Abstract
Nitric acid became commonly available in the seventeenth century. Since then, it held the interest of chemists, especially those interested in the art of dyeing. Due to what is now called the xanthoproteic reaction (from Greek xanthós, describing shades of yellow), nitric acid produces a stable yellow colouration in proteinaceous materials, such as wool, silk, and bones. The chemistry of this reaction is well understood today. Less well-known is that it held the interest of dyers in the past. Dyers considered the ability of nitric acid to give a yellow colour to certain substances a solution to giving materials a durable, that is, a lasting, yellow colour. Yellow, indeed, posed a problem in the art of dyeing. Before the discovery of synthetic dyes in the mid-nineteenth century, there were no organic yellow dyes with long-term colour stability. Using historical dyeing manuals and chemistry treatises, combined with our practical engagement with the processes they describe, this paper traces how, between the seventeenth and nineteenth centuries, dyers explored nitric acid while examining the durability of yellow colourations. Based on these explorations into nitric acid, the chemical arts developed theories about the nature of colour, and about the causes for its relative permanence.
Introduction
Yellow posed a particular problem in the art of dyeing fabric a long-lasting colour. Before the discovery of synthetic dyes in the mid-nineteenth century, not many organic yellow dyes presented long-term colour stability to visible or ultraviolet light and other environmental factors. Eighteenth and nineteenth-century treatises on the art of dyeing show that much effort was made to remedy the non-durability of yellow textiles. In search of permanent colours, chemists recorded complicated experiments with different types of mordants and other additives and documented the exploration of new materials imported from Southeast Asia and the Americas. It is against this backdrop of searching for a lasting yellow colour that chemists began to explore nitric acid for giving a permanent yellow stain to proteinaceous substances, such as wool, silk, and bones, or to heighten the colour and permanence of certain organic dyes. Today, the reaction of nitric acid with proteinaceous substances is known as the xanthoproteic reaction, a chemical colour reaction that is used to test for the presence or absence of certain proteins that contain aromatic amino acids, such as tryptophan, tyrosine or phenylalanine. Nitric acid, reacting with aromatic compounds, forms the corresponding nitrated products, that typically have a characteristic yellow colour.
This paper traces how, between the seventeenth and nineteenth centuries, chemists, and especially those interested in the art of dyeing, explored nitric acid in their examination of the durability of yellow colourations. It shows that, based on their explorations into nitric acid, the chemical arts developed theories about the nature of colour, and specifically about the causes for its relative permanence. Our practical engagement with some of these historical processes in the context of a modern chemistry lab helped us gain a better understanding of the nature and different shades of yellow produced by nitric acid on the various substances explored in the historical sources – ranging from silk and dyes to wood. By visualising some procedures difficult to put into words, these experiments also offer readers a better understanding of the often-complex chemical processes explored by the historical actors discussed in this paper.
Finding a durable yellow for cloth
In terms of permanence, the most popular yellow dye in the art of textile dyeing came from the plant known as weld (Reseda luteola). Dyeing with weld has a long history, some of which has been discussed in other papers in this volume.Footnote1 In the eighteenth and nineteenth centuries, at the height of chemical explorations of the colouration of textiles, dyeing manuals often present weld as a benchmark for the durability of yellow dyes. In his 1815 Chemical Essays, Samuel Parks explains that “the most permanent yellow dye we know of is procured from the weld.”Footnote2 Parks makes this remark when discussing the influence of the extraction temperature of dye from quercitron bark on the final colour it communicates to the textile. He argues that unlike quercitron dye – a New World dye, obtained from North American Eastern Black Oak trees (Quercus velutina) – weld can be extracted at all temperatures without its colour being impacted:
I have often made experiments on extracting the colour from this plant [i.e. Reseda luteola], but I never observed that the beauty of the dye was in the least impaired by boiling.Footnote3
Yellow communicated to wool by weld has little permanency, if the wool be not previously prepared by some mordant. For this purpose, alum and tartar are used, by means of which that plant gives a very pure yellow, which has the advantage of being permanent.Footnote4
But despite weld’s relative durability compared to other yellow dyes, giving textiles a permanent yellow colour with plant dyes remained problematic as in the end none of the yellow plant dyes are particularly long-lasting. Eighteenth and nineteenth-century treatises on the art of dyeing show that much effort was made to remedy the non-durability of yellow textiles. In search of permanent colours, they record complicated experiments with different types of mordants and other additives and document the exploration of new materials imported from Southeast Asia and the Americas such as the quercitron bark mentioned above. Quercitron dye was not considered as brilliant as weld, but treatises mention that it was believed to be at least as durable if used under the right circumstances.Footnote5 It is against this backdrop of searching for a lasting yellow colour that chemists begin to explore nitric acid for giving a permanent yellow stain to proteinaceous substances, such as wool, silk, and bones, and for heightening the colour and durability of certain organic dyes on textiles. These explorations with acid dyes were based on earlier knowledge about nitric acid and its role in producing permanent colours.
Salts and colour permanence
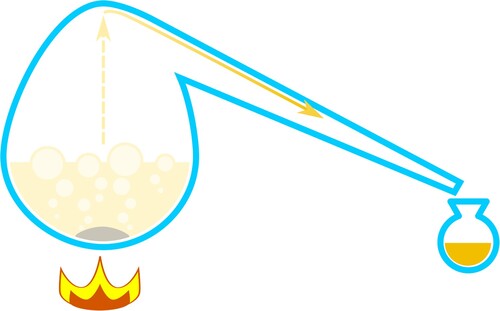
While nitric acid was probably a known chemical substance since the medieval period, it had become commonly available by the seventeenth century.Footnote6 The earliest discussion of nitric acid as a colouring agent for certain materials can be found in the work of Johann Rudolph Glauber (1604–1670). In his Philosophical Furnaces (Furni novi philosophici, 1646–1649, translated into English in 1651), Glauber provides detailed information on how to prepare nitric acid, a substance he calls aqua fortis, and explains that the spirit of paper and linen cloth “tingeth the nailes, skin and hair with a yellow colour.”Footnote7 In his 1653 Miraculum mundi, Glauber is the first to include information about nitre in its fixed form (i.e. potassium nitrate) and volatile form (i.e. nitric acid) and lists some of its many uses.Footnote8 It is interesting that in subsequent works, Glauber considers the making of aqua fortis common knowledge and refuses to describe its production for fear of repeating himself. In his 1656 Explicatio of the Miraculum mundi, for instance, Glauber argues that it needs no proof how “Aqua-fortis may be prepared of Nitre by distillation” because “the way of making it being every where known, as a thing common” ().Footnote9
Figure 1. Schematic representation of Glauber’s method for preparing nitric acid: sulphuric acid and saltpeter (potassium nitrate) are distilled from a retort; the product coming out of the beak is nitric acid, whose purity depends on the quality of the sulphuric acid, while potassium bisulphate is formed as a by-product and remains in the retort. © G. Montanari.
In the Explicatio of the Miraculum mundi, Glauber presses the usefulness of nitre and sets forth its many practical applications in thirty-nine points. These uses range from waterproofing the clothes of “Souldiers, Merchants, Travellers, Carriers, Fisherman and others, who are much in the open Air” with a special varnish made from linseed oil cooked with nitre; to the blackening of certain hard woods with aqua fortis to make an imitation of ebony; and its use by engravers to etch lines on the copper plate “which otherwise would require a long time to be engraven.”Footnote10 Significant for our present discussion, in addition to its ability to create a yellow stain, Glauber also points out that nitric acid played an important role in bringing out and preserving colour in silk dyed with red dyes obtained from the dried bodies of female scale insects, such as dyer’s kermes (primarily from Kermes vermilio) and cochineal (from Old World insects such as Porphyrophora polonica, Porphyrophora hamelii, and later from New World scale insects such as Dactylopius coccus). Glauber refers to those who practice this art in point four of his overview of the uses of nitre:
Point IV. Embroiderers may put any durable Colour they please upon the Silk with which they work.
It may be known, that Nitre, as being a depurated salt, will easily induce colours, and constantly preserve them, yea, exalt them, which many know, who dye cloth with rich grain colours, as Scarlet, Crimson, &c. When they add the Spirit of Nitre in the boiling, to aluminate it (as the Dyers call it) the colour is wonderfully exalted, and made much more fiery, so that it may be sold dearer than common Crimson or Scarlet. This spirit of Nitre also tingeth Ashes, Nails, or Hoofs, Quills, or Feathers, with a golden colour.Footnote11
that the Infusion or Decoction of some Plants, as of Brazil, Senna, &c. will be heighten’d into a redish colour, by putting Alkalizate Salts, as of Tartar, or of Pot-ashes, in the Water that extracts their Tinctures: Whereas acid Spirits, at least some of them, will much impair, if not destroy their colour; as a little Aqua fortis will immediately turn a red Tincture of Brazil, made in fair Water, into a pale yellow.Footnote14
Staining bones, horns, ivory, and wood with nitric acid
Glauber mentioned the ability of nitric acid to impart a yellow stain to certain animal substances. For Boyle, such permanent stains became an argument for proving the porosity of these materials, as he sets forth in his Essay of the Porousness of Animal Bodies (1684):
Bones, Hornes, and the parts of the like Substance, being those that are granted to be the most solid of the Bodies of Animals, I come in the last place to shew by particular Experiments that these also have their Pores.Footnote15
The like durableness I found in the Purple spots, that I sometimes purposely made on my Nails, by letting some little drops of the solution of Gold in Aqua Regia dry upon them, which I now and then did, to observe the way of the Nails growth. For if the stain were made near the root of the nail, it would be still, though very slowly, thrust on by the new matter, till after some weeks it arrived to the further end of the Nail, and was fit to be pared off with it.Footnote18
In addition to the staining of animal substances yellow or other colours, seventeenth-century treatises also mention the ability of nitric acid to stain woods yellow. One of the earliest treatises to describe this practice is Stalker and Parker’s 1688 Treatise on Japaning and Varnishing. The second recipe in the section on the “Dyeing or Staining of Wood, Ivory, &c.” explains how to stain wood “a fine yellow.”Footnote19 We learn that white wood such as “Burr or knotty Ash” should be made smooth and then warmed. The aqua fortis is applied with a brush over the wood and then you should “hold it to the fire, as you do Japan-work, until it leaves smoking.” Next, you have to sand the wood again because the aqua fortis, Stalker and Parker explain, “will make it very rough.” The stained wood is finally polished and varnished and then polished again:
you’ll find no outlandish wood surpass it; for the curled and knotty parts admit of so much Variety, being in some places hard, in others soft and open-grained, to which Aqua fortis gives a deeper colour, than to the harder and more resisting parts.Footnote20
Figure 3. (a) Jacques Lamarre (gunsmith), Flintlock Sporting Gun of Empress Margarita Teresa of Spain (1651–1673), ca. 1670–73, Metropolitan Museum of Art, New York. (b) Detail of (a).

Seventy years later, Robert Dossie (1717–1777) provides a similar recipe for staining wood yellow with aqua fortis in his Handmaid to the Arts (1758). Like Stalker and Parker, Dossie explains that the wood should be warm when the aqua fortis is applied and “held to the fire immediately afterwards.”Footnote21 He also includes the warning that you must make sure that your aqua fortis is not too strong, “or that it be sparingly used,” because “otherwise a brown, sometimes even blackish may be the result.” Dossie concludes that the varnishing of wood stained yellow with nitric acid is required “to render any of these stains more beautiful and durable.”Footnote22
The effect of nitric acid on certain woods, which is still well known in today’s woodworking circles, may reasonably be derived from the nitration of polyphenols (such as tannins) naturally present in wood.Footnote23 To verify this hypothesis and the effect that heating has on the final colouration of the wood, we compared the effect of nitric acid on solutions with different concentrations of tannic acid, before and after heating. Using lab-grade tannic acid (purchased from Sigma-Aldrich), we prepared a 10%, 5%, and 2.5% tannic acid solution in distilled water, to which 2 mL of 68% nitric acid was added. The solutions were then heated to a simmer, turning them distinctly more yellow than the untreated solutions (). Nitric acid can attack an activated aromatic ring (such as a phenol) to form a yellow-coloured nitro-derivative, in a way not dissimilar to the xanthoproteic reaction used to dye silk or wool yellow. Of course, different woods contain different amounts and types of tannins, resulting in slightly different colourations. Additionally, there are other components (such as lignin and cellulose)Footnote24 which can react with nitric acid to form other reaction products that can determine the final colour and texture of the treated wood. From our experiments, we found that heating the mixture (as Stalker and Parker and Dossie recommend doing with the wood) is necessary to get a brighter yellow colour.
Durable yellows from trees
It is possible that the artisanal practice of staining of wood yellow with nitric acid led the German physician and chemist Mathias Brunnwiser to propose a theory about the nature of dyes in plant materials, wood especially. In his 1771 Entdeckung verschiedener vegetabilischen Farbmaterialien, Seiden- und Wollenzeuge schön und dauerhaft gelb zu färben (Discovery of Various Vegetable Dyestuffs to Dye Silk and Wool Textiles a Beautiful and Durable Yellow), Brunnwiser points out that the dyeing substances that give a yellow colour – and particularly deliver a good, beautiful, durable colour, unchangeable in the air and sun (besonders aber gute, schöne, beständige, in der Luft und Sonne unveränderliche Farbe) – are to his knowledge not to be found in such large numbers that more were not desirable.Footnote25 Brunnwiser observes that wood changes colour when exposed to air, and he is keen to find out where these colours come from and how he can produce them by artificial means. Brunnwiser argues that woods potentially contain all colours. He bases this theory on the fact that, depending on the acidic solution applied to wood, it assumes different colours, which to Brunnwiser are a mixture of yellow, blue, and red. According to Brunnwiser, the colours displayed in berries, leaves, and so forth exist first in the wood of the plant (but are disguised by an alkali) before they become visible. He sets out to prove this theory by producing a yellow colour in wool, silk, and camel’s hair with nitric acid.
Brunnwiser’s experiments procced as follows: he first applies nitric acid to wood and then pours water on it. He then cooks the wood in this liquid and as soon as it is brought to a boil, samples of wool, silk, and camel’s hair are added. These samples are cooked until the colour penetrates the textiles in a beautiful and even manner. Finally, they are rinsed in cold water.
According to Brunnwiser this process produces yellow textiles that are at least as good in terms of beauty, gloss, and appearance as yellow textiles from East India and France, and others shown to him in the shops. He also argues for their superior durability, in that the yellow samples are not changed, or damaged in colour or other properties, by exposure to sunlight or air.Footnote26 In other words, in addition to being beautiful, his yellow dye is more durable. Brunnwiser concludes his treatise by mentioning several other durable yellows that have recently been discovered, but, he argues, even though these may be durable and beautiful, they are not easy to obtain and therefore cannot be used for “general use” (allgemeinen Gebrauche).Footnote27 This, of course, is the advantage of his own invention, which is derived from commonly known and easily manageable trees such as Weidenholz (from the different species of the genus Salix, i.e. willow). Brunnwiser goes so far as to suggest that his discovery could meet the demands of entire countries and replace all other yellow dyes. Here the problem of yellow dyeing has become one of resources: since the wood that delivers the yellow colourant is easily cultivated, Brunnwiser’s beautiful yellow dye is not just durable but also cheap.
Colour permanence and combustion in Berthollet
It would soon be discovered that Brunnwiser’s presumed breakthrough was based on the wrong interpretation of what caused the yellow colour in his textiles. Twenty years after the publication of Brunnwiser’s treatise, the renowned chemist Claude Louis Berthollet gave an extensive explanation of why nitric acid stains silk yellow in his Elements of the Art of Dyeing (Élements de l’art de la teinture, 1791). In the section dealing with the causes “Of the Yellow Colour produced in Animal Substances by the Nitric and oxygenated Muriatic Acids,” Berthollet engages with Brunnwiser’s theory by explaining why it is not correct. Berthollet points out that it is not dye from the wood that stains the textile, as Brunnwiser had suggested, but the nitric acid that he used to first stain the wood a yellow colour.Footnote28
Before the section on the yellowing effects of nitric acid on animal substances, Berthollet first discussed “the Action of different Substances, particularly of Air and Light on Colours.” Berthollet’s rationale for putting these two topics together is that he considers them related. According to Berthollet “the stability of colour consists in its power of resisting the action of vegetable acids, alkalis, soap, and more especially that of air and light.”Footnote29 Berthollet’s theory of colour permanency revolves around “combustion.” From several practical observations and experiments, he explains how air causes the combustion of plant- and animal-derived colouring matters thus changing these dyestuffs into red, yellow or “fawn” (fauve) colours:
it [the air] first combines with them, renders them weaker and paler, and by degrees occasions a slight combustion, by means of which, the hydrogen, which entered in their composition is destroyed; they change to a yellow, red, or fawn colour; their attraction for the fabric seems to diminish; they separate from it, and are carried off by water.Footnote30
Colouring substances therefore resist the action of the air more or less, according as they are more or less disposed to unite with oxygen, and thereby to suffer more or less quickly, a smaller or greater degree of combustion. Light favours this effect, which in many cases is not produced without its assistance; but the colouring matter in its separate state, is much more prone to this combustion, than when united to a substance, such as alumina, which may either defend it by its own power of resisting combustion, or by attracting it strongly, weaken its action on other substances, which is the chief effect of mordants; and finally, this last compound acquires still greater durability, when it is capable of combining intimately with the fabric.Footnote32
Berthollet warns that his observations should not lead the reader to infer that “all yellow colours are owing to the carbonic part of the colouring substance,” as very different compounds are capable of producing the same colour.Footnote35 He also warns the reader not to infer that there cannot be examples where oxygen unites with colouring substances, “without weakening their colour, or changing it to yellow (sans en affoiblir la couleur ou sans la faire passer au jaune).”Footnote36 To illustrate this last point, Berthollet gives two examples. He argues that the chapter on indigo shows that this blue dye can change its colour to green when it unites “with an alkali, with lime or a metallic oxyd” but can regain its colour “when it recovers a small portion of the oxygen which it had lost.”Footnote37 The second example concerns Tyrian purple. According to Berthollet, “The liquor of the whelk employed to dye purple, is naturally yellowish; but when exposed to the air, and more especially to the sun, it quickly passes through various shades, and at length assumes that colour so precious in the eyes of the ancients.”Footnote38
Dyeing silk yellow with nitric acid
It is therefore combustion that Berthollet considers to be behind the colour change in silk when exposed to nitric acid:
I therefore suppose that the yellow colour arises from a commencement of combustion, as explained in the preceding chapter [on “the Action of different Substances, particularly of Air and Light on Colours”]. This combustion being very slight, it does not sensibly weaken the silk; but if the acid is a little too strong, or the immersion too long continued, or if the whole of it is not carried off by careful washing, the silk immediately becomes weak, and in vulgar language, which is here quite accurate, is burnt (elle est brûlée).Footnote39
Berthollet explains that something similar must happen through the action of oxygenated muriatic acid [i.e. chlorine] but also that it differs in ways he does not yet fully comprehend.Footnote40 The colour of chlorine on silk is of a much lighter yellow and it has a much stronger action on the silk:
it soon weakens, and even dissolves it: if the silk be left for some time in this fluid, the yellow which at first appeared grows lighter; in conformity to what I have remarked, that oxygen by being accumulated, is capable of disguising the yellow colour occasioned by the combustion, which it had at first produced.Footnote41
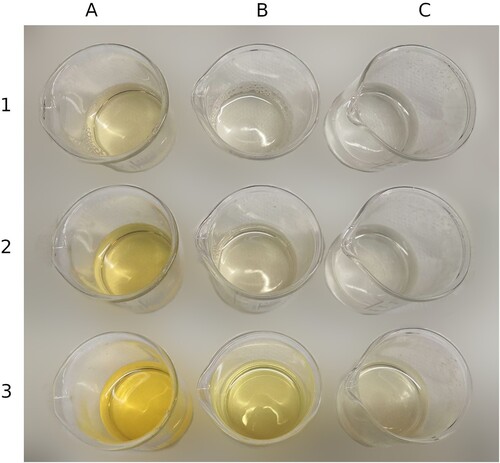
Based on the experiments described by Berthollet, we tried dyeing some silk crêpe.Footnote42 We found that when using silk crêpe and concentrated nitric acid at room temperature the colour change is instant; however, the silk fibres are damaged to the point of losing structural integrity and turn gel-like (in a process called acidic denaturation of proteins) after just a few seconds. Using diluted nitric acid (30% and 20%) at room temperature for an overnight soak, we obtained a yellow colouration without overtly damaging the fibres; however, the colour is significantly brighter when using the more concentrated acid. Weaker solutions (6% and 9%) did not result in any dyeing under the same conditions; however, by heating the dyeing bath we were able to see the colour change, reaching a similar shade even though the reaction was significantly slower with the more diluted solution. To further investigate the role of heat and time on the shade of yellow obtained, we repeated the experiment using weak nitric acid solutions (6.5% and 3.25%) and heating them at 70°C, monitoring the time frame necessary to get a yellow colouration without damaging the silk fibres (). For each solution, four pieces of silk crêpe were added at the same time. We retrieved a piece of silk from the soak every fifteen minutes. From this experiment, we found that a minimal colouration appears at the 45-minute mark, and a bright colour is not achieved even after 60 min when using the 3.25% solution. On the other hand, the 6.5% solution results in a fully bright colour just after 30 min, and this colour did not change for the rest of allotted time.
Figure 5. Results of the dyeing experiment using diluted nitric acid at 70°C and monitoring the effects of time on the achieved colour. © G. Montanari.

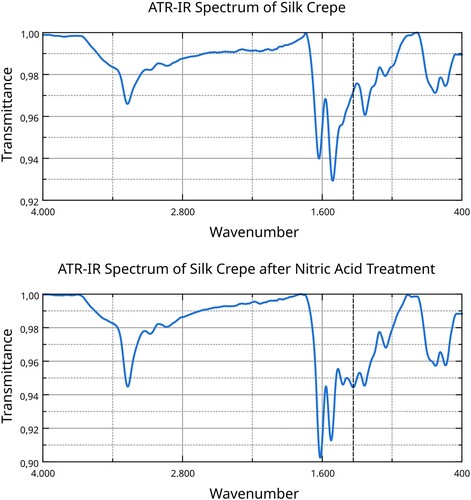
To verify whether this colour derives from a nitration of the silk fibres, we used Attenuated Total Reflectance Infra-Red (ATR-IR) Spectroscopy, a technique that shows the chemical bonds present in a sample. It is quite apparent that after treating the silk with nitric acid a new band appears at 1333 cm−1, which is consistent with the asymmetrical N-O bond stretching of a nitro-group (). As nitro-groups are not naturally present in silk proteins, it must derive from the treatment. The rest of the peak pattern is consistent with the IR spectrum of silk proteins as presented in scientific literature.Footnote43
Figure 6. ATR-IR spectra of the silk before and after nitric acid treatment; dashed line represents the signal at 1333 cm−1. Data analysis and visualisation by the Authors.
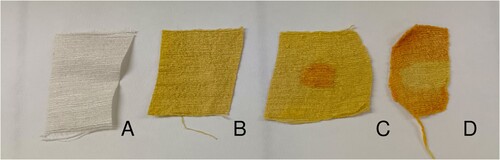
We then focused on the colour change to orange that Berthollet cites in passing and ascribes to an alkaline treatment.Footnote44 To this end, we prepared some lye, filtering water through a bed of ashes four times, changing the ashes every time.Footnote45 This resulted in a pale yellow solution. We soaked the silk samples in the lye for five minutes. This lye proved fully capable of changing the silk from yellow to orange; even applying a single drop dramatically changed the colour (). We presumed that this colour change depends on the deprotonation of weak acid groups present in the fibre. To confirm this, as it would make it an acid–base reaction, we applied a 2 M hydrochloric acid solution to the silk, and the colour reverted to yellow almost instantly, confirming our hypothesis.
Figure 7. Silk tussah, undyed (A), after nitric acid treatment (B), with orange spot made by adding a drop of lye (C), treated with nitric acid and lye and yellow spot caused by a drop of hydrochloric acid (D). © G. Montanari.
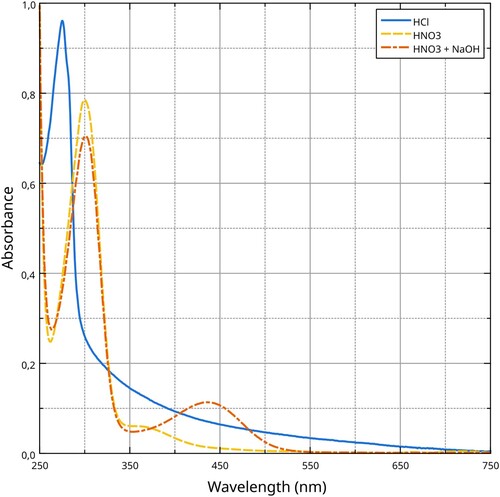
To further investigate the chemical changes caused by the acidic and alkaline treatments, we collected the UV–Vis absorption spectraFootnote46 of dissolved silk proteins in HCl (hydrochloric acid), HNO3 (nitric acid), and the nitrated solution after NaOH (sodium hydroxide) was added to it ().Footnote47 HCl has no oxidising or nitrating properties, therefore the spectrum shows only a band at about 275 nm, consistent with the absorption of aromatic amino acids, in particular tyrosine and tryptophan.Footnote48 The silk dissolved in nitric acid shows a band with a maximum at about 300 nm, with a shift that is consistent with the effects of nitration,Footnote49 moreover a smaller band is present with a maximum at about 360 nm, caused mainly by the nitration of tyrosine,Footnote50 that spills into the visible region of the spectrum (above 400 nm), and thus is responsible for the yellow colour of the solution when it is sufficiently concentrated. When sodium hydroxide is added to the nitrated solution, the band at 300 nm remains the same, however the band at 360 nm disappears and a new band appears with a maximum at about 440 nm, which is responsible for the orange colouration.Footnote51
Figure 8. UV–Vis absorption spectra of silk proteins dissolved in different media. Data analysis and visualisation by the Authors.
Berthollet’s ideas about the degree of combustion being responsible for the relative permanence of colours were not shared by his contemporary Edward Nathaniel Bancroft (1772–1842). Bancroft proposes a different theory in his Experimental Researches Concerning the Philosophy of Permanent Colours, and the Best Means of Producing Them, by Dyeing, Calico Printing (1794). He in fact argues that, in “thus ascribing the decays of vegetable and animal colouring matters generally, to effects or changes similar to those of combustion, Berthollet has, I think, gone farther than is warrantable by facts.” To explain this further, Bancroft refers to the fact that an alkaline treatment can often undo a colour alteration caused by acids and which alteration he therefore does not consider the result of a mere destructive force:
The nitric, sulphuric, and other acids containing oxygene, have the power not only of weakening, but of rendering latent for a time, the colours of many tingent matters; not however by any effect which can properly be denominated a combustion, but rather by a change in their several affinities or attractions, for particular rays of light in preference to other rays; but none of their parts being destroyed, or carried away, the addition of an alkali, or of a calcareous carbonate, will generally undo such alteration, and restore the original colour, by decomposing and neutralizing the acid or oxygene which had caused the alteration.Footnote52
in the same manner, colourless nitric acid, when applied to wool, silk, fur, or the skins of animals, their nails, horns, &.c., renders them all not only yellow, but orange, and even aurora-coloured. M. Berthollet thinks, these changes are produced by a kind of combustion; but I am persuaded they are the result of a combination of the oxygene with the nitrogene, which he has proved to be a constituent part of all animal substances.Footnote53
Bancroft argues that, rather than combustion, the effect of nitric acid on animal substances is the combination of oxygen with the “azote” (from a-zote “not living”), namely nitrogen, which Bancroft points out can be found in all animal substances – precisely those materials that are coloured by nitric acid. With this explanation, in fact, Bancroft comes very close to our modern understanding of the xanthoproteic reaction, which, as we have seen, is due to reaction with certain amino acids found in proteins. Bancroft further illustrates his point by asking whether, if the “yellow colour upon silk had indeed been the effect of combustion,” it should not also be produced “on linens, and cottons, and vegetable substances, which contain either little or no azote, but a great portion of the basis of charcoal, and ought therefore to be more liable to be acted upon in the way of combustion, than animal substances?”Footnote54
Indigo bandanas
Berthollet had discussed the effects of nitric acid on animal substances at the beginning of his work because it connected to his combustion theory of colour permanency. In addition to its importance for his theoretical framework, he also remarks that “the yellow colour they produce in wool, and more especially in silk, might merit a place among the processes of the art.”Footnote55 The practice does indeed appear in several more practically-oriented dyeing treatises, including under the name of “Spirit Yellows” in the 1850 The Dyer’s Instructer.Footnote56 The recipe in this treatise explains that silk is dyed a yellow colour “by passing” it through “strong Nitric Acid, and then through a little Soda or Soda ashes in warm water. This will produce a good full Yellow or light Orange.”Footnote57 But beyond being discussed in several dyeing manuals, it is not entirely clear whether the method of staining silk with nitric acid would have been used in practice, or on what scale.
At least one practice documented in the sources suggests that nitric acid stains might have been of some importance in the production of silk bandanas in imitation of Indian tie-dyed silk bandanas. The making of these imitation bandanas is described in several treatises, but a noticeably early example can be found in volume 39 of The Cyclopaedia; Or, Universal Dictionary of Arts, Sciences and Literature (1819) in the section dealing with printing on calico, a technique which had gained immense popularity at this time.Footnote58 The entry in question first describes a method of discharge printing a pattern on textiles coloured with Turkey-red. Discharge printing is a technique used to create a design on a textile by chemically stripping certain dyes from a previously dyed cloth. After having explained how to discharge Turkey-red dye by means of citric acid, the entry goes on to explain another method of discharge printing. This method creates a yellow pattern on silk handkerchiefs dyed blue with indigo using “the nitrous, and sometimes the nitro-muriatic acid”:
Aqua-fortis, or nitro-muriatic acid, of such strength as suitable for the kind of blue which is intended to be discharged, is mixed with either gum tragacanth, or with flour paste, to a proper consistence, and in this form is printed on the silk, by means of a common block, on which the intended pattern is cut. The consequence of this is, that wherever the acid attaches, there the original colour [of the indigo] is discharged, and a yellow dye is produced in its place. The pieces are then steamed, by passing them over a vessel containing boiling water, which gives brilliancy to the colour and finishes the operation.
Very different from the earlier treatises we discussed, which were mainly interested in the underlying chemical principles of the practice of dyeing, this encyclopaedia describes the use of nitric acid to stain silk yellow in the popular practice of making silk imitation bandanas. Here, interestingly, the nitric acid works together with indigo, discharging its colour and replacing it with yellow through its reaction with the silk. Since the silk handkerchiefs described in these encyclopaedias were products in daily use, they appear not to have survived in great numbers. But the silk handkerchief in , now in the collection of the Victoria and Albert Museum, London, is likely to have been produced using the method described above.
Figure 9. Handkerchief, ca. 1880, Printed in England, probably in Manchester, in imitation of an Indian tie-dyed handkerchief (bandana), © Victoria and Albert Museum, London.

One problem with nitric acid that might have impacted its success as a permanent yellow stain for silk is that it is damaging to the fabric. In his treatise Silk Dyeing, Printing, and Finishing (1892), George Henry Hurst points out that nitric acid “destroys silk” but that “moderately dilute nitric acid gives it a yellow colour, due to the production of xanthoproteic acid.” But, even with dilute nitric acid, Hurst asserts that the silk is still “considerably weakened by the operation.”Footnote60
Concentrated nitric acid indeed damages the silk fibre’s structure, as we learned from our experiments. But as Hurst pointed out, even treating the silk with diluted acid and then lye results in damage. We noticed that the lye used to turn the colour of the silk orange appeared bright yellow when the sample was removed. This lye was analysed by UV–Vis spectroscopy and revealed an absorption band at 280 nm, consistent with the presence of aromatic amino acids ().
Figure 10. UV–Vis absorption spectra of lye before (new) and after (used) using it to dye orange the nitrated silk. Data analysis and visualisation by the Authors.
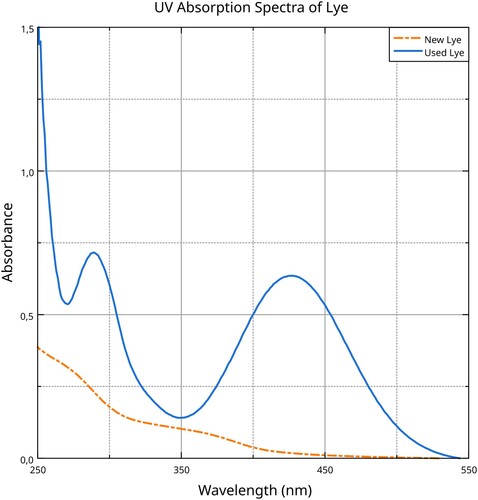
The shape of the absorption profile is comparable to the one we collected by dissolving silk in nitric acid and treating the solution with NaOH (sodium hydroxide), with a shift towards lower wavelengths that is consistent with the slightly different natures of the two solutions.
Finding nitrated amino acids dissolved in the lye means that the silk fibre underwent partial hydrolysis. This breaking off of amino acid from the structure of the fibres makes them overall more fragile and prone to breaking.
Indeed, our practical explorations not only helped us better understand what happens to silk when it is exposed to nitric acid, the nature of its colour change, and the types of degradation that may occur, but they also helped in interpreting the ideas and concerns of our historical actors when using this substance to produce colour or to examine the nature of colour. But our experiments also generated many more questions than we could answer in this paper. How strong was nitric acid in different time periods? How would this have influenced its use? How widespread was its application in the textile industry? And as this paper explores durable colours generated by a volatile substance, our material engagement with nitric acid also raised important questions about its material legacy. How can we identify the use of nitric acid on historical artefacts, and how could our own experiments help with its identification such as for instance in the case of silk bandanas or stained woods? Ironically, artefacts dyed with this presumably more durable yellow colour have not survived in great numbers. Even when they do survive, it may not be obvious that their yellow colour was produced by the methods described in this paper.
Conclusion
The xanthoproteic reaction makes what is possibly the most permanent colour change in animal fibres, as it acts directly on their chemical structure. But unlike most other dyes, nitric acid induces a reversible change in colouration. The historical protagonists discussed in this paper understood that there was something special about the permanence of the stains made with nitric acid on animal substances. It led Glauber to posit a link between the durability of colour on textiles and nitric acid; Boyle to consider the permanence of nitric acid stains on his own skin as an argument for the structural characteristics of solid animal bodies; and Brunnwiser to believe that nitric acid would help him extract a more durable (and cheap) yellow colour from wood. Brunnwiser had certainly hoped for a large-scale application of his alleged discovery, while Berthollet suggested there might be a place for the technique of colouring silks yellow with nitric acid in the art of dyeing. Yet beyond the block printing of indigo bandanas, the use of nitric acid to dye textile materials does not seem to have been implemented at an industrial scale, possibly due to its tendency to damage the textile coupled with the difficulties that using such a highly reactive material entails. With the contemporary push towards environmental sustainability in every field, including chemistry with the definition of the twelve principles of green chemistry in 1998, the production and use of nitric acid has become an even more freighted topic.Footnote61
Nonetheless, perhaps we can learn another lesson from this history of making yellow last with nitric acid – or, rather, it may spur us in a new direction. The chemists discussed in this paper were interested in the relative stability of colours and theorised about the underlying causes of this permanence. An important part of their theoretical interest in colour permanency was driven by a shared cultural desire for stable colours that had been shaped over centuries. What, however, if impermanence had been at the heart of their explorations? We believe that the answer to this question gains importance in the context of our current environmental crisis. Although, today, materials science develops new dyes for industry that are less polluting than many of the synthetic dyestuffs currently in use, the durability of these newly developed substances is still a key concern.Footnote62 But what if colours are allowed to fade? What if, in fact, this becomes a token of their appreciation? How would this shape contemporary explorations of dyes and pigments in chemistry or materials science? And how differently coloured might our world look?
Acknowledgements
We want to extend our thanks to Lucia Maini, Marianna Marchini, Matteo Martelli, and the DURARE team (Jan van Daal, Grace Kim-Butler, Clara Mikellides De Chiaro, Henrike Scholten) for their support and helpful comments in the development of this paper; we also thank Jennifer Rampling for her invaluable editorial expertise.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Marjolijn Bol
Marjolijn Bol is Associate Professor in Technical Art History at Utrecht University and PI of the ERC Starting Project DURARE. Her research intersects with historical studies of craft, heritage, knowledge, and the environment with a special focus on performative methods (reconstruction) and written sources on art technology. Her current ERC research project investigates the impact of artisans’ and patrons’ ambitions to craft, own, and theorise durable objects on the long-term development of the visual and decorative arts. Her recent publications include The Varnish and the Glaze (Chicago, 2023) and the edited volume The Matter of Mimesis (Brill, 2023). E-mail: [email protected].
Giacomo Montanari
Giacomo Montanari is a research fellow for the project “AlchemEast in the West: Graeco-Arabic Alchemy in Western Europe” (PI Professor Matteo Martelli) at the University of Bologna. Coming from a background in chemistry and in pharmaceutical sciences, his research involves the investigation of historical technological practices with a focus on experimental replications. E-mail: [email protected].
Notes
1 See the Introduction and the paper by Caterina Manco and Matteo Martelli in this volume.
2 Samuel Parks, Chemical Essays: Principally Relating to the Arts and Manufactures of the British Dominions, 5 vols. (London: Baldwin, Cradock, and Joy, 1815) vol. 1, 363, n. 227.
3 Parks, Chemical Essays, 363, n. 227.
4 Claude Louis Berthollet, Élements de l’art de la teinture, 2 vols. (Paris: Firmin Didot, 1791) vol. 2, 297; and for the English translation that came out almost immediately after, see Elements of the Art of Dyeing, trans. William Hamilton, 2 vols. (London: Stephen Couchman, 1791) vol. 2, 261.
5 See, for instance, James Smith, The Panorama of Science and Art; Embracing the Principal Sciences and Arts; The Methods of Working in Wood and Metal; and a Miscellaneous Selection of Useful and Interesting Processes and Experiments (London: Caxton Press, 1828) vol. 2, 538–39 and 542–43; Edward Bancroft, Philosophy of Permanent Colours and the Best Means of Producing Them, by Dyeing, Calico Printing, &c., 2 vols. (Philadelphia: Thomas Dobson, 1814), vol. 1, 281–82; and Berthollet, Elements of the Art of Dyeing, vol. 1, 286–87.
6 There are several publications on the history of nitric acid, see for instance: Hermann Kopp, Geschichte der Chemie, 4 vols. (Braunschweig: F. Vieweg und Sohn, 1845) vol. 3, 225–31; Robert Forbes, A Short History of the Art of Distillation: From the Beginnings Up to the Death of Cellier Blumenthal (Leiden: Brill, 1970), 86–87; and Vladimír Karpenko, “Some Notes on the Early History of Nitric Acid: 1300-1700,” Bulletin for the History of Chemistry 34 (2009): 105–16.
7 Johann Rudolph Glauber, Furni novi philosophici oder Der Beschreibung einer Neu-erfundenen Distillir-Kunst … 5 vols. (Amsterdam: Johann Fabel, 1646–1649), vol. 1, 74 and vol. 2, 65–67; for the first English translation, see Johann Rudolph Glauber, A Description of New Philosophical Furnaces (London: Richard Coates, 1651), 32 and 96–98.
8 See also William R. Newman and Lawrence M. Principe, Alchemy Tried in the Fire: Starkey, Boyle, and the Fate of Helmontian Chymistry (Chicago, IL: University of Chicago Press, 2002), 242.
9 Johann Rudolph Glauber, Explicatio tractuli, qui miraculum mundi inscribitur (Amsterdam: Joannem Janssonium, 1656), 39; for the first English translation, see Johann Rudolph Glauber, The Works of the Highly Experienced and Famous Chymist, John Rudolph Glauber … , trans. Christopher Packe (London: Thomas Milbourn, 1689), 179.
10 Glauber, Explicatio tractuli, qui miraculum mundi, 39–69; Glauber, The Works, 179–85.
11 Glauber, Explicatio tractuli, qui miraculum mundi, 41; Glauber, The Works, 179.
12 Pierre Thibaut dit le Lorrain, Cours de Chymie (Paris: Thomas Iolly, 1667), 275; for the first English translation, see Pierre Thibaut dit le Lorrain, The Art of Chemistry as it is now Practiced (London: Starkey, 1675), 267–68.
13 For Boyle’s understanding of the nature and composition of nitre, see Simon Duffy, “The Difference Between Science and Philosophy: The Spinoza-Boyle Controversy Revisited,” Paragraph 29, no. 2 (2006): 115–38.
14 Robert Boyle, Some Considerations Touching the Usefulnesse of Experimental Naturall Philosophy (Oxford: H. Hall, 1663), 126–27.
15 Robert Boyle, Essay of the Porousness of Animal Bodies (London: S. Smith, 1684), 56.
16 Boyle, Porousness of Animal Bodies, 56–57.
17 Boyle, Porousness of Animal Bodies, 56–57.
18 Boyle, Porousness of Animal Bodies, 56–57.
19 John Stalker and George Parker, A Treatise of Japaning and Varnishing Being a Compleat Discovery of Those Arts with the Best Way of Making all Sorts of Varnish … (Oxford: Richard Wood, 1688), 82–83.
20 Stalker and Parker, A Treatise of Japaning and Varnishing, 83.
21 Robert Dossie, Handmaid to the Arts (London: J. Nourse, 1758), 435.
22 Dossie, Handmaid to the Arts, 435.
23 See for instance the following blogs discussing the use of nitric acid as a stain for wood on a piece of furniture and on the wooden parts of rifles: https://millcrek.wordpress.com/2012/01/30/new-project-8-finishing-up/" \o "https://millcrek.wordpress.com/2012/01/30/new-project-8-finishing-up (accessed 16 November 2023) and https://americanlongrifles.org/forum/index.php?topic=60515.0 (accessed 16 November 2023).
24 Cellulose remains white after nitration; however, it consumes part of the nitric acid reacting with it, which in turn can affect the resulting colouration of the stained wood.
25 Mathias Brunnwiser, Entdeckung verschiedener vegetabilischen Farbmaterialien, Seiden- und Wollenzeuge schön und dauerhaft gelb zu färben (Kehlheim, 1771), 343–44. Translations are by the authors of this paper.
26 Brunnwiser, Entdeckung verschiedener vegetabilischen Farbmaterialien, 346–47.
27 Brunnwiser, Entdeckung verschiedener vegetabilischen Farbmaterialien, 349.
28 Berthollet, Élements de l’art de la teinture, vol. 1, 65–71; Elements of the Art of Dyeing, vol. 1, 65–71.
29 Berthollet, Élements de l’art de la teinture, vol. 1, 45; Elements of the Art of Dyeing, vol. 1, 45.
30 Berthollet, Élements de l’art de la teinture, vol. 1, 51–52; Elements of the Art of Dyeing, vol. 1, 52–53.
31 Berthollet, Élements de l’art de la teinture, vol. 1, 52; Elements of the Art of Dyeing, vol. 1, 53.
32 Berthollet, Élements de l’art de la teinture, vol. 1, 59–60; Elements of the Art of Dyeing, vol. 1, 60.
33 Berthollet, Élements de l’art de la teinture, vol. 1, 60; Elements of the Art of Dyeing, vol. 1, 60–61.
34 Berthollet, Élements de l’art de la teinture, vol. 1, 60; Elements of the Art of Dyeing, vol. 1, 61.
35 Berthollet, Élements de l’art de la teinture, vol. 1, 60; Elements of the Art of Dyeing, vol. 1, 61.
36 Berthollet, Élements de l’art de la teinture, vol. 1, 60; Elements of the Art of Dyeing, vol. 1, 61.
37 Berthollet, Élements de l’art de la teinture, vol. 1, 61; Elements of the Art of Dyeing, vol. 1, 61–62.
38 Berthollet, Élements de l’art de la teinture, vol. 1, 61; Elements of the Art of Dyeing, vol. 1, 61–62.
39 Berthollet, Élements de l’art de la teinture, vol. 1, 71; Elements of the Art of Dyeing, vol. 1, 71.
40 For Berthollet’s discovery of chlorine, which he calls “oxygenated muriatic acid,” see Ludwig Mond, “Address to the Chemical Section of the British Association. Liverpool, 1896,” Chemical News and Journal of Industrial Science 74 (1896): 156–58.
41 Berthollet, Élements de l’art de la teinture, vol. 1, 72; Elements of the Art of Dyeing, vol. 2, 72.
42 For his experiments with nitric acid on silk, Berthollet refers to the treatise of Friedrich Gmelin, Tingendo per nitri acidum sive nudum, sive terra, aut metallo saturatum serico (Erfurt: G. A. Keyser, 1785). For Berthollet’s discussion of Gmelin, see Berthollet, Élements de l’art de la teinture, vol. 1, 67–68; Elements of the Art of Dyeing, vol. 1, 67–68.
43 See S. Vahur, A. Teearu, P. Peets, L. Joosu, and I. Leito, “ATR-FT-IR Spectral Collection of Conservation Materials in the Extended Region of 4000-4080 cm–1,” Anal Bioanal Chem 408, no. 13 (2016): 3373–79, doi:10.1007/s00216-016-9411-5; Y. Ji et al., “DFT-Calculated IR Spectrum Amide I, II, and III Band Contributions of N-Methylacetamide Fine Components,” ACS Omega 5, no. 15 (2020): 8572–78, https://pubs.acs.org/doi/10.1021/acsomega.9b04421; and P. R. Laity, S. E. Gilks, and C. Holland, “Rheological Behaviour of Native Silk Feedstocks,” Polymer 67 (2015): 28–39. doi:10.1016/j.polymer.2015.04.049.
44 Berthollet, Élements de l’art de la teinture, vol. 1, 67: “Si on la trempe dans une dissolution alkaline, elle prend un belle couleur orangée.”
45 We used distilled water and ashes made from the wood of local trees in Bologna, provided to us by Lucia Maini, Department of Chemistry “Giacomo Ciamician,” University of Bologna. The mode of preparation was taken from Zosimos of Panopolis, Collection des anciens alchimistes grecs, ed. M. Berthelot and C.É. Ruelle, 3 vols. (Paris: Steinheil, 1888), vol. 2, 372–73.
46 UV-Vis absorption spectroscopy shows which wavelenghts of visible (380–750 nm) and ultraviolet light (100–380 nm) are absorbed by a compound or a mixture. Due to technical limitations of the instrument used, the spectra were collected between 250 and 750 nm.
47 UV–Vis absorption spectroscopy shows which wavelenghts of visible (380–750 nm) and ultraviolet light (100–380 nm) are absorbed by a compound or a mixture. Due to technical limitations of the instrument used, the spectra were collected between 250 and 750 nm.
48 G.H. Beaven and E.R. Holiday, “Ultraviolet Absorption Spectra of Proteins and Amino Acids,” Advances in Protein Chemistry 7 (1952): 319–86.
49 See for example the data provided by UV–Vis spectrophotometer manucturer Shimadzu at: https://www.shimadzu.co.kr/service-support/technical-support/technical-information/uv-ap/apl.html (accessed 16 November 2017).
50 K.C. Bible, S.A. Boerner, and S.H. Kaufmann, “A One-Step Method for Protein Estimation in Biological Samples: Nitration of Tyrosine in Nitric Acid,” Analytical Biochemistry 267 (1999): 217–21.
51 400 nm corresponds to violet light, while 440 nm to blue light; as the visible colour corresponds to the complementary colour to the absorbed wavelengths, the first solution is yellow and the second orange.
52 Edward Nathaniel Bancroft, Experimental Researches Concerning the Philosophy of Permanent Colours, and the Best Means of Producing Them, by Dyeing, Calico Printing, 2 vols. (London: T. Cadell, Jun. and W. Davies, 1794), vol. 1, 49–50.
53 Bancroft, Experimental Researches, 32.
54 Bancroft, Experimental Researches, 32.
55 Berthollet, Élements de l’art de la teinture, vol. 1, 65; Elements of the Art of Dyeing, vol. 1, 65.
56 David Smith, The Dyer’s Instructer: Comprising Practical Instructions in the Art of Dyeing Silk, Cotton, Wool, and Worsted and Woollen Goods (London: Simpkin & Marshall, 1850), 85 [no. 4].
57 Smith, The Dyer’s Instructer, 85 [no. 4].
58 Abraham Rees, The Cyclopaedia; Or, Universal Dictionary of Arts, Sciences and Literature, 39 vols. (London: Longman, Hurst, Rees, Orme, & Brown, 1819), vol. 39, s.v. “Printing, Calico.”
59 For another detailed description of the process, see Rudolf Wagner, A Handbook of Chemical Technology (London: J. & A. Churchill, 1872), 616. Here the bandanas are called “mandarins.”
60 George Henry Hurst, Silk Dyeing, Printing, and Finishing (George Bell & Sons: London, 1892), 9.
61 P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice (Oxford: Oxford University Press, 1998), 30. More environmentally friendly methods for the production of nitric acid are being explored: see for example: https://www.nitricacidaction.org (accessed 16 November 2023) and https://www.international-climate-initiative.com/en/iki-media/news/the-way-to-a-climate-friendly-nitric-acid-industry (accessed 16 November 2023).
62 A plethora of papers could be cited here, but we keep to three telling examples that illustrate this point: Luqman Jameel Rather, Sabiyah Akhter, Rayees Ahmad Padder, Qazi Parvaiz Hassan, Mohammad Hussain, Mohd Ali Khan, and Faqeer Mohammad, “Colorful and Semi Durable Antioxidant Finish of Woolen Yarn with Tannin Rich Extract of Acacia nilotica Natural Dye,” Dyes and Pigments 139 (2017): 812–19; Mohammad Shahid, Yuyang Zhou, Ren-Cheng Tang, Guoqiang Chen, and Waseem A. Wani, “Colourful and Antioxidant Silk with Chlorogenic Acid: Process Development and Optimization by Central Composite Design,” Dyes and Pigments 138 (2017): 30–38; and Yinchun Fang, Xinhua Liu, Hongliang Zheng, and Hailong Liu, “Eco-friendly Colorization of Textile Originating from Polydopamine Nanofilm Structural Color with High Colorfastness,” Journal of Cleaner Production 295 (2021). doi:10.1016/j.jclepro.2021.126523 (accessed 16 November 2023).