;42(1):67–82. doi:10.1080/0361073X.2016.1108784.
- Power JD, Petersen SE. Control-related systems in the human brain. Curr Opin Neurobiol. 2013;23(2):223–228. doi:10.1016/j.conb.2012.12.009.
- Campbell J, Sharma A. Cross-Modal Re-Organization in adults with early stage hearing loss. PLoS One. 2014;9(2):e90594. doi:10.1371/journal.pone.0090594.
- Du Y, Buchsbaum BR, Grady CL, et al. Increased activity in frontal motor cortex compensates impaired speech perception in older adults. Nat Commun. 2016;7(1):12241. doi:10.1038/ncomms12241.
- Eckert MA, Kamdar NV, Chang CE, et al. A cross-modal system linking primary auditory and visual cortices: evidence from intrinsic fMRI connectivity analysis. Hum Brain Mapp. 2008; Jul29(7):848–857. doi:10.1002/hbm.20560.
- Peelle JE. Listening effort: how the cognitive consequences of acoustic challenge are reflected in brain and behavior. Ear Hear. 2018; Mar/Apr39(2):204–214. doi:10.1097/AUD.0000000000000494.
- Peelle JE, Troiani V, Wingfield A, et al. Neural processing during older adults’ comprehension of spoken sentences: age differences in resource allocation and connectivity. Cereb Cortex. 2010; Apr20(4):773–782. doi:10.1093/cercor/bhp142.
- Belkhiria C, Vergara RC, Martín S, et al. Cingulate cortex atrophy is associated with hearing loss in presbycusis with cochlear amplifier DysfunctionData_sheet_1.PDF. Front Aging Neurosci. 2019;11:97. doi:10.3389/fnagi.2019.00097.
- Fitzhugh MC, Hemesath A, Schaefer SY, et al. Functional connectivity of heschl’s gyrus associated with age-related hearing loss: a resting-state fMRI study. Front Psychol. 2019;10:2485. doi:10.3389/fpsyg.2019.02485.
- Fortunato S, Forli F, Guglielmi V, et al. A review of new insights on the association between hearing loss and cognitive decline in ageing. Acta Otorhinolaryngol Ital. 2016;36(3):155–166. doi:10.14639/0392-100X-993.
- Sergeyenko Y, Lall K, Liberman MC, et al. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci. 2013; Aug 2133(34):13686–13694. doi:10.1523/JNEUROSCI.1783-13.2013.
- Tyler RS, Summerfield AQ. Cochlear implantation: relationships with research on auditory deprivation and acclimatization. Ear Hear. 1996; Jun17(3 Suppl):38s–50s. doi:10.1097/00003446-199617031-00005.
- Willott JF. Physiological plasticity in the auditory system and its possible relevance to hearing aid use, deprivation effects, and acclimatization. Ear Hear. 1996; Jun17(3 Suppl):66s–77s. doi:10.1097/00003446-199617031-00007.
- Noble W, Gatehouse S. Effects of bilateral versus unilateral hearing aid fitting on abilities measured by the speech, spatial, and qualities of hearing scale (SSQ). Int J Audiol. 2006; Mar45(3):172–181. doi:10.1080/14992020500376933.
- Karawani H, Jenkins K, Anderson S. Neural and behavioral changes after the use of hearing aids. Clin Neurophysiol. 2018; Jun129(6):1254–1267. doi:10.1016/j.clinph.2018.03.024.
- Dunn CC, Tyler RS, Oakley S, et al. Comparison of speech recognition and localization performance in bilateral and unilateral cochlear implant users matched on duration of deafness and age at implantation. Ear Hear. 2008; Jun29(3):352–359. doi:10.1097/AUD.0b013e318167b870.
- Seeber BU, Baumann U, Fastl H. Localization ability with bimodal hearing aids and bilateral cochlear implants. J Acoust Soc Am. 2004; Sep116(3):1698–1709. doi:10.1121/1.1776192.
- Ching TY, Incerti P, Hill M. Binaural benefits for adults who use hearing aids and cochlear implants in opposite ears. Ear Hear. 2004; Feb25(1):9–21. doi:10.1097/01.AUD.0000111261.84611.C8.
- Kitterick PT, Smith SN, Lucas L. Hearing instruments for unilateral severe-to-Profound sensorineural hearing loss in adults: a systematic review and Meta-Analysis. Ear Hear. 2016; Sep-Oct37(5):495–507. doi:10.1097/AUD.0000000000000313.
- Silman S, Gelfand SA, Silverman CA. Late-onset auditory deprivation: effects of monaural versus binaural hearing aids. J Acoust Soc Am. 1984; Nov76(5):1357–1362. doi:10.1121/1.391451.
- Neuman AC. Late-onset auditory deprivation: a review of past research and an assessment of future research needs. Ear Hear. 1996; Jun17(3 Suppl):3s–13s. doi:10.1097/00003446-199617031-00002.
- Cisneros-Franco JM, Ouellet L, Kamal B, et al. A brain without brakes: reduced inhibition is associated with enhanced but dysregulated plasticity in the aged rat auditory cortex. eNeuro. 2018; Jul-Aug5(4):ENEURO.0051-18.2018. doi:10.1523/ENEURO.0051-18.2018.