Abstract
Four Lake Trout Salvelinus namaycush morphs were identified from Isle Royale, Lake Superior; the morphs differed in shape, traits linked to feeding and locomotion, buoyancy, and physical habitat use. Lean, humper, and siscowet Lake Trout generally conformed to previous descriptions, and we report, for the first time, quantitative evidence of a fourth morph, previously described anecdotally as the “redfin.” Jackknife classification of individuals to morphs based on body shape were 94% correct. High variation within and low variation among morphs led to moderately low percent agreement among visual identifications and high uncertainty in Bayesian model groupings of morphs. Eight linear measures of phenotypic traits linked to feeding (i.e., head and eyes) and locomotion (i.e., fin lengths and caudal peduncle shape) varied among morphs, consistent with specialized adaptations for trophic and physical resource use. Habitat differed among morphs with leans being most abundant in the 0–50-m depth stratum and siscowets most abundant in two deeper strata (50–100 and 100–150 m). Differences in capture depth and percent buoyancy reflected physical habitat and known trophic resource partitioning among morphs. While the historical fingerprint of morphological and ecological diversity in Lake Superior Lake Trout persists, it is unknown whether the contemporary low level of differentiation is due to ecological release without subsequent reorganization or to a complete breakdown of differentiation.
Received July 11, 2013; accepted February 7, 2014
Salmonines have radiated into diverse assemblages of morphs or ecological variants in postglacial ecosystems. For example, sympatric populations of Powan (or European whitefish) Coregonus lavaretus (Amundsen et al. Citation2004), Lake Whitefish C. clupeaformis (Lindsey Citation1981; Bernatchez Citation2004), ciscoes Coregonus spp. (Todd and Smith Citation1992; Mehner et al. Citation2012), and Arctic Char Salvelinus alpinus (Klemetsen Citation2010; Reist et al. Citation2012) have evolved to occupy shallow and deep waters, as well as benthic and pelagic habitats. By comparison, the analogous diversity that occurs in North American Lake Trout S. namaycush is less well documented, and in the Laurentian Great Lakes much of that diversity has been lost.
Understanding patterns of Lake Trout diversity in Lake Superior will further the ongoing efforts to re-establish the species and its morphs in Lakes Huron, Michigan, Erie, and Ontario. Identification of morphs is also critical to assessment and management of the Great Lakes Lake Trout fishery, especially if exploitation rates differ among morphs. An updated description of Lake Trout diversity is also timely because siscowet (a deepwater Lake Trout morph) abundance has been increasing in Lake Superior during the last decade (Bronte et al. Citation2003; Bronte and Sitar Citation2008), and pilot fisheries to harvest these fish for lipid extraction (i.e., docosahexaenoic and eicosapentaenoic acids) are underway. The extent to which a new deepwater fishery will alter the composition of Lake Trout diversity is unknown, but reduced siscowet abundance has been postulated to positively affect coregonine abundance (Kitchell et al. Citation2000; Bronte et al. Citation2010). Finally, a study of the diversity of a remnant population of Lake Trout in Lake Superior provides insights into the ecological consequences of a century of food web and fish community alteration in the Laurentian Great Lakes.
Historically, multiple Lake Trout (also called lake char or lake charr) morphs occurred in the Laurentian Great Lakes (Roosevelt Citation1865; Thomson Citation1883; Goode Citation1884; Rakestraw Citation1968). The accounts of 17th century Jesuit missionaries and early Great Lakes fishers described regionally distinct Lake Trout populations that showed considerable morphological and ecological variation between inshore and offshore habitats within a region and distinct morphs occurring among regions (Roosevelt Citation1865; Goode Citation1884; Sweeny Citation1890; Thurston Citation1962; Rakestraw Citation1968; Organ et al. Citation1979; Coberly and Horrall Citation1980; Loftus Citation1980; Goodier Citation1981; Toupal et al. Citation2002). Early accounts described Lake Trout morphs that were visually distinct and easily identified; naturalists and fishers recognized up to 10 morphs in the Canadian waters of Lake Superior (Goodier Citation1981). Similar diversity occurs in several other large North American lakes (Blackie et al. Citation2003; Alfonso Citation2004; Zimmerman et al. Citation2006, Citation2007; Hansen et al. Citation2012; Chavarie et al. Citation2013).
Dramatic anthropogenic and ecological changes during the past century have altered Great Lakes food webs and Lake Trout were especially affected (Lawrie and Rahrer Citation1973; Muir et al. Citation2012a). By the mid-1960s, much of the historic Lake Trout diversity was lost due to Sea Lamprey Petromyzon marinus predation and overfishing (Holey et al. Citation1995; Krueger and Ihssen Citation1995). Lake Superior was the only Great Lake to retain some of its original Lake Trout diversity (Krueger et al. Citation1995); how much diversity remains in the lake and how remnant diversity is currently organized is unknown.
Beginning in the 1960s, quantitative methods were used to describe three Lake Trout morphs in Lake Superior: a “lean” morph that typically occupies shallow water <70 m deep, a “humper” morph that is thought to occupy offshore, midwater shoals, or banks, and a “siscowet” morph that occupies waters >100 m deep (Peck Citation1975; Moore and Bronte Citation2001; Bronte et al. Citation2003). The three morphs can be distinguished by body fat content (Thurston Citation1962; Eschmeyer and Phillips Citation1965), growth (Rahrer Citation1965; Burnham-Curtis and Bronte Citation1996), external morphology (Khan and Qadri Citation1970; Goodier Citation1981; Moore and Bronte Citation2001), osteology (Burnham-Curtis and Smith Citation1994), spawning condition and timing (Eschmeyer Citation1955; Rahrer Citation1965; Bronte Citation1993), and genetic structure (Guinand et al. Citation2003; Goetz et al. Citation2010; Guinand et al. Citation2012).
Lean, humper, and siscowet Lake Trout persist in the waters surrounding Isle Royale, Lake Superior (Rakestraw Citation1967; Moore and Bronte Citation2001; ), and we present evidence herein of a fourth morph, the redfin. As part of an ongoing study of North American Lake Trout diversity, we revisited these Isle Royale populations for the purpose of expanding sample sizes and locations over those sampled in the past, and to apply new discriminatory methods to the problem of assessing phenotypic and ecological variation at this site. Our research objectives were as follows: (1) determine how many Lake Trout morphs occur at Isle Royale; (2) determine whether the grouping method (i.e., statistical versus visual) altered the classification of individuals to morphs; and (3) compare morphological, ecological, and physiological characteristics among morphs. Isle Royale was selected for this study because its offshore location, national park status, and small, nearshore fishery make it less perturbed than mainland areas; therefore, we expected to encounter greater Lake Trout diversity in the waters surrounding the island than along the mainland shoreline.
METHODS
Fish collections.—A total of 738 Lake Trout were sampled at Isle Royale (48°00′N, 88°50′W) during August 2006 and 2007 (). Gill-net sampling effort was distributed across 0–50-m (eight net sets), 50–100-m (six net sets), and 100–150-m (six net sets) depth strata because those ranges roughly correspond to the known habitat for lean, humper, and siscowet morphs, respectively. Gill-net gangs were 183 m long by 1.8 m high and made of nylon, with 30.5-m panels of stretch mesh sizes ranging from 50.8 to 114.3 mm, in 12.7-mm increments. All gill nets were deployed on the bottom for approximately 24 h.
Field processing procedures.—A calibrated digital image of the left side of each individual was captured as described Muir et al. (Citation2012b). Sex and maturity were recorded and TL (mm) was measured on each fish. Weight of the fish in air (Wa), and weight of the fish in water (Ww) were measured (grams) on whole fresh fish using a Pesola spring scale (see Zimmerman et al. Citation2006 for methods).
Treatment of data.—Statistical procedures (significance level, α = 0.05) were conducted using R (version 2.15.1; www.r-project.org), SigmaPlot 11 (Systat Software, San Jose, California), and the Thin Plate Spline suite (TPS; State University of New York at Stony Brook; http://life.bio.sunysb.edu/morp). Fish less than 430 mm TL were omitted from the analyses to avoid confounding effects of ontogenetic shifts in body morphology and food habits at that size (sensu Zimmerman et al. Citation2009); therefore, morphological variation should not have been associated with differences in size or age among individuals. Sexes were pooled for all analyses because no differences in TL (U = 42908, P = 0.06), buoyancy (U = 43327, P = 0.31), or relative weight (U = 42635, P = 0.09) were detected. In addition, decades of research has shown that Lake Trout are not sexually dimorphic morphologically (Martin and Olver Citation1980; Gunn Citation1995; Esteve Citation2005; Esteve et al. Citation2008). Kolmogorov–Smirnov tests were used to assess normality of the error distributions for all variables and a Levene's test assessed homogeneity of variance for buoyancy, relative weight, and linear phenotypic measures. Significant departures from normality were transformed to normalize the error distribution; where transformations were ineffective, nonparametric tests were used.
Lake Trout morphs.—We were interested in how to best differentiate Lake Trout morphological variation and whether morphs could be reliably identified in the field; therefore, three different methods were used to assess the number of Lake Trout morphs occurring at Isle Royale (objective 1). The methods used to identify morphs were: (1) a Bayesian cluster analysis of geometric head shape, (2) a Bayesian cluster analysis of geometric body shape, and (3) visual identification. The first two analyses quantified head and body shape using geometric morphometric methods (Zelditch et al. Citation2004; Zimmerman et al. Citation2006) implemented in TPS.
Twenty sliding semilandmarks and eight homologous landmarks were digitized on images for 593 fish to characterize head shape (). Homologous landmarks represent corresponding parts, such as a fin insertion, on each individual. By contrast, sliding semilandmarks are placed along outlines or curves to be combined with landmark points (Rohlf Citation2009). The procedure involves first sliding the semilandmark to the left or right along a curve to minimize the amount of shape change between each specimen and the Procrustes average (i.e., consensus specimen) of all the specimens. Following Zimmerman et al. (Citation2009), the semilandmarks on the head were anchored by LM-1 and LM-12 representing the head length from snout (LM-1) to opercle (LM-12). The posterior semilandmarks (LM-11 and LM-13) were selected as the vertical extension of LM-12 to the dorsal and ventral surface of the fish, respectively. These extensions were drawn perpendicular to the total length of each fish to ensure that they represented the same location relative to the posterior point of the opercle. Sixteen homologous and four sliding semilandmarks were digitized on whole-body images for 571 fish to characterize overall body shape (). Following Zimmerman et al. (Citation2007), the semilandmarks on the body represented the belly curvature at 20, 30, 40, and 50% of total length and were anchored by LM-1 and LM-10. Of the total 738 fish sampled, fish were removed from these analyses either because they were <430 mm TL or because full body images were of insufficient quality or improperly oriented for shape analysis (see Muir et al. Citation2012b).
![FIGURE 2. (a) Landmark order and placement for digitizing head shape. Semisliding landmarks (black dots) were placed at 10 evenly spaced partitions between the anterior tip of the snout and the posterior edge of the opercle. Homologous landmarks (white dots) indicate the anterior terminus of upper (1) and lower jaw (23); anterior (25), center (26), and posterior (27) of the orbit; posterior terminus of maxilla (24); pectoral fin insertion (28); and the posterior of the opercle (12). (b) Semisliding landmarks (black dots) were placed at 0.20, 0.30, 0.40, and 0.50×standard length (SL) to quantify body depth. Homologous landmarks (white dots) indicated the anterior terminus of upper jaw (1), posterior tip of maxilla (2), center of eye (3), top of cranium at midpoint of eye (4), posterior of neurocranium (5), anterior insertion of dorsal fin (6), posterior insertion of dorsal fin (7), anterior insertion of adipose fin (8), dorsal insertion of caudal fin (9), midpoint of hypural plate (10), ventral insertion of caudal fin (11), posterior insertion of anal fin (12), anterior insertion of anal fin (13), anterior insertion of pelvic fin (14), dorsal insertion of pectoral fin (19), and isthmus of branchiostegal membrane (20; c.f., Zimmerman et al. Citation2009). (c) Eight linear phenotypic characteristics were measured on each specimen as follows: (1) caudal peduncle depth (CPD): least vertical depth of the caudal peduncle; (2) caudal peduncle length (CPL): distance along the horizontal axis of the body between the posterior of the anal fin and the caudal flexure; (3) head length (HLL): distance from the tip of the premaxilla to the posterior margin of the opercle; (4) maxilla length (MXL): anterior point of premaxillae to posterior end of the maxilla; (5) orbital length (OOL): distance between anterior and posterior fleshy margins of the orbit; (6) pectoral fin length (PCL): measured from the insertion of outermost ray to farthest tip of fin; (7) pelvic fin length (PVL): measured from the insertion of outermost ray to farthest tip of fin; and (8) preorbital length (POL): tip of the premaxilla to the anterior fleshy margin of the orbit. [Figure available online in color.]](/cms/asset/fd78cf26-97c9-48c1-a243-fa6581e4e729/utaf_a_900823_f0002_oc.jpg)
Landmark data were used to scale each individual and obtain centroid size and partial warp scores using TPSrelw for the head and body shape in two separate analyses. Partial warp scores were retained as new size-free shape variables. The head shape analysis produced 26 shape variables and the body shape analysis produced 18 variables; therefore, principal component analysis (PCA) using singular value decomposition reduced the dimensionality to four variables, i.e., the first four principal components (PCs), for each data set. The first four PCs from the head shape analysis and from the body shape analysis described greater than 60% of the variation in shape and were retained for further analysis.
Morphological groups were identified using a Bayesian clustering package implemented in R (MCLUST; Fraley and Raftery Citation2009). Unlike previous discriminatory methods used to assess Lake Trout diversity in Lake Superior, MCLUST offers the powerful advantage that a priori assignment of individuals to groups is not required. Resulting groups are therefore not biased by our preconceived notions. Two MCLUST models (EII and VII; see Fraley and Raftery Citation2009 for model descriptions) were fit to the first two to four PCs for the head and body shape data in three independent analyses, resulting in six models for head shape and six models for body shape.
The “best” of the models, representing the most likely number of groups on the basis of head and body shape, respectively, were identified using Bayesian Information Criterion (BIC). The BIC value is the maximized log-likelihood for the model, the data dimensions, and the number of model components; the larger the BIC, the stronger the support for the model and number of clusters (Fraley and Raftery Citation2009). The “best” model for head shape and body shape, that is, the model that could, at a minimum, discriminate lean and siscowet Lake Trout (because leans and siscowets are most morphologically distinct) and had the highest BIC was selected to assign individuals to morphological groups and quantify uncertainty in model assignments. This method provided one set of group assignments on the basis of head shape and a second set of group assignments on the basis of body shape.
The third group assignment involved visual interpretation of digital images by three experienced Lake Trout biologists (A. M. Muir, C. R. Bronte, and C. C. Krueger). Ideally, Isle Royale commercial fishers rather than biologists would have identified the morphs, and fresh specimens rather than digital images would have been used. However, few fishers familiar with the morphs remain regionally due to attrition of the fishery and an aged remnant of fishers. Visual identification by gross examination of fresh fish was not feasible given the sampling logistics, and one advantage of digital images is that specimens can be scaled and easily compared side by side. Each fish was independently assigned by the biologists to one of the four morphological groups as follows: (1) lean, (2) humper, (3) siscowet, and (4) other. The “other” group contained all fish that were not assigned to lean, humper, or siscowet groups. One of the biologists created another group (total of five groups) because a fourth distinctive morph was thought to exist. Snout angle and length, eye size and position, paired fin length, and caudal peduncle depth and length were the primary characteristics considered when visually assigning fish to morphological groups (Burnham-Curtis Citation1993; Burnham-Curtis and Smith Citation1994). The visual assignments were not blind because fish size information from the digital image was available to the biologists; however, no other data were provided during interpretation. Upon completion of independent visual group assignments, the biologists conferred while reviewing the images in an attempt to achieve consensus for all assignments that disagreed. During the process of reconciling visual identifications to arrive at a consensus, the three biologists concurred that that the new group (i.e., fourth morph) identified by one of the biologists was likely valid due to consistency in characteristics used to identify this morph (i.e., a redfin group; see Results and Discussion). Percent agreement was calculated as the proportion of visual assignments that were the same among all three biologists and was used to assess consistency in visual assignment of specimens to morphs.
Visual and model group assignments were reconciled to attain a final morph assignment for each fish. When visual and model assignments disagreed, the decision rules that follow were used to reduce subjectivity in the final group assignments: (1) if the head and body model assignments agreed, but differed from the visual consensus, the model assignment was used; (2) if the head and body model assignments disagreed, but either of the two model assignments agreed with the visual assignment, then the visual assignment was used; (3) if the head and body model assignments agreed, but visual consensus was not reached, the model assignment was used; and (4) if the head and body model assignments differed and visual consensus could not be achieved, then the fish was removed from further analyses. This reconciled assignment of Lake Trout to morphs was referred to as the “overall group assignment” and was used in all subsequent analyses. Percent agreement among all five assignments (i.e., three visual, one head-shape, and one body-shape assignment) was used as an indicator of the certainty of the overall group assignment.
Validity of group assignments.—To assess the validity of group assignments (objective 2), we conducted a jackknife classification test, compared percent agreement of visual assignments among the three analysts, analyzed uncertainty in model assignment of individuals to groups, and assessed how biotic (i.e., body condition) and abiotic (depth and sampling site) variables influenced model uncertainty and related body shape of individuals in a group.
A discriminate function analysis (DFA) tested how well head and body shape grouped individuals. Wilks lambda (λ) tested for significance among the groups, where λ = 0 represented complete separation and λ = 1 represented no separation among groups. The first four PCs from the head and the body shape analysis were used in two separate DFAs and the validity of the resulting group structure was assessed using jackknife classification where successful individual assignment back to groups was quantified.
A chi-square goodness-of-fit statistic tested whether overall percent agreement (i.e., among all five assignments: head shape, body shape, and three visual assignments) differed among morphs. On the basis of our experience assigning morphs in the field, we predicted that lean and siscowet Lake Trout should have the greatest percent agreement among the assignment methods compared with other morphs. Two separate Kruskal–Wallis one-way ANOVA on ranks with Dunn's method of pairwise multiple comparisons assessed how uncertainty in head- and body-shape model assignments varied among morphs. Model uncertainty was defined as 1 − the probability of the most likely group for each individual fish being assigned on the basis of its head or body shape (Fraley and Raftery Citation2009). We predicted these results would follow the same pattern as the percent agreement for visual assignments, indicating that lean and siscowet Lake Trout are morphologically distinct and easy to classify compared with other morphs.
Two analyses tested how biotic and abiotic factors influenced model uncertainty in group assignments. First, we predicted that uncertainty in assignments from the body shape model would be positively associated with fish body condition because misclassification could occur for lean Lake Trout with high body condition and siscowets with low body condition (i.e., uncertainty would increase with fat leans and skinny siscowets). Relative weight (Wr) of each fish was used as a measure of body condition and calculated as Wr = (W/Ws)×100, where W was the weight of an individual Lake Trout and Ws was a length-specific standard weight. Length-specific standard weight was predicted by a standard weight–length regression for 58 Lake Trout data sets: log10Ws = −5.681 + 3.2462 log10TL (Piccolo et al. Citation1993). A general linear model (GLM) tested the prediction that body condition influenced group assignment, where overall group assignment was a factor, log10Wr was the independent variable, and log10 uncertainty from the body shape model assignments was the dependent variable.
Second, we predicted that uncertainty in model group assignments would vary spatially and by depth because in heterogeneous habitats, lean, humper, and siscowet Lake Trout may co-occur, leading to increased model uncertainty, whereas in homogeneous habitats, only a single morph may occur leading to reduced model uncertainty. A GLM tested how uncertainty in body-shape model assignments varied among morphs as a function of space and capture depth. This analysis was limited to the body-shape model uncertainty because that model identified all four morphs. Each net set was used as a variable that represented both space and depth.
Phenotypic and ecological characteristics of morphs.—The geometric shape analysis quantifies gross body shape, but provides little information about specific functional adaptations in morphology relating to feeding or locomotion. For this reason, eight linear phenotypic characters linked to feeding and locomotion, and known to vary among Lake Trout morphs (Zimmerman et al. Citation2006, Citation2007), were quantified using the calibrated linear measurement tool in TPSdig (). Phenotypic characteristics measured on each specimen were: (1) caudal peduncle depth (CPD), the least vertical depth of the caudal peduncle; (2) caudal peduncle length (CPL), the distance along the horizontal axis of the body between the posterior of the anal fin and the caudal flexure; (3) head length (HLL), the distance from the tip of the premaxilla to the posterior margin of the opercle; (4) maxilla length (MXL), the distance from the anterior point of the premaxillae to posterior end of the maxilla; (5) orbital length (OOL), the distance between anterior and posterior fleshy margins of the orbit; (6) pectoral fin length (PCL), the distance from the insertion of outermost ray to farthest tip of fin; (7) pelvic fin length (PVL), the distance from the insertion of outermost ray to farthest tip of fin; and (8) preorbital length (POL), the distance from the tip of the premaxilla to the anterior fleshy margin of the orbit. Phenotypic measures were corrected for size using a series of ANCOVA, where individual phenotypic traits were ln transformed and treated as independent variables, TL was the dependent variable, and morph was a covariate (Reist Citation1985). Size-corrected phenotypic measures were compared among morphs in a separate ANOVA; pairwise multiple comparisons used the Holm–Sidak method.
Buoyancy accounts for differences in soft and hard tissues that affect the specific gravity of the fish and is positively correlated with body lipid content and depth of capture in many fishes. Fishes that use deep water, or undergo diel vertical migrations to exploit migrating prey, tend to be lighter in water due to high body lipid content; that is, they have high buoyancy (Alexander Citation1993; Eshenroder et al. Citation1998; Eshenroder and Burnham-Curtis Citation1999; Zimmerman et al. Citation2006). Percent buoyancy (B) was calculated for each fish as follows:
where Wa is the weight of fish measured in air and Ww is the weight of the fish measured in water (this formula corrects an error in the formula given by Krause et al. Citation2002). A two-factor ANOVA compared percent buoyancy among and within morphs and depth strata. Holm–Sidak post hoc pairwise tests of estimated marginal means compared morphs within and among depth strata. To account for the effect of fish size on buoyancy, prior to analysis buoyancy was log10 normalized, regressed on TL, and the residuals were retained as size-adjusted measures of buoyancy.
TABLE 1. The number of principle components (PCs) from an ordination of head or body shape used to assign individual Lake Trout to morphological groups (PCs used), percentage of variation explained by those PCs (% variation explained), type of multivariate clustering model (MCLUST model), mean model uncertainty in group assignments (Mean uncertainty±SE); group assignments were only made using the best (highest Bayesian information criterion [BIC; indicated by bold italics]) of the two models in each model pair, and the associated BIC.
Catch per effort (CPE; determined as catch per net) was used as a relative index of fish abundance in each depth stratum. A two-factor ANOVA that included the interaction term between morph and depth strata compared ln+1(CPE) among depth strata (Hansen et al. Citation2012). Catch rates for each morph were separated using Holm–Sidak post hoc comparisons to substantiate differences in catch rates for the different morphs across depth strata. Least-squares geometric mean CPE was estimated using ANOVA with a morphotype×depth interaction.
RESULTS
Head Shape Group Assignments
A model that used the first four PCs from an ordination of geometric head shape discriminated three Lake Trout morphs that corresponded to classic descriptions of lean, humper, and siscowet (model H3; ). Model H3 was the best on the basis of BIC, but uncertainty in model assignment was slightly higher (0.20), compared with model H1 (0.12) and model H2 (0.11; ). The first four PCs accounted for 72% of the variation in head shape. Principal components 1 and 2 accounted for 38% and 14% of the variation in head shape, respectively, whereby narrower, more pointed heads (i.e., lean morph) clustered on the left side of the biplot and shorter, deeper heads with large eyes (i.e., siscowet morph) clustered on the right side of the biplot ().
Body Shape Group Assignments
A model that used the first four PCs from an ordination of geometric body shape discriminated four Lake Trout morphs (model B3; ). Three of the morphs identified corresponded to classic descriptions of lean, humper, and siscowet (). The fourth group shared similarities with a previously described “redfin” morph (Rakestraw Citation1968; Organ et al. Citation1979; Loftus Citation1980; Goodier Citation1981; ); therefore, this group is referred to as “redfin” hereafter (see Discussion for rationale). Model B3 was considered the best on the basis of BIC and had the lowest uncertainty (0.17) compared with models B1 (0.25) and B2 (0.20; ).
![FIGURE 4. The first two principle components of geometric body shape plotted for MCLUST model group assignments; grey triangles = sicsowet, black dots = humper, grey dots = putative redfin, plus symbols (+) = lean. The outlines represent body shape variation along PC 1 and PC 2. [Figure available online in color.]](/cms/asset/a61d2aff-6eab-4a05-a706-f4df85c9697c/utaf_a_900823_f0004_oc.jpg)

The first four PCs accounted for 66% of the variation in body shape. Principal components 1 and 2 accounted for 35% and 14% of the variation in body shape, respectively. Principal component 1 primarily separated siscowet and humper from lean and redfin on the basis of fin and eye positions, as well as snout shape; whereas, PC 2 discriminated smaller and leaner morphs (i.e., lean and humper) from larger, deeper-bodied morphs (i.e., siscowet and redfin; ).
Visual Group Assignments
Complete agreement was achieved among the three Lake Trout biologists for 54% of the sample (n = 310 of 570), 67% agreement was achieved for about one-quarter of the sample (n = 141 of 570), and no agreement was achieved for about 21% of the fish (n = 119 of 570). Average percent agreement among the three biologists was highest for lean (90%) compared with siscowet (84%) and humper (69%). Agreement was low (15%) for redfin, but that is because only one of the biologists initially visually identified this group (see Methods). If four a priori groups had been used when doing visual assignments, percent agreement for redfins would have been higher than that observed.
Reconciling Head, Body, and Visual Group Assignments
An overall group assignment (consensus) was achieved for 95% of the fish analyzed using established criteria (see Methods). On the basis of a DFA, groups were well discriminated by head shape (λ = 0.19, P < 0.05) and body shape (λ = 0.12, P < 0.05). Jackknife classification of body shape assignments resulted in 94, 90, 99, and 93% of lean, humper, siscowet, and redfin, respectively, being assigned back to their respective groups. Jackknife classification of head shape assignments was also high (mean = 97% for all morphs), but this model only discriminated lean, humper, and siscowet.
TABLE 2. Sample size (n) and mean±SE phenotypic measures for lean, humper, siscowet, and redfin Lake Trout morphs sampled from Isle Royale, Lake Superior. Values with shared lowercase letters are not significantly different. Reported measures are not size-corrected, but statistical analyses reported were done on size-corrected data; TL (mm), Ww = wet weight (kg). Different-sized circles represent the relative values of each variable. The size of the filled circles reflects character size (or length) relative to the other morphs. Unfilled circles show the size and position of the eyes. [Table available online in color.]
Mean percent agreement among the five group assignments (i.e., two models and three visual assignments) was 70%. Percent agreement was zero for 5% of the sample (n = 28 of 621) and 100 for 25% of the sample (n = 155 of 621). Uncertainty in head-shape (H2 = 0.32, P = 0.85) and body-shape (H2 = 0.98, P = 0.61) model assignment of individuals to groups was not related to percent agreement among visual group assignments, which suggests that difficulty in assigning individuals to morphs varied among grouping methods (i.e., statistical versus visual).
Validity of Group Assignments
Percent agreement among the five grouping methods (i.e., two models and three visual assignments) differed among the four morphs (χ20.05, 21 = 153.07, P < 0.001). Overall percent agreement was highest for lean and siscowet (approximately 75%) and lowest among humper (59%) and redfin (49%) Lake Trout. Uncertainty in the head-shape model group assignments also differed among morphs (H3 = 9.25, P = 0.03) and was greatest for the humper. Uncertainty in body-shape model group assignments differed among morphs (H3 = 34.70, P < 0.001) and was highest for redfin (median = 0.18) and much lower for lean (median = 0.08) Lake Trout than for all other groups (Dunn's method: all P < 0.05).
Body condition differed among the four morphs (H3 = 97.68, P < 0.001). The fourth group, redfin, had the highest Wr (median = 96.7), and the lean group had the lowest Wr (median = 81.2), with humper (median = 84.9) and siscowet (median = 90.2) falling in between the two (all P < 0.05). Uncertainty in body shape model assignments of individuals to morphs was related to body condition (t = −2.45, P = 0.02), but that relationship was weak for leans where uncertainty increased as relative weight increased (t = 5.12, P < 0.001), and almost no relationship was apparent for the other morphs. Uncertainty in body-shape model group assignments varied by net set (F62, 484 = 2.62, P < 0.001), but that variation was primarily limited to redfin Lake Trout in three sets (t = 2.25, P = 0.03); therefore, spatial variation in model uncertainty was also minimal.
Phenotypic and Ecological Characteristics of Morphs
Linear phenotypic measures differed among morphs (all F3, 521 > 17, all P < 0.001; ). With the exceptions that follow, all pairwise multiple comparisons were significant: caudal peduncle length did not differ between lean and redfin, head length and maxilla length did not differ between lean and siscowet, and orbital length did not differ between lean and humper or between siscowet and humper. Relative to the other morphs, lean Lake Trout have a large head, long snout (POL), small eyes, long and narrow caudal peduncle, and short paired fins. Humper Lake Trout have a small head, short snout, short maxilla, large eye, and short and narrow caudal peduncle. Although humper appear long and skinny posteriorly, the peduncle itself is actually short relative to the other morphs. Siscowets have a large head, short snout, long maxilla, large eye, short and deep caudle peduncle, and moderately long paired fins. By contrast, redfin Lake Trout were the most robust morph, having the largest head, snout, eyes, longest and deepest caudal peduncle, and much longer pelvic and pectoral fins than the other morphs.
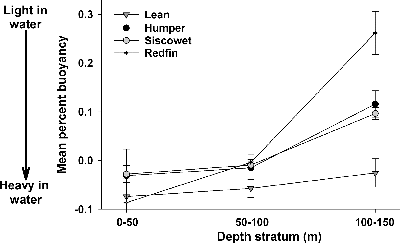
Percent buoyancy differed among morphs and depth strata (significant interaction: F6, 539 = 3.4, P = 0.002). Lean Lake Trout were equally buoyant regardless of capture depth; however, humper, siscowet, and redfin were significantly more buoyant in the two deeper depth strata (50–100 and 100–150 m) compared with the shallow depth stratum (0–50 m). Within the 0–50-m and 50–100-m depth strata, buoyancy did not differ among the four morphs (all P > 0.05). Lean Lake Trout were heavier (i.e., less buoyant) and redfin were lighter (i.e., more buoyant) than all other morphs within the 100–150-m depth stratum. Buoyancy did not differ between siscowet and humper at any depth stratum (all P > 0.05), suggesting skinny siscowet compared with historical data.
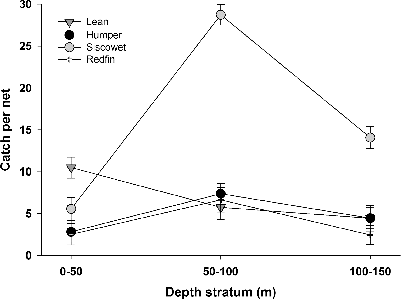
Catch per effort (as catch per net) varied by depth, but that relationship differed among morphs (significant interaction: F3, 63 = 3.61, P < 0.05). Least-squares mean CPE±SE was lowest in the shallow depth (1.51±1.19) and highest in the middepth (9.46±1.15) compared with the deep depth (5.10±1.16; all P < 0.05; ). Overall, siscowet was the most abundant morph occurring in samples from Isle Royale (all P < 0.001; ). Lean and siscowet Lake Trout were caught in most sets, but CPE for leans was greatest in the shallow depth (significantly greater than redfin based on Holm–Sidak test: t = 3.03, P = 0.02) while siscowet CPE was greater than CPE for all other morphs in the mid- and deep depths (all t > 3.5, all P < 0.01). Redfin were primarily caught in two sets in ∼80 m of water (i.e., middepth stratum, 50–100 m)―32% of redfin were caught near the southwest end of Amygdaloid Island (; 48°07′55″N, 88°40′56″W) and 27% were caught on Fisherman's Reef (47°49′25″N, 89°24′55″W) southwest of the Rock of Ages Lighthouse. A few humpers were caught in several sets, but they were most abundant at one site in ∼85 m of water near the mouth of Siskiwit Bay (i.e., 21% of humpers were caught near Menagerie Island: 47°57′06″N, 88°44′19″W).
DISCUSSION
Contemporary versus Historical Diversity
Moderate distinction among Isle Royale Lake Trout morphs in this study contrasts with historical anecdotal accounts that document highly distinct and visually obvious morphs (Rakestraw Citation1967) in Lake Superior as well in other less-perturbed lakes (Blackie et al. Citation2003; Zimmerman et al. Citation2006, Citation2007; Northrup et al. Citation2010). One hypothesis that would explain this lack of discrimination and high variation is that ecological reorganization has occurred from a century of food web change, anthropogenic development, fishery exploitation, and stocking that has caused the breakdown of reproductive and ecological isolating mechanisms that have lead to homogenization and reduced differentiation of morphs (Ecological Reorganization Hypothesis). Altered fish community composition, particularly replacement of native with nonnative planktivores, has changed the way energy is transferred through the food web, and this breakdown of resource partitioning may be partly responsible for a reversal in selective forces maintaining diversity among morphs (sensu Taylor et al. Citation2006; Vonlanthen et al. Citation2012).
Few quantitative data on historical Lake Trout diversity exist, but multiple lines of evidence has led us to propose that Lake Trout diversity in Lake Superior has decreased over the past century: (1) occurrence of well defined diversity in less-perturbed lakes, (2) historical anecdotal accounts of well-defined diversity from Great Lakes populations prior to perturbations, (3) dramatic declines in siscowet fat content and changes in population dynamics during the past 50 years, and (4) contemporary loss of genetic diversity.
Great Bear Lake, Northwest Territories, in northern Canada contains the most striking example of diversity across the Lake Trout range, where as many as four well-defined, shallow-water (i.e., <30 m) morphs occur (Blackie et al. Citation2003; Chavarie et al. Citation2013). Great Slave Lake, Northwest Territories, contains distinct deep- and shallow-water Lake Trout morphs similar to siscowet and lean Lake Trout from Lake Superior, respectively (Zimmerman et al. Citation2006). Shallow (lean) and deepwater (humper-like) Lake Trout from Lake Mistassini, Quebec, can be readily differentiated by body shape, fin length, buoyancy, and body color (Zimmerman et al. Citation2007) as well as life history characteristics, such as age, growth, and maturity (Hansen et al. Citation2012). In all of these examples, morphs are more discrete and easier to recognize than the current collections from Isle Royale, Lake Superior.
Unfortunately, measures of quantitative changes in Great Lakes Lake Trout morphology through time are not possible due to the lack of precollapse (1950s) data. Anecdotal accounts from Isle Royale fishers document many Lake Trout morphs, including lean, bank-trout (aka, banker or humper), siscowet, racer, Rock of Ages trout, redfin, silver-grey, channel salmon, and salmon-trout (Rakestraw Citation1968; Organ et al. Citation1979; Coberly and Horrall Citation1980; Loftus Citation1980; Goodier Citation1981; Toupal et al. Citation2002). To illustrate the uniqueness of two morphs, Goode (Citation1887) quoted an 1880 letter, written by the chairman of the Minnesota Fish Commission:
“The amateur is likely to confound the Namaycush [i.e., lean Lake Trout] with the Siscowet [i.e., deepwater Lake Trout], but when the differences are once pointed out, no confusion of the two again arises. The fishermen recognize them before taken from the water when hauling the nets; even the Indian children know them at a glance.”
This contrasts with the lack of strong distinction observed in our study and the difficulty among biologists in differentiating the morphs.
Paterson et al. (Citation2009) indicated temporal declines in Lake Trout (all morphs combined) muscle energy density in Lakes Erie, Ontario, Huron, and Superior between 1995 and 2004. Lake Superior siscowet (mean = 30% of wet weight [WW]; range = 10–48% WW) were historically three times as fatty as lean Lake Trout (mean = 9% WW, range = 2–19% WW; Eschmeyer and Phillips Citation1965). By contrast, siscowet Lake Trout sampled during 2009 from Grand Marais, Munising, and Marquette, Lake Superior, had mean percent lipid that did not differ from that of historical lean Lake Trout (mean = 9% WW, range = 2–15% WW; R. Kinnunen, Michigan State University, unpublished data). This represents a 72% decline in siscowet body lipid content between 1954 and 2009. Trends in body lipid content, a key ecological adaptation, are likely associated with a siscowet population that is expanding in abundance and bathymetric distribution in the face of a declining cisco forage base (Bronte et al. Citation2003, Citation2010; Bronte and Sitar Citation2008). These changes reflect shifts to a more common resource use and are therefore expected to increase the ecological variation within morphs and reduce the diversity among morphs, making them less distinct compared with those in historical populations.
A loss of genetic diversity among Lake Trout in Lake Superior was attributed to demographic declines in abundance by the 1950s, extirpation of localized stocks, and hatchery stocking (Guinand et al. Citation2003, Citation2012). Guinand et al. (Citation2012) demonstrated losses in genetic diversity within Lake Trout morphs since the population collapse (ca. 1950), but reported that contemporary morphs remained genetically distinct in Lake Superior.
Historically, the Lake Superior food web was simple: energy was transferred from primary and secondary producers through shallow- and deepwater coregonines and sculpins (Myoxocephalus thompsonii, Cottus ricei, and C. cognatus) to Lake Trout and Burbot Lota lota (Dryer et al. Citation1965). Beginning in the 1800s, the Lake Superior food web underwent dramatic changes that included the overharvest of Lake Trout and ciscoes (Hile et al. Citation1951; Smith Citation1964; Lawrie and Rahrer Citation1973; Selgeby Citation1982; Bronte and Sitar Citation2008; Bronte et al. Citation2010) that reduced abundance and diversity of these fishes, invasion by predatory Sea Lamprey (Heinrich et al. Citation2003), and the expansion of nonnative Rainbow Smelt Osmerus mordax (Anderson and Smith Citation1971). Taken together, these changes altered the Lake Superior food web, and Lake Trout diversity was especially affected.
A century of ecological change probably altered selection pressures on Lake Trout morphs thereby reducing ecological barriers among morphs. Such reversals in diversity are not uncommon. For example, eutrophication reduced ecological opportunity leading to a speciation reversal in European whitefishes Coregonus spp. counteracting previous adaptive radiations (Vonlanthen et al. Citation2012). Similarly, a benthic and limnetic Threespined Stickleback Gasterosteus aculeatus species pair from a small lake in British Columbia, collapsed into a hybrid swarm in less than a decade, possibly due to introduction of the exotic crayfish, Pascifasticus lenisculus (Taylor et al. Citation2006). These examples demonstrate how quickly (i.e., within a decade) and easily (i.e., phosphorous loading) thousands of years of ecological adaptation and reproductive isolating mechanisms can be eliminated. We have demonstrated that at least some of the historic morphological and ecological variation in Lake Superior Lake Trout persists, but whether this diversity is in a state of ecological release and subsequent reorganization or is headed towards complete breakdown and homogenization is unknown.
Extant Lake Trout Morphs
Lean, humper, and siscowet morphs from Isle Royale identified herein generally conform to historical descriptions. On the basis of scant historical data, we identified a fourth morph consistent in external gross morphology and habitat use with the colloquial “redfin” from Lake Superior (Rakestraw Citation1968; Organ et al. Citation1979). Redfins have also been described from Lakes Michigan and Huron (Coberly and Horrall Citation1980; Loftus Citation1980).
Adult redfins averaged 5 to7 kg in Lake Michigan (Coberly and Horrall Citation1980), 5 kg and greater in Lake Huron (Loftus Citation1980), and up to 20 kg at Isle Royale, Lake Superior (Toupal et al. Citation2002). They had red flesh, red or red and yellow fins, sometimes covered in spots, and often dark dorsal coloration (Loftus Citation1980; Goodier Citation1981). Fishers described the Isle Royale redfin as being large with a big head and large pectoral fins (Toupal et al. Citation2002). Similarly, the redfins we caught attained a large size (maximum length = 868 mm; maximum weight = 5.3 kg) and were robust in appearance, with dark dorsal coloration, a large head and eyes, a long and deep caudal peduncle, and long and broad paired fins that were red. The redfins we caught were most abundant in moderate depths (∼80 m) with few caught in water less than 50 m deep, similar to observations on Lake Huron (Loftus Citation1980).
Great Lakes commercial fishers observed redfins in spawning condition from August to mid-September, similar to the humper (Rahrer Citation1965), but prior to the October–November spawning period of lean Lake Trout (Organ et al. Citation1979; Coberly and Horrall Citation1980; Loftus Citation1980; Goodier Citation1981; Toupal et al. Citation2002) and siscowets. Redfins were thought to spawn over rocky substrate at depths of 1–18 m in Lake Huron (Loftus Citation1980) and often on the same shoals used by lean Lake Trout in Lake Superior (Goodier Citation1981). Redfin spawn around Isle Royale at Passage Island, Gull Island, and from Rainbow Point to Siskiwit Island () over numerous shoals in 9–15 m of water. Fishers caught redfins at 13 areas around Isle Royale, including “Redfin Island,” named for the prevalence of the morph (; Toupal et al. Citation2002).
Phenotypic and Ecological Characteristics of Morphs
Phenotypic and ecological characteristics differed among morphs (objective 3). Consistent with previous studies (Alfonso Citation2004; Zimmerman et al. Citation2006, Citation2007; Blackie et al. Citation2012; Chavarie et al. Citation2013), we found evidence of resource polymorphisms, defined as different morphologies within sympatric populations of a species associated with feeding or habitat (Skulason and Smith Citation1995). Resource polymorphism, often along depth and trophic axes, is common among northern fishes dispersing into novel environments after the last glacial retreat (Robinson and Wilson Citation1994) and was previously hypothesized to explain the maintenance of Lake Trout diversity in Lake Superior (Eshenroder Citation2008).
Water depth is a primary ecological gradient driving the differentiation of Lake Trout (Eshenroder and Burnham-Curtis Citation1999; Eshenroder Citation2008). Lake Superior is the deepest of the Great Lakes, with a maximum depth of 407 m, and approximately 80% of its waters are greater than 50 m deep (Eshenroder and Lantry Citation2012). The deepwater pelagic zone has relatively constant year-round temperature (∼4°C) and extends outward from approximately the 80-m water depth (Horns et al. Citation2003) up to the thermocline, but does not include hypolimnetic waters just off the bottom or warmer epilimnetic waters above the thermocline (Eshenroder and Lantry Citation2012). The physical characteristics of the water masses provide contrasting niches of shallow- and deepwater epilimnetic, deepwater hypolimnetic, and deepwater pelagic habitats, each with divergent selection pressures.
In response to differing selection pressures in deep- and shallow-water habitats, Lake Trout morphs appear to have evolved to have two different mechanisms for buoyancy regulation: hydrodynamic and hydrostatic lift (Eshenroder et al. Citation1999). We propose that lean Lake Trout rely more on hydrodynamic lift via sustained swimming and a greater reliance on the swim (gas) bladder to maintain neutral buoyancy and move through the water column. Relative to the other morphs, lean Lake Trout had low buoyancy (i.e., low lipid), shorter paired fins, a more streamlined body shape, and a long, narrow caudal peduncle, all of which are adaptations for pelagic swimming (Webb Citation1984; Bond Citation1996). By contrast, siscowet and redfin morphs had high buoyancy (i.e., high lipid content), longer paired fins, and thick and short caudal peduncles, suggesting that they relied more on hydrostatic lift as a mechanism to maintain buoyancy and move vertically in the water column. A thick caudal peduncle and large fins are adaptive for beat-and-glide locomotion, for slowing descent due to hydrostatic pressure when foraging in pelagia, and for maneuvering over rocky substrate when foraging near the bottom (Webb Citation1984). Humpers inhabit moderate water depths (∼100 m: Moore and Bronte Citation2001) and are often associated with offshore shoals or banks. These are typically areas of strong current. Humpers are only slightly fat compared with lean Lake Trout, have small paired fins, and a short, narrow peduncle, characteristics that are adaptive for hydrodynamic lift. Humper gas bladders tend to fill when brought to the surface from deep water, which suggests a physiological limitation to vertical ascent; therefore, vertical migration could be restricted in humpers due to gas exchange limitations.
Trophic resource use is the second primary ecological gradient thought to generate and maintain Lake Trout diversity. Using known morphological differences among morphs and differences in food habits between lean and siscowet (Dryer et al. Citation1965; Peck Citation1975; Conner et al. Citation1993; Harvey and Kitchell Citation2000; Hrabik et al. Citation2006; Ray et al. Citation2007; Gamble et al. Citation2011), we can infer how trophic resource use relates to morphological diversity among morphs. Our knowledge of redfin and humper food habits is incomplete and requires further study.
Eye size and relative position on the head are trophically linked traits (Barel Citation1983; van der Meer and Anker Citation1984) that differed among Lake Trout morphs at Isle Royale. Humpers and siscowets have relatively large eyes positioned high on the head, an arrangement that provides improved binocular vision (Bond Citation1996) and improved light gathering ability providing more sensitive low-light vision (van der Meer and Anker Citation1984). Vertically migrating, low-light, visual predators that live in deep water, such as humpers, siscowets (Hrabik et al. Citation2006), and possibly redfins, tend to have eyes that are larger than those of lean Lake Trout and could be adaptive for feeding from below on Mysis (humper morph) and ciscoes (siscowet and redfin morphs). By contrast, leans have relatively small eyes that are positioned low on the side of head providing a wide, lateral field of view, which is adaptive for shallow-water or pelagic predation on fish (Bond Citation1996).
Validity of Group Assignments
The four Lake Trout morphs were identified visually and by a body-shape model; redfin were not identified by a head-shape model. We expected head shape to be more reliable than other grouping methods because it is conserved, reflecting differences in bony structure of the skull and less influenced by body or reproductive condition. Contrary to our prediction, geometric morphometric size correction and scaling successfully accounted for differences in relative body condition among individuals and resulting group classifications were sensitive to these differences. Similarly, key visual characteristics used to group individuals were associated with the head, fins, and peduncle, rather than midbody areas most affected by body condition. These data reinforce the notion that condition or body shape, such as midbody depth, are not indicative of body lipid content, and therefore, should not be used when visually identifying Lake Trout morphs (see ).
Consistent with visual identifications, variation in head and body shape among morphs was high, resulting in incomplete separation of groups. We determined that 52% and 49% of the variation in head and body shape, respectively, was accounted for in an ordination of geometric shape variates, which is comparable but less than the 63% and 73% reported by Zimmerman et al. (Citation2009) for similar-sized lean and siscowet-like Lake Trout from Great Slave Lake. This difference means that the major axis of shape variation is less dominant for Isle Royale Lake Trout, resulting in weak separation relative to Great Slave Lake.
Management Implications
Understanding ecological diversity within Lake Trout has management implications for conservation and restoration in the Laurentian Great Lakes. Historically, some of the highest Lake Trout diversity in Lake Superior was reported in the waters surrounding Isle Royale (Hubbs and Lagler Citation1949; Rakestraw Citation1967, Citation1968; Toupal et al. Citation2002). Restoration of Lake Trout morphs at Isle Royale appears to be an ongoing process, which involves the differential use of habitat (i.e., water depth) and the expression of different body morphologies under conditions of low total mortality. This process demonstrates the resilience of extant, residual wild Lake Trout populations to perturbations. Unsuccessful Lake Trout restoration efforts, which are hampered by other impediments not related to diversity (see Bronte et al. Citation2003), primarily have relied on stocking the lean morph, which addresses population re-establishment of only a small proportion of the habitat available in the Great Lakes (Krueger and Ihssen Citation1995). Future efforts should be broadened to include other morphs (e.g., humper morph; Bronte et al. Citation2008; Markham et al. Citation2008) with timing and placement consistent with the ecology and life history of the morph. Overlap in depth use among morphs means that future fisheries will likely be “mixed stock” or at least “mixed morph,” as they were historically, and are now being observed in Lake Superior today. The management of a fishery that targets one morph over another will have to consider the impacts on nontarget morphs as well as the ecological and genetic processes contributing to the differences among morphs.
ACKNOWLEDGMENTS
We thank Stewert Sivertson and Enar and Betty Strom for their knowledge of Lake Superior and its fishes, and for their hospitality at Washington and Barnam islands. Jonathan Pyatskowit provided cheerful and able assistance with field work. Thanks to Scott Miehls (U.S. Geological Survey) and Sarah Seegert and Christina Haska (Great Lakes Fishery Commission) for their assistance collecting morphological data. Thanks to Shawn Sitar (Michigan Department of Natural Resources) and two anonymous reviewers for constructive feedback on the manuscript. Funding for this project was provided by the Great Lakes Fishery Commission through the Fishery Research Program. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the U.S. Fish and Wildlife Service.
REFERENCES
- Alexander, R.M. 1993. Buoyancy. Pages 75–97 in D.H. Evans, editor. The physiology of fishes, 2nd edition. CRC Press, Boca Raton, Florida .
- Alfonso, N.R. 2004. Evidence for two morphotypes of Lake Charr, Salvelinus namaycush, from Great Bear Lake, Northwest Territories, Canada. Environmental Biology of Fishes 143:21–32.
- Amundsen, P., T. Bohn, and G.H. Vaga. 2004. Gill raker morphology and feeding ecology of two sympatric morphs of European whitefish (Coregonsu lavaretus). Annales Zoologici Fennici 41:291–300.
- Anderson, D.E., and L.L. J. Smith. 1971. Factors affecting abundance of Lake Herring (Coregonus artedii Lesueur) in Western Lake Superior. Transactions of the American Fisheries Society 4:691–707.
- Barel, C.D. N. 1983. Towards a constructional morphology of cichlid fishes (Teleostei, Peciformes). Netherlands Journal of Zoology 33:357–424.
- Bernatchez, L. 2004. Ecological theory of adaptive radiation. An empirical assessment from Coregonine fishes (Salmoniformes). Pages 175–207inA.P. Hendry, editor. Evolution illuminated, salmon and their relatives. Oxford University Press, New York.
- Blackie, C.T., P. Vecsei, and P.A. Cott. 2012. Contrasting phenotypic variation among river and lake caught Cisco from Great Slave Lake: evidence for dwarf and large morphs. Journal of Great Lakes Research 38:798–805.
- Blackie, C.T., D.J. Weese, and D.L. G. Noakes. 2003. Evidence for resources polymorphism in the Lake Charr (Salvelinus namaycush) of Great Bear Lake, Northwest Territories, Canada. Ecoscience 10:509–514.
- Bond, C.E. 1996. Biology of fishes. Saunders College Publishing, Forth Worth, Texas .
- Bronte, C.R. 1993. Evidence of spring spawning Lake Trout in Lake Superior. Journal of Great Lakes Research 19:625–629.
- Bronte, C.R., M.P. Ebener, D.R. Schreiner, D.S. DeVault, M.M. Petzold, D.A. Jensen, C. Richards, and S.J. Lozano. 2003. Fish community change in Lake Superior, 1970–2000. Canadian Journal of Fisheries and Aquatic Sciences 60:1552–1574.
- Bronte, C.R., C.C. Krueger, M.E. Holey, M.L. Toneys, R.L. Eshenroder, and J.L. Jonas. 2008. A guide for the rehabilitation of Lake Trout in Lake Michigan. Great Lakes Fishery Commission, Miscellaneous Publication 2008-01, Ann Arbor, Michigan .
- Bronte, C.R., M.H. Hoff, O.T. Gorman, W.E. Thogmartin, P.J. Schneeberger, and T.N. Todd. 2010. Decline of the shortjaw cisco in Lake Superior: the role of overfishing and risk of extinction. Transactions of the American Fisheries Society 139:735–748.
- Bronte, C.R., and S.P. Sitar. 2008. Harvest and relative abundance of siscowet Lake Trout in Michigan waters of Lake Superior, 1929–1961. Transactions of the American Fisheries Society 137:916–926.
- Burnham-Curtis, M.K. 1993. Interlacustrine speciation of Salvelinus namaycush: an investigation of genetic and morphological variation and evolution of the Lake Trout in the Laurentian Great Lakes. Doctoral dissertation. University of Michigan, Ann Arbor.
- Burnham-Curtis, M.K., and C.R. Bronte. 1996. Otoliths reveal a diverse age structure for humper Lake Trout in Lake Superior. Transactions of the American Fisheries Society 125:844–851.
- Burnham-Curtis, M.K., and G.R. Smith. 1994. Osteological evidence of genetic divergence of Lake Trout (Salvelinus namaycush) in Lake Superior. Copeia 1994:843–850.
- Chavarie, L., K.L. Howland, and W.M. Tonn. 2013. Sympatric polymorphism in Lake Trout: the coexistence of multiple shallow-water morphotypes in Great Bear Lake. Transactions of the American Fisheries Society 142:814–823.
- Coberly, C.E., and R.M. Horrall. 1980. Fish spawning grounds in Wisconsin waters of the Great Lakes. University of Wisconsin, Sea Grant Institute, WIS-SG-80-235, Madison.
- Conner, D.J., C.R. Bronte, J.H. Selgeby, and H.L. Collins. 1993. Food of salmonine predators in Lake Superior, 1981–1987. Great Lakes Fishery Commission Technical Report 59.
- Dryer, W.R., L.F. Erkkila, and C.L. Tetzloff. 1965. Food of Lake Trout in Lake Superior. Transactions of the American Fisheries Society 94:169–176.
- Eschmeyer, P.H. 1955. The reproduction of Lake Trout in southern Lake Superior. Transactions of the American Fisheries Society 84:47–74.
- Eschmeyer, P.H., and A.M. Phillips Jr. 1965. Fat content of the flesh of siscowets and Lake Trout from Lake Superior. Transactions of the American Fisheries Society 94:62–74.
- Eshenroder, R.L. 2008. Differentiation of deep-water Lake Charr Salvelinus namaycush in North American lakes. Environmental Biology of Fishes 83:77–90.
- Eshenroder, R.L., R.L. Argyle, and L.M. TeWinkel. 1998. Evidence for buoyancy regulation as a speciation mechanism in Great Lakes Ciscoes. Archive fur Hydrobiologie 50:207–217.
- Eshenroder, R.L., and M.K. Burnham-Curtis. 1999. Species succession and sustainability of the Great Lakes fish community. Pages 145–184 in W.W. Taylor and C.P. Ferreri, editors. Great Lakes fishery policy and management: a binational perspective. Michigan State University Press, East Lansing.
- Eshenroder, R.L., and B.F. Lantry. 2012. Recent changes in succesional state of the deep-water fish communities of Lakes Michigan, Huron, and Ontario and management implications. Pages 137–165inW.W. Taylor, A.J. Lynch, and N.J. Leonard, editors. Great Lakes fisheries policy and management: a binational perspective, 2nd edition. Michigan State University Press, East Lansing.
- Eshenroder, R.L., V.G. Sideleva, and T.N. Todd. 1999. Functional convergence among pelagic sculpins of Lake Baikal and deepwater Ciscoes of the Great Lakes. Journal of Great Lakes Research 25:847–855.
- Esteve, M. 2005. Observations of spawning behaviour in Salmoninae: Salmo, Oncorhynchus and Salvelinus. Reviews in Fish Biology and Fisheries 15:1–21.
- Esteve, M., D.A. McLennan, and J.M. Gunn. 2008. Lake Trout (Salvelinus namaycush) spawning behaviour: the evolution of a new female strategy. Environmental Biology of Fishes 83:69–76.
- Fraley, C., and A.E. Raftery. 2009. MCLUST version 3 for R: normal mixuture modeling and model-based clustering. University of Washington, Technical Report 504, Seattle.
- Gamble, A.E., T.R. Hrabik, J.D. Stockwell, and D.L. Yule. 2011. Trophic connections in Lake Superior part I: the offshore fish community. Journal of Great Lakes Research 37:541–549.
- Goetz, F., D. Rosauer, S. Sitar, G. Goetz, C. Simchick, S. Roberts, R. Johnson, C. Murphy, C.R. Bronte, and S. Mackenzie. 2010. A genetic basis for the phenotypic differentiation between siscowet and lean Lake Trout (Salvelinus namaycush). Molecular Ecology 19(4):176–196.
- Goode, G.B. 1884. The fisheries and fishery industries of the United States. U.S. Commission of Fish and Fisheries, Washington, D.C.
- Goode, G.B. 1887. American fishes: a popular treatise upon the game and food fishes of North America. L. C. Page, Boston.
- Goodier, J.L. 1981. Native Lake Trout (Salvelinus namaycush) stocks in the Canadian waters of Lake Superior prior to 1955. Canadian Journal of Fisheries and Aquatic Sciences 38:1724–1737.
- Guinand, B., K.S. Page, M.K. Burnham-Curtis, and K.T. Scribner. 2012. Genetic signatures of historical bottlenecks in sympatric Lake Trout (Salvelinus namaycush) morphotypes in Lake Superior. Environmental Biology of Fishes 95:323–334.
- Guinand, B., K.T. Scribner, K.S. Page, and M.K. Burnham-Curtis. 2003. Genetic variation over space and time: analyses of extinct and remnant Lake Trout populations in the upper Great Lakes. Proceedings of the Royal Society B Biological Sciences 270:425–433.
- Gunn, J.M. 1995. Spawning behaviour of Lake Trout: effects on colonization ability. Journal of Great Lakes Research 21(Supplement 1):323–329.
- Hansen, M.J., N.A. Nate, C.C. Krueger, M.S. Zimmerman, H.G. Kruckman, and W.W. Taylor. 2012. Age, growth, survival, and maturity of Lake Trout morphotypes in Lake Mistassini, Quebec. Transactions of the American Fisheries Society 141:1492–1503.
- Harvey, C.J., and J.F. Kitchell. 2000. A stable isotope evaluation of the structure and spatial heterogeneity of a Lake Superior food web. Canadian Journal of Fisheries and Aquatic Sciences 57:1395–1403.
- Heinrich, J.W., K.M. Mullett, M.J. Hansen, J.V. Adams, G.T. Klar, D.A. Johnson, G.C. Christie, and R.J. Young. 2003. Sea lamprey abundance and management in Lake Superior, 1957–1999. Journal of Great Lakes Research 29(Supplement 1):566–583.
- Hile, R., P.H. Eschmeyer, and G.F. Lunger. 1951. Status of the Lake Trout fishery in Lake Superior. Transactions of the American Fisheries Society 80:278–312.
- Holey, M.E., R.W. Rybicki, G.W. Eck, E.H. Brown, J.E. Marsden, D.S. Lavis, M.L. Toneys, T.N. Trudeau, R.M. Horrall. 1995. Progress toward Lake Trout restoration in Lake Michigan. Journal of Great Lakes Research 21(Supplement 1):128–151.
- Horns, W., C.R. Bronte, T.R. Busiahn, M.P. Ebener, R.L. Eshenroder, T. Gorenflo, N. Kmiecik, W. Mattes, J.W. Peck, M. Petzold, D.R. Schreiner. 2003. Fish-community objectives for Lake Superior. Great Lakes Fishery Commission, Special Publication 03-01, Ann Arbor, Michigan .
- Hrabik, T.R., O.P. Jensen, S.J. D. Martell, C.J. Walters, and J.F. Kitchell. 2006. Diel vertical migration in the Lake Superior pelagic community. I. Changes in vertical migration of coregonids in response to varying perdation risk. Canadian Journal of Fisheries and Aquatic Sciences 63:2286–2295.
- Hubbs, C.L., and K.F. Lagler. 1949. Fishes of Isle Royale, Lake Superior, Michigan. Papers of the Michigan Academy of Science, Arts, and Letters 33:73–134.
- Khan, N.Y., and S.U. Qadri. 1970. Morphological differences in Lake Superior Lake Char. Journal of the Fisheries Research Board of Canada 27:161–167.
- Kitchell, J.F., S.P. Cox, C.J. Harvey, T.B. Johnson, D.M. Mason, K.K. Schoen, K. Aydin, C. Bronte, M. Ebener, M. Hansen, M. Hoff, S. Schram, D. Schreiner, and C.J. Walters. 2000. Sustainability of the Lake Superior fish community: interactions in a food web context. Ecosystems 3:545–560.
- Klemetsen, A. 2010. The charr problem revisited: exceptional phenotypic plasticity promotes ecological speciation in postglacial lakes. Freshwater Reviews 3:49–74.
- Krause, A.E., R.L. Eshenroder, and L.J. Begnoche. 2002. Buoyancy differences among two deepwater Ciscoes from the Great Lakes and their putative ancester. Archiv fuer Hydrobiologie Special Issues in Advanced Limnologie 57:233–242.
- Krueger, C.C., and P.E. Ihssen. 1995. Review of genetics of Lake Trout in the Great Lakes: history, molecular genetics, physiology, strain comparisons, and restoration management. Journal of Great Lakes Research 21(Supplement 1):348–363.
- Krueger, C.C., M.L. Jones, and W.W. Taylor. 1995. Restoration of Lake Trout in the Great Lakes: challenges and strategies for future management. Journal of Great Lakes Research 21(Supplement 1):547–558.
- Lawrie, A., and J.F. Rahrer. 1973. Lake Superior: a case history of the lake and it fisheries. . Great Lakes Fishery Commission Technical Report 19.
- Lindsey, C.C. 1981. Stocks are chameleons: plasticity in gill rakers of coregonid fishes. Canadian Journal of Fisheries and Aquatic Sciences 38:1497–1506.
- Loftus, D.H. 1980. Interviews with Lake Huron commercial fishermen. Ontario Ministry of Natural Resources, Lake Huron Fisheries Assessment Unit Report 1-80, Owen Sound.
- Markham, J., A. Cook, T. MacDougall, L. Witzel, K. Kayle, C. Murray, M. Fodale, E. Trometer, F. Neave, J. Fitzsimons, J. Francis, and M. Stapanian. 2008. A strategic plan for the rehabilitation of Lake Trout in Lake Erie, 2008–2020. Great Lakes Fishery Commission, Miscellaneous Publication 2008-2, Ann Arbor, Michigan .
- Martin, N.V., and C.H. Olver. 1980. The Lake Charr, Salvelinus namaycush. Pages 205–277inE.K. Balon, editor. Charrs: salmonid fishes of the genus Salvelinus. Dr W. Junk, The Hague, The Netherlands.
- Mehner, T., S. Busch, C. Clemmesen, I.P. Helland, F. Hölker, J. Ohlberger, and M.A. Peck. 2012. Ecological commonalities among pelagic fishes: comparison of freshwater ciscoes and marine herring and sprat. Marine Biology 159:2583–2603.
- Moore, S.A., and C.R. Bronte. 2001. Delineation of sympatric morphotypes of Lake Trout in Lake Superior. Transactions of the American Fisheries Society 130:1233–1240.
- Muir, A.M., C.C. Krueger, and M.J. Hansen. 2012a. Re-establishing Lake Trout in the Laurentian Great Lakes: past, present, and future. Pages 533–588inW.W. Taylor, A.J. Lynch, and N.J. Leonard, editors. Great Lakes fisheries policy and management: a binational perspective, 2nd edition. Michigan State University Press, East Lansing.
- Muir, A.M., P. Vecsei, and C.C. Krueger. 2012b. A perspective on perspectives: a method toward reducing variation in digital shape analysis. Transactions of the American Fisheries Society 141:1161–1170.
- Northrup, S., M. Connor, and E.B. Taylor. 2010. Population structure of Lake Trout (Salvelinus namaycush) in a large glacial-fed lake inferred from microsatellite DNA and morphological analysis. Canadian Journal of Fisheries and Aquatic Sciences 67:1171–1186.
- Organ, W.L., G.L. Towns, M.O. Walter, R.B. Pelletier, and D.A. Riege. 1979. Past and presently known spawning grounds of fishes in the Michigan coastal waters of the Great Lakes. Michigan Department of Natural Resources, Fisheries Division Technical Report 79-1, Ludington.
- Paterson, G., D.M. Whittle, K.G. Drouillard, and G.D. Haffner. 2009. Declining Lake Trout (Salvelinus namaycush) energy density: are there too many salmonid predators in the Great Lakes? Canadian Journal of Fisheries and Aquatic Sciences 66:919–932.
- Peck, J.W. 1975. Brief life history accounts of five commercial salmonid fishes in Lake Superior. Michigan Department of Natural Resources, Fisheries Division Fisheries Research Report 1821, Ludington.
- Piccolo, J.J., W.A. Hubert, and R.A. Whaley. 1993. Standard weight equation for Lake Trout. North American Journal of Fisheries Management 13:401–404.
- Rahrer, J.F. 1965. Age, growth, maturity, and fecundity of humper Lake Trout, Isle Royal, Lake Superior. Transactions of the American Fisheries Society 94:75–83.
- Rakestraw, L. 1967. Post-columbian history of Isle Royale. Part II: fisheries. Master's thesis. Michigan Technological University, Houghton.
- Rakestraw, L. 1968. Commercial fishing on Isle Royale. Isle Royale Natural History Association with the National Park Service, Houghton, Michigan. . Available: http://www.nps.gov/history/history/online_books/isro/rakestraw/index.htm. (May 2014).
- Ray, B.A., T.R. Hrabik, M.P. Ebener, O.T. Gorman, D.R. Schreiner, S.T. Schram, S.P. Sitar, W.P. Mattes, and C.R. Bronte. 2007. Diet and prey selection by Lake Superior Lake Trout during spring 1986–2001. Journal of Great Lakes Research 33:104–113.
- Reist, J.D. 1985. An empirical evaluation of several univariate methods that adjust for size variation in morphometric data. Canadian Journal of Zoology 63:1429–1439.
- Reist, J.D., M. Power, and J.B. Dempson. 2012. Arctic Charr (Salvelinus alpinus): a case study of the importance of understanding biodiversity and taxonomic issues in northern fishes. Biodiversity 14:45–56.
- Robinson, B.W., and D.S. Wilson. 1994. Character release and displacement in fishes: a neglected literature. American Naturalist 144:596–627.
- Rohlf, J.F. 2009. tpsDig, digitize landmarks and outlines, version 2.14. State University of New York–Stony Brook, Stony Brook. . Available: http://life.bio.sunysb.edu/morph/. (May 2014).
- Roosevelt, R.B. 1865. Superior fishing: or, the Striped Bass, trout, and black bass of the northern States. Carleton Publisher, New York.
- Selgeby, J.H. 1982. Decline of Lake Herring (Coregonus artedii) in Lake Superior: an analysis of the Wisconsin herring fishery, 1936–1978. Canadian Journal of Fisheries and Aquatic Sciences 39:554–563.
- Skulason, S., and T.B. Smith. 1995. Resource polymorphisms in vertebrates. Trends in Ecology and Evolution 10:366–370.
- Smith, S.H. 1964. Status of the deepwater Cisco population of Lake Michigan. Transactions of the American Fisheries Society 93:155–163.
- Sweeny, R.O. 1890. The siskiwit. Transactions of the American Fisheries Society 19:84–87.
- Taylor, E.B., J.W. Boughman, M. Groeneboom, M. Sniatynski, D. Schluter, and J.L. Gow. 2006. Speciation in reverse: morphological and genetic evidence of the collapse of a Three-spined Stickleback (Gasterosteus aculeatus) species pair. Molecular Ecology 15:343–355.
- Thomson, J. 1883. A trout trip to St. Ignace Island. Pages 97–117inC.F. Orvis and A.N. Cheney, editors. Fishin with the fly: sketches by lovers of the art with illustrations of standard flies. C.F. Orvis, Manchester, UK.
- Thurston, C.E. 1962. Physical characteristics and chemical composition of two subspecies of Lake Trout. Journal of the Fisheries Research Board of Canada 19:39–44.
- Todd, T.N., and G.R. Smith. 1992. A review of differentiation in Great Lakes Ciscoes. Polskie Archiwum Hydrobiologii 39:261–267.
- Toupal, R.S., R.W. Stoffle, and M.N. Zedirio. 2002. The Isle Royale folkefiskerisamfunn: familier som levde av fishke—an ethnohistory of the Scandinavian folk fishermen of Isle Royale National Park. . Report prepared for The National Park Service Midwest Regional Office and Isle Royale National Park, Houghton, Michigan.
- van der Meer, H.J., and G.C. Anker. 1984. Retinal resolving power and sensitivity of the photopic system in seven haplochromine species (Cichlidae, Teleostei). Netherlands Journal of Zoology 34:197–209.
- Vonlanthen, P., D. Bittner, A.G. Hudson, K.A. Young, R. Müller, B. Lundsgaard-Hansen, D. Roy, S. Di Piazza, C.R. Largiader, and O. Seehausen. 2012. Eutrophication causes speciation reversal in whitefish adaptive radiations. Nature 482:357–362.
- Webb, P.W. 1984. Body form, locomotion and foraging in aquatic vertebrates. American Zoologist 24:107–120.
- Zelditch, M.L., D.L. Swiderski, D.H. Sheets, and W.L. Fink. 2004. Geometric morphometrics for biologists: a primer. Elsevier Academic Press, New York.
- Zimmerman, M.S., C.C. Krueger, and R.L. Eshenroder. 2006. Phenotypic diversity of Lake Trout in Great Slave Lake: differences in morphology, buoyancy, and habitat depth. Transactions of the American Fisheries Society 135:1056–1067.
- Zimmerman, M.S., C.C. Krueger, and R.L. Eshenroder. 2007. Morphological and ecological differences between shallow- and deep-water Lake Trout in Lake Mistassini, Quebec. Journal of Great Lakes Research 33:156–169.
- Zimmerman, M.S., S.N. Schmidt, C.C. Krueger, M.J. Vander Zanden, and R.L. Eshenroder. 2009. Ontogenetic niche shifts and resource partitioning of Lake Trout morphotypes. Canadian Journal of Fisheries and Aquatic Sciences 66:1007–1018.

![FIGURE 1. Lake Trout sampling locations (solid triangles) at Isle Royale, Lake Superior. [Figure available online in color.]](/cms/asset/70a516a5-bf8b-43de-b38b-41b5201ade10/utaf_a_900823_f0001_oc.jpg)
![FIGURE 3. The first two principle components of geometric head shape plotted for MCLUST model group assignments; grey triangles = lean, black dots = humper, grey dots = siscowet. The outlines above the plot represent the head shape variation along PC 1. [Figure available online in color.]](/cms/asset/b92de31d-cb66-44cc-a9d4-73f13fbaa884/utaf_a_900823_f0003_oc.jpg)