Abstract
We used coded wire tag data to compare spawner age structure and seasonal patterns of age-specific size at date among fish harvested in the ocean from the four seasonal run timings (fall, late-fall, winter, and spring) of Chinook Salmon Oncorhynchus tshawytscha from the Central Valley, California, and we examined differences between the fall-run fish (the most abundant run) from the Sacramento and San Joaquin River basins. The runs varied in their ocean size at a common age and date, and within each run, monthly mean ocean sizes appeared to stop increasing when spawners began to return to freshwater. Despite support for multiple hypotheses, no single factor explained all of the variation among and within runs. Ocean size at a common date was well explained by a “juvenile head-start” hypothesis, predicting larger sizes for the spring and fall runs due to earlier ocean entry. Month of spawner return was well explained by a “premature adult migration” hypothesis, predicting earlier returns (within years, regardless of age) by winter- and spring-run fish spawning further upstream. However, neither release timing nor spawning elevation could fully explain observed patterns in spawner age structure, such as an unusually high occurrence of age-2 San Joaquin River fall-run spawners and the near absence of age-4 or older spawners in the winter run. Larger smolt size might explain earlier maturation by the San Joaquin versus Sacramento River fall run, but smolt size could not explain patterns in age structure across runs. Metabolic costs of holding upstream with large size might explain the lack of older spawners among the winter run but are inconsistent with the late-fall run having the highest frequency of age-4 and older spawners. Our results demonstrate multiple pathways by which differences both within and among the runs may contribute to differences in their fishery vulnerability and demographic decoupling, which could contribute to a stabilizing portfolio effect.
Received August 29, 2016; accepted February 2, 2017 Published online April 27, 2017
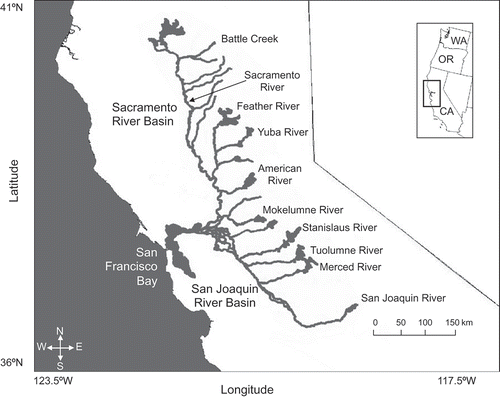
Chinook Salmon Oncorhynchus tshawytscha from the Central Valley of California display four distinct life histories, which are classified on the basis of their spawning run times (Fisher Citation1994). This diversity of run timing leads to the unusual distinction that adult salmon are present year-round in the Central Valley system (Fisher et al. Citation1991), which consists of the Sacramento and San Joaquin River basins (), with multiple salmon-bearing tributaries. A high diversity of life histories may provide a “portfolio effect,” buffering stock complexes against environmental variation (Greene et al. Citation2010; Moore et al. Citation2010; Schindler et al. Citation2010). Despite this diversity, damming, water diversions, habitat degradation, harvest, and other anthropogenic influences have reduced the abundance of all Chinook Salmon runs in the Central Valley. The winter run (endangered) and spring run (threatened) have been particularly impacted (Yoshiyama et al. Citation1998) because they historically spawned in upper watershed habitats that are now made inaccessible by dams. Although spring-run salmon historically made up the bulk of returns to the San Joaquin River (Fry Citation1961; Yoshiyama et al. Citation1998), all natural spring-run populations in the San Joaquin appear to have been extirpated (Myers et al. Citation1998), and no more than 4 of the 18 or 19 identified historical populations of Central Valley spring-run Chinook Salmon remain (Lindley et al. Citation2007). Winter-run Chinook Salmon have been extirpated from their historical habitat in spring-fed streams of the McCloud and Pit rivers, and today spawning and rearing are confined to the Sacramento River below Shasta Dam (Winship et al. Citation2013).
FIGURE 1. Map of the Central Valley, California, showing locations of the Sacramento and San Joaquin River basins and major salmon-bearing tributaries.
It is important to understand the life history diversity among run timings because Chinook Salmon returns to the Central Valley, now dominated by the fall run, have become increasingly variable (Satterthwaite and Carlson Citation2015), with (recent) record-high returns followed by closure of the fishery in 2008–2009. Life history diversity within runs may also be important. For example, Carlson and Satterthwaite (Citation2011) showed that correlations in stock abundance among rivers were lower when comparing rivers from the San Joaquin River basin to rivers from the Sacramento River basin than when comparing rivers within basins. This may reflect differences in age at maturation among fish from the different basins (Myers et al. Citation1998), resulting in responses to the ocean environment on different time scales. A better mechanistic understanding of life history differences between and within runs at multiple life stages would improve our understanding of the extent to which restoration and other fisheries and water management activities could contribute to population diversity and a strengthened portfolio effect in this system.
Most research exploring factors contributing to population diversity in salmon population complexes has focused on factors that can be observed in freshwater, such as rearing habitat, migration timing, and the age structure of spawners (e.g., Hilborn et al. Citation2003; Greene et al. Citation2010). The portfolio effect literature has paid less attention to processes in the ocean, such as growth rates or spatial distributions, but differences in how stocks experience the ocean also have the potential to influence life history diversity among constituent populations through, for example, the effects of ocean environment on maturation rates or the effects of location and body size on exposure to harvest by fisheries.
Earlier work with Chinook Salmon suggests that aspects of their freshwater and ocean ecology can be correlated, leading to distinct life history syndromes referred to as “ocean-type” and “stream-type” fish (Healey Citation1991), designations which are commonly applied to more northerly Chinook Salmon populations. In general, ocean-type fish have short freshwater residence as juveniles and spawn shortly after returning to freshwater. Young ocean-type fish in the ocean are larger (due to earlier entry into the productive ocean environment) and initially grow faster than stream-type fish (Healey Citation1991). By contrast, stream-type fish have extended freshwater residence as juveniles and thus have a delayed ocean entry. Stream-type adults hold in freshwater for extended periods before spawning, and they are smaller and initially grow more slowly in the ocean than ocean-type fish (Healey Citation1991).
However, classification of all Chinook Salmon in the California Central Valley as ocean type or stream type is difficult. Fall-run Chinook Salmon seem to follow an ocean-type life history (minimal juvenile period in freshwater; spawning soon after their return to freshwater). Characterizing the remaining runs as ocean type or stream type is not straightforward. For example, Williams (Citation2006:24) considers the spring run a mix of mostly ocean-type and some stream-type fish, while Healey (Citation1991:319) only labels them as stream type. According to Fisher (Citation1994), the spring run may spend anywhere from 3 to 15 months in freshwater as juveniles, consistent with either classification, while peak spawning comes 3–4 months after peak migration, consistent with the stream-type classification. Healey (Citation1991:319) considers winter-run Chinook Salmon to exhibit a mix of ocean- and stream-type characteristics, returning immature and holding before spawning (stream type) while spending only a short juvenile period in freshwater (ocean type). Fisher (Citation1994) states that winter-run fish spend anywhere from 5 to 10 months in freshwater as juveniles, consistent with the stream-type classification. Conversely, the late-fall run, classified by Moyle (Citation2002:254) as primarily stream type, spends an extended juvenile period in freshwater (7–13 months; Fisher Citation1994) but mostly spawns shortly after migration (Fisher Citation1994), which is characteristic of ocean-type fish. Thus, California runs display a mix of ocean- and stream-type traits, suggesting (at least in some cases) weaker correlations between different parts of the life cycle and the need to consider how differences during the ocean phase of the life cycle influence diversity among and within runs.
Similar to other systems, there are well-documented differences in juvenile rearing and seasonal migration timing among the four Central Valley Chinook Salmon runs (Fisher Citation1994; Yoshiyama et al. Citation1998; Moyle Citation2002). By contrast, relatively little is known about how the runs differ in their ocean ecology (e.g., size at age or spatial distribution), and published information on spawner age structure and maturation rates is limited. Satterthwaite et al. (Citation2013) used information from fishery recoveries of tagged hatchery fish to infer that all Central Valley Chinook Salmon populations were rarely encountered in ocean fisheries north of Oregon and that late-fall and winter-run fish appeared particularly restricted to the south of Point Arena, California. Fisher (Citation1994) presents information on spawner ages in Central Valley Chinook Salmon populations; however, he does not include information on methodologies or data sources. Otherwise, knowledge about these details of Central Valley Chinook Salmon ecology seems limited to management documents and the personal knowledge of managers.
Objectives
Our first objective is to further the understanding of Central Valley Chinook Salmon life histories by (1) quantifying age-specific patterns of ocean size at date among and within runs; (2) quantifying patterns in spawner age structure; and (3) exploring how these patterns correlate with aspects of their juvenile life history (emigration timing and smolt size) as well as spatial distribution in the ocean. We then evaluate the consistency of patterns observed with various hypotheses for drivers of life history variation (see Hypotheses section below). Note that we use coded wire tag (CWT) data derived almost entirely from hatchery-produced fish (), so we must use information on fish released by hatcheries as a proxy for juvenile rearing ecology. Our analyses are necessarily of correlations rather than causation; nevertheless, they may help inform on the potential for variation in life history and ocean ecology within and among runs to contribute to a strengthened portfolio effect in Central Valley Chinook Salmon.
TABLE 1. Source hatcheries and fish facilities in California whose production and marking of Chinook Salmon contributed to this analysis. For the purposes of this report, we include Mokelumne River Hatchery among the Central Valley fall run but not the San Joaquin River basin fall run, since the Mokelumne River receives water and fish from the Sacramento River via the Delta Cross Channel. Start and end years refer to the earliest and latest brood years from which coded wire tags were recovered in the sport fishery and included in the size-at-age analysis (including harvest through 2010); these years do not necessarily coincide with the initiation or termination of hatchery production. In addition to the years listed, winter-run fish from Coleman National Fish Hatchery (NFH) were recovered from brood year 1978, and a small number of tags from natural-origin fish were recovered, including 60 Butte Creek spring-run tags in 1998–2004, 68 fall-run tags from the Yuba River in 1983–1987, 31 fall-run tags from the Feather River in 1997–2004, and 54 fall-run tags from the Mokelumne River in 1990–1999.
Hypotheses
We investigate several mechanisms (see list of hypotheses in ) that might drive the variation in life histories observed within and among runs. Because earlier entry into the ocean results in fish experiencing a longer period of rapid growth, we predict under the “juvenile head start” hypothesis that early ocean entrants will be larger at a particular time of year than later ocean entrants of the same age (in terms of calendar years since parent spawning) and that early ocean entrants will mature earlier (Hankin Citation1990), possibly because they more rapidly reach a threshold size for maturation (Mangel Citation1994). Under the juvenile head start hypothesis, we would expect that runs with more cumulative time spent in the ocean at a given age would display larger sizes at age in the ocean than runs with later ocean entry and would mature earlier as a result. Tattam et al. (Citation2015) reported that larger smolts within a cohort tended to mature at earlier ages, and we explored whether a similar pattern applied across runs under the “smolt size” hypothesis, which predicts that the fish that are larger at release would mature earlier. We also explored the “premature adult migration” hypothesis (sensu Quinn et al. Citation2016), specifically the salmon making the “best of a bad situation” explanation. Quinn et al. (Citation2016) suggest that populations constrained by access to the spawning grounds due to low flows or high temperatures may initiate their upstream migrations early to avoid those negative conditions, thus sacrificing opportunities for further growth in the ocean by returning earlier within the calendar year but with no selective pressure to return at younger ages.
TABLE 2. List of hypotheses and predictions relating timing of life history events, ocean size, and maturation schedules of the different seasonal runs of Central Valley Chinook Salmon (WR = winter run; SR = spring run; FR = fall run; LFR = late-fall run).
Based on consideration of the suitability of the freshwater environment for large adults, we hypothesized that fish with extended adult holding times (hence, the “adult holding time” hypothesis) in freshwater prior to spawning would face selection against the metabolic costs imposed by a large body size (balanced against other selective advantages of a larger body size). This would tend to select for return at younger ages, minimizing cumulative ocean mortality risk. Under the adult holding time hypothesis, we would expect younger spawners and possibly smaller size at age or size at date in the ocean for runs with longer holding periods in freshwater prior to spawning, whereas under the premature adult migration hypothesis, fish spawning further upstream would tend to return earlier within a year but would not be expected to display a difference in size at age/size at date or age at spawning.
METHODS
Study system
The four runs of Central Valley Chinook Salmon are named on the basis of their adult migration times. Differences among the runs in their freshwater ecology are detailed elsewhere (Vogel and Marine Citation1991; Fisher Citation1994; Yoshiyama et al. Citation1998; Williams Citation2006), and depicts the timing of key events in the life cycle of each run. Fall-run adults spawn in main-stem and lower tributary habitat shortly after their return migration (October–December), and juveniles emigrate during the following spring. Spring-run adults hold in upstream habitats for an extended period after their return, spawning in August–October, with juveniles emigrating the following spring, somewhat earlier than the fall run. Late-fall-run adults spawn in main-stem habitat shortly after returning from the ocean (January–March), with juveniles often rearing for a year or more before emigrating to the ocean. Winter-run adults historically spawned in high-elevation, spring-fed sites (currently, coolwater releases from Shasta Dam serve a similar purpose) after a short holding period (May–July); juveniles rear and emigrate the following fall through early winter. In the ocean, all stocks are subject to commercial and recreational fisheries that do not directly discriminate among them, although minimum size limits may reduce impacts on the winter run (O’Farrell et al. Citation2012). Stock-specific ocean harvest data based on tagged hatchery-origin fish (Weitkamp Citation2010; Satterthwaite et al. Citation2013) or genetically identified fish (Bellinger et al. Citation2015) indicate that Central Valley fall-run fish are most often contacted off California and southern Oregon and are very rarely contacted north of Cape Falcon, Oregon. Satterthwaite et al. (Citation2013) documented similarity in the spatial recovery patterns for hatchery-origin Central Valley fall and spring runs, while late-fall and winter runs were restricted more to the south, mostly south of Point Arena, California (39°N). In a follow-up paper, Satterthwaite et al. (Citation2015) documented similar patterns in the recovery of natural-origin spring-run and winter-run fish identified using genetic methods.
FIGURE 2. Diagram showing the timing of key events in the life cycle of each Central Valley Chinook Salmon run, along with the aging convention used here (BY = brood year; OA = ocean age; SA = spawner age; PPR = peak parental return; PPS = peak parental spawning; JE = juvenile emergence; numbers denote ages; E/RP = emigration/release peak). Fingerling releases (as are typical) of the spring run are denoted by “(f)”; “ye” indicates the potential emigration of spring-run yearlings. Asterisks represent the return of spawning adults (with the number of asterisks indicating age); “ee” denotes the potential return of unusually early late-fall emigrants as age-1 spawners. Shading indicates the starting month for the comparison of ocean size at age or size at date. Italics indicate ages that are rarely observed.
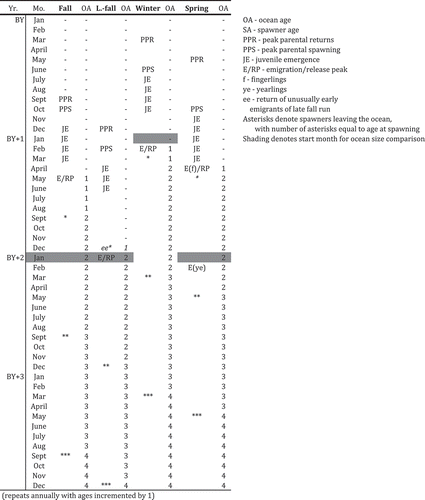
Standardized comparisons of fish body size across runs is made difficult by the different birth dates of fish and thus the potential to make comparisons at either a common calendar date or a common developmental age (which could depend on the chosen starting stage: e.g., fertilized egg versus emergent fry). The timing of events such as fertilization or emergence can be variable and are usually reported with low precision. In addition, we wanted to compare patterns in size at a common date to illustrate whether seasonal patterns in apparent growth (Harvey et al. Citation2014; see also the Supplement available in the online version of this article) were similar across runs and to compare runs at a common date to reflect their simultaneous relative exposure to fisheries or potential competitive interactions or availability as prey. We therefore compared body size based on months elapsed from January of the first year after the end of the run-specific emergence period reported by Fisher (Citation1994). This period starts 1 year after the brood year (the year of parent return) for the winter run and 2 years after the brood year for the remaining runs (see for details).
Fish were also assigned numeric ages based on fishery management conventions for Central Valley Chinook Salmon. Age at spawning is calculated as the calendar year of return minus the brood year (which we define as the year of parent return). Fall-run fish have an assumed birth date of September 1 (O’Farrell et al. Citation2010), spring-run fish have an assumed birth date of May 1 (Grover et al. Citation2004), and winter-run fish have an assumed birth date of March 1 (O’Farrell et al. Citation2012). All of these birth dates were assigned on the basis of the timing of spawner returns from the ocean (not spawning per se), so we assumed a December 1 birth date for the late-fall run based on their return timing as reported by Fisher (Citation1994). To facilitate comparisons with age-specific ocean harvest data (e.g., O’Farrell et al. Citation2012, Citation2013; Satterthwaite et al. Citation2013), ocean ages can also be calculated. For fish in the ocean during the calendar year prior to their birth date, ocean ages are calculated as the calendar year minus the brood year, and fish remaining in the ocean are 1 year older after their birth date, corresponding to their numeric age at their next spawning opportunity (birth dates are denoted in and ).
Data sources
Logistical challenges to observing fish in the ocean and in assigning ocean-recovered fish to their source stocks contribute to our relative lack of understanding of ocean ecology compared to freshwater ecology of these fish. Coded wire tag data (Johnson Citation1990; Lapi et al. Citation1990; Nandor et al. Citation2010) provide our largest current source of information on specific runs of Chinook Salmon (and Coho Salmon O. kisutch). The CWTs are 1-mm-long pieces of wire with an etched code inserted into the nasal cartilage of fish. These codes allow associating fish with individual release groups, thus facilitating the tracking of individual fish back to run of origin, and provide additional information on brood year (allowing unambiguous calculation of ages) and release-specific data (e.g., time of release, release location, and properties of fish at the time of release). California and Oregon state agencies attempt to sample at least 20% of all ocean harvest for CWTs, recording information on the port of landing, size, and release group determined for each coded-wire-tagged fish that was sampled. The CWT program provides many years’ data on the ocean ecology of these stocks, with recoveries dating back as far as 1978 for Sacramento River fall-run Chinook Salmon. In Central Valley stocks, CWT marking is almost exclusively done with hatchery-produced fish. Data from CWTs are used extensively in the management of California salmon fisheries (Goldwasser et al. Citation2001; Mohr Citation2006; O’Farrell et al. Citation2012), and ocean recoveries of fish with CWTs provide information on run-specific size at age for all four runs.
Our analyses were all based on records of coded-wire-tagged fish from databases maintained by the Regional Mark Processing Center (www.rmpc.org). To estimate the size distribution of ocean fish, we queried “Standard Reporting, All Recoveries” for all recoveries of Chinook Salmon sourced from the Central Valley of California and captured in the ocean recreational fisheries off the coast of California or Oregon. To determine release characteristics of fish recovered from the different runs, we queried the “All Releases” database for information on all releases from the Central Valley, sorted by “tag code or release ID” to facilitate matching up with the tag codes of individual fish harvested in the ocean. We obtained data on minimum legal size from the California Department of Fish and Wildlife (Allen Grover, personal communication). Almost all CWT recoveries are from hatchery stocks, with sources as described in . Hatcheries varied through time in which runs they produced and how extensively they tagged fish; thus, the duration of available time series varies across populations.
Empirical model of size at date
We fit sizes for each month independently to allow for complex shapes of the “growth” curve due to multiple effects on mean size that could vary both seasonally and with fish size, including growth, natural mortality, harvest, and maturation, as illustrated by our conceptual model (see Supplement). Our empirical estimates of size at age were made separately for each run and for each month on the basis of coded-wire-tagged fish harvested in the recreational fishery off the coast of California and Oregon. Because of minimum size limits, length data from harvested fish reflect a truncated sample of ocean size distribution. We restricted our analysis to the recreational fishery because it had smaller minimum size limits, and thus the analysis was less prone to computational issues that result when fitting a truncated normal distribution to data that do not span both sides of the fitted mode.
Because we estimated size for each month separately, modeling years as fixed effects would generate a very cumbersome model structure, especially if allowing for interaction terms. Furthermore, the method for fitting truncated distributions performs poorly if truncation restricts sampling to only one side of the mode (Goldwasser et al. Citation2001), so for some years in which all fish came from fisheries with high size limits, the estimates are not reliable. In addition, classic techniques of model selection, such as Akaike’s information criterion scores (Burnham and Anderson Citation2002), might lead to selection of an overly complicated model, since not all recovered fish are truly independent samples (e.g., many came from the same release group, and there is great potential for temporal and spatial pseudoreplication), and model selection techniques often break down in the face of such data because they allow for huge changes in likelihoods (Browne Citation2000). However, based on previous knowledge of the system (Wells et al. Citation2006), we were aware that growth was anomalous in some years, typically correlated with El Niño conditions. We therefore fit monthly sizes using a Bayesian hierarchical model described fully in Satterthwaite et al. (Citation2012). In brief, we assumed that mean length μ of fish from a particular run r of age a harvested in month m of year y during which the environment is in state E could be modeled as
where x is interannual mean length in years below the environmental threshold; I(·) is an indicator function, taking the value of 1 if · is true and 0 otherwise; g1 is a threshold environmental state above which mean length is increased by g2; and η is a random effect of year (all variables used are defined in ). We used the Northern Oscillation Index (NOI) for g1, with smaller fish in years with low NOI (Satterthwaite et al. Citation2012). We assume that deviations in mean length for individual years from the long-term mean are normally distributed,
TABLE 3. List of variables used in this paper.
We also assume that the SD in fish length (σ) varies across years, with
We chose this formulation rather than a gamma for ease of interpretation; in practice, we determined that had a gamma been fit, its shape would have been similar to a normal distribution (Satterthwaite et al. Citation2012), with the fitted normals having negligible support below zero.
The likelihood associated with a particular set of observed lengths li for fish of run r and age a taken from fisheries with size limits in month m of year y is given by
where Φ(·) is the cumulative probability distribution function for the normal density function φ(·).
For the size-at-age model, we used all ocean tag recovery data available at the time of analysis. Overall, size-at-age/size-at-date estimates were informed by 31,467 Central Valley fall-run tag recoveries (24,521 Sacramento River and 3,451 San Joaquin River, with the balance from the Mokelumne River), 5,655 late-fall-run recoveries, 408 winter-run recoveries, and 4,120 spring-run recoveries, based on the releases reported in .
Spawner information
We constructed an index of spawner age distributions based on recoveries of coded-wire-tagged spawners at their source hatchery or in spawner or carcass surveys of the river on which the source hatchery was located. The spawner age distribution for returns from a given cohort reflects both life history variation (how many fish ultimately would have spawned at different ages, if they escaped harvest) and variations in harvest intensity (fewer old spawners will be recovered from cohorts where young ages were subject to high harvest mortality) or natural mortality. The age structure for a given return year will also be influenced by variation in brood year strength. Thus, we calculated the age distribution of spawners returning for each brood year, combining across return years. While this approach does not avoid confounding effects of varying mortality and harvest, with the result that temporal changes in age structure cannot be attributed to changing maturation rates per se, it does allow comparison across runs within a brood year. We excluded brood years with less than 25 spawner tag recoveries and excluded spawner age information from brood years after 2004 to avoid the effects of anomalous ocean conditions and fishery restrictions associated with the “collapse” that closed fisheries in 2008–2009 and severely restricted fisheries in 2010 (Carlson and Satterthwaite Citation2011); we also excluded postcollapse brood years because data on returning spawners from all possible ages were not yet available for the most recent brood years. Similarly, there were years in which some hatcheries did not mark fish or did not report results from the sampling of returning spawners, thus compromising the age structure information on multiple brood years. Consequently, information is available from different brood years for different runs. To facilitate direct comparisons among runs, we calculated aggregate age structure for brood years 1998–2004, for which data were available for all runs. We also calculated the Shannon diversity index as –1 times the sum across all age-classes of the proportion of fish in that age-class times the natural logarithm of that proportion (Shannon Citation1948) to reflect the diversity in cumulative age structure over this time period.
Consultation with local experts and our own colleagues’ experiences in attempting cohort reconstructions (similar to O’Farrell et al. Citation2012) based on Central Valley Chinook Salmon escapement data highlighted significant problems with historical sampling practices and/or reporting (see also Baker and Morhardt Citation2001), so we attempted to confirm the patterns we found in coarse-scale CWT recoveries by referencing against site-specific studies conducted by local experts selecting the best available data sources.
Release information
For each coded-wire-tagged spawner recovered at its source hatchery or from adjacent spawning surveys, we determined its release date based on its tag code or release identification number and the Regional Mark Information System tagged releases data set. When data were available, we determined the mean weight at release as well. We calculated the mean Julian day of release for fish in each run based on an average of the release date for each recovered fish (thus, different release groups are weighted in proportion to how often they survived to spawn; see Huber and Carlson Citation2015 for description of release practices unweighted by return rates). We calculated Julian day such that day 1 represented release on January 1 of the year after the brood year. Therefore, some fish that were released during their brood year were assigned negative release dates. To evaluate the juvenile head start hypothesis, we calculated the time elapsed between mean release date and two reference points later in the life cycle: (1) the common size-at-date reference point of January 1 of the year after emergence and (2) the nominal time of peak spawner return corresponding to age-2 spawners.
We did not attempt to adjust release times to approximate ocean entry time because of the uncertainties and variability in transit time due to the diversity of release locations and release characteristics (Huber and Carlson Citation2015) and limited published information on transit time. However, this is unlikely to have a major impact on our results. Michel et al. (Citation2013) documented travel times of approximately 3 weeks for late-fall releases from the upstream Coleman National Fish Hatchery, and studies in process for other stocks indicate that transit times for the fall and spring runs may be approximately 1 week faster (C. Michel and A. Ammann, National Marine Fisheries Service, personal communication); some fish from these runs are released downstream, accelerating their ocean entry even further. Acoustically tagged winter-run fish showed transit times similar to those of the late-fall run, although winter-run fish may display holding behavior that delays their ocean arrival (C. Michel and A. Ammann, personal communication). This is unlikely to confound our results since we already characterize the winter run as a late-arriving stock, and the longer transit time of the late-fall run versus the fall run or spring run reinforces the difference in mean release time.
Hypothesis testing
As this is a primarily descriptive paper, we focus on estimating means and credible intervals for size at age/size at date rather than statistical hypothesis testing, and we reference against other literature on spawner ages and maturation rates to confirm patterns detected in spawner age structure. When comparing the fall run between the Sacramento and San Joaquin River basins, we excluded the Mokelumne River—although it is a tributary to the San Joaquin River, it receives Sacramento River water (and potentially fish) via the Delta Cross Channel (Carlson and Satterthwaite Citation2011). We first document patterns in size at age/size at date and age structure to increase general understanding of this system, and we then evaluate the extent to which observed patterns are consistent with our hypotheses.
RESULTS
Patterns of Size at Date
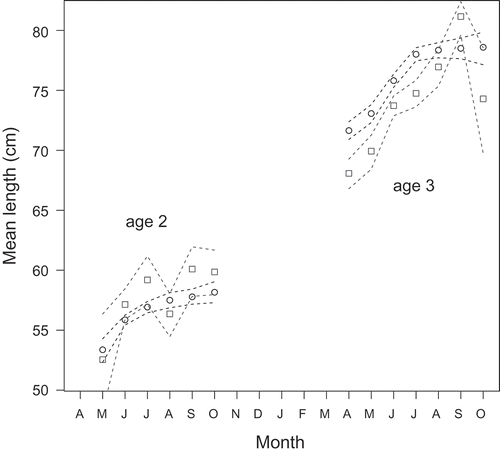
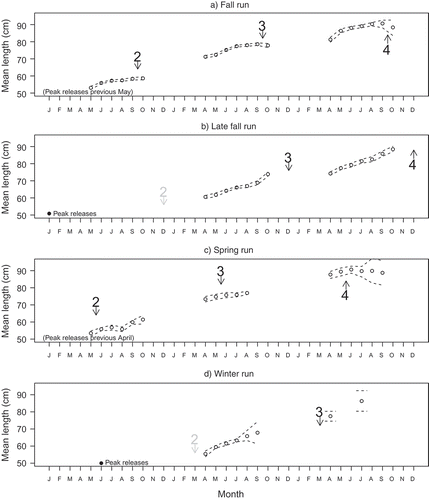
We compared runs on the basis of the posterior median and credible intervals calculated for mean size in typical (i.e., NOI not anomalously low) years—that is, the posterior estimate of x + g2 from equation (1). Fall-run and spring-run Chinook Salmon were consistently larger than late-fall-run and winter-run fish () of the same age at the same time of year. For all runs, mean size at date increased nonlinearly through time but did not appear to follow a von Bertalanffy growth curve. Different runs had different periods with the fastest increase in mean size. For all runs (where data were available during the time spawners return), the increase in mean size slowed, stopped, or even switched to a decrease during or after the time period when spawners returned to freshwater, especially for age-3 fish (; see also Supplement). This pattern can be directly observed in the slowing increase in mean size of the fall run in September–October and can be inferred for the remaining runs by comparison with patterns in other runs at common dates. For the late-fall run, there was very little change in mean size of age 4 in April compared to age 3 in October, indicating essentially no net change in length over the unsampled winter gap (when spawners are returning), despite the potential for size to increase during that period as evidenced by the other runs. For the spring run, mean size increased minimally in May–July, but a clear increase can be observed for age-2 fish during April–October and substantial increases in mean size must have occurred during the later fall and winter gaps. Age-4 winter-run fish were smaller than would be expected if mean size continued to increase over the winter at the same rate observed for age-3 fish in April–September.
FIGURE 3. Monthly mean size (TL) at age for coded-wire-tagged Chinook Salmon recovered in the ocean for each Central Valley run. Circles are posterior medians; dotted lines are 68% credible intervals. In all cases, the x-axis starts with January of the calendar year following the end of the emergence period reported by Fisher (Citation1994). Arrows denote peak migration periods (adult return to freshwater) as identified by Fisher (Citation1994), labeled with the corresponding age at the subsequent spawning event, with arrows and numbers in gray for spawner ages that are rarely observed. Note that the spawning event may be separated from migration by an extended freshwater holding period and that migration timing can spread out over several months. For example, Vogel and Marine (Citation1991) report spring-run migration extending into early October. The timing of peak hatchery releases (see text) is denoted near the x-axis.
Within the fall run, fish sourced from the San Joaquin River basin were generally larger at age 2 than Sacramento River basin fish (), although small sample sizes led to overlapping credible intervals; for age-3 fish, the relationship was reversed, with Sacramento River basin-sourced fish generally larger and credible intervals nonoverlapping for most of the year.
Spawner Ages
The longest time series of spawner ages was available for the Sacramento River fall run (), which displayed annual variability but a strong predominance of age-3 fish in most years. While age 3 was the predominant age of returning spawners for all runs (), age-2 spawners were consistently encountered in the fall run, especially among Mokelumne and San Joaquin River-sourced fish (). However, in some years, a very large proportion of late-fall-run spawners were age 2 (), and overall the late-fall run displayed the greatest variability in spawner age structure and the highest average contribution of age-4 fish. Winter-run spawners were dominated by age 3 and had the simplest age structure (). Sacramento River spring-run spawners from Feather River Hatchery were predominantly age 3, with consistent contributions from age-4 fish and periodic contributions of age-2 fish (). Cumulative age structure for the 1998–2004 brood years showed the highest proportion of age-2 fish in San Joaquin River fall run, the highest proportion of age-4 and older fish in the late-fall run, the lowest proportion of age-4 and older in the winter run, and a spring-run age composition intermediate between those of the Sacramento River and San Joaquin River fall run (). The winter run had the lowest diversity in age structure (Shannon index = 0.37; ), followed by the spring run (0.77), with fall and late-fall runs being similarly diverse (0.87–0.94).
TABLE 4. Cumulative age structure (%) of tagged Chinook Salmon spawners returning to Central Valley hatcheries for each run (and for each basin within the fall run) for brood years 1998–2004 combined. The final column reports the sample size N (number of tags recovered). The diversity index is calculated as –1 times the sum over all age-classes of the proportion of spawners in that age-class times the natural logarithm of that proportion.
FIGURE 5. Age structure of returning spawners, by brood year, for Central Valley Chinook Salmon of the different run timings (and different basins for fall-run fish): (a) Sacramento River basin fall run; (b) San Joaquin River basin fall run; (c) late-fall run; (d) winter run; and (e) spring run. For each run, only brood years for which tagging, sampling, and reporting allowed for the possibility of recovering age-2 through age-4 fish are presented; this results in different temporal representation of the various runs. The dashed vertical line indicates the start of the 1998–2004 brood year period common to all stocks.
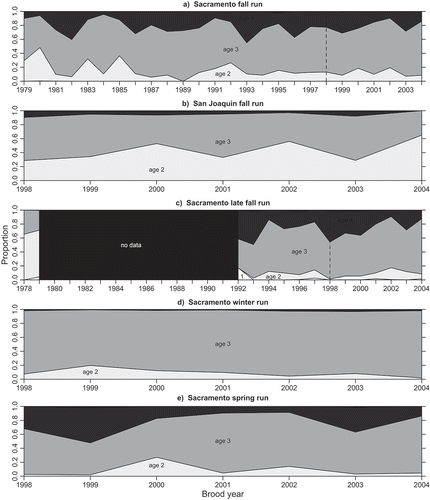
Literature results were highly consistent with our findings for the fall, late-fall, and winter runs. Neillands (Citation1995; as cited in Myers et al. Citation1998) also reported larger contributions of age-2 spawners in the San Joaquin River fall run as compared to other stocks. Maturation rate estimates reported from cohort reconstructions were consistent with younger spawners in the winter run and older spawners in the late-fall run as compared to the Sacramento River fall run as a baseline. Age-3 maturation rate estimates for Sacramento River fall-run fish range from 30% to 78% (Palmer-Zwahlen et al. Citation2006), while Cramer and Demko (Citation1996) estimated age-3 female maturation rates below 25% in 10 of 13 years for the late-fall run (mean = 19%; maximum = 54%) and O’Farrell et al. (Citation2012) estimated age-3 maturation rates of 85–100% with a mean of 95% for the winter run.
Comparisons with spring-run age composition were less straightforward. Reports from Butte Creek (Ward et al. Citation2003, Citation2004; McReynolds et al. Citation2005, Citation2006, Citation2007; Garman and McReynolds Citation2008, Citation2009) indicated a predominance of age-3 spawners in 2001, 2002, 2004, 2005, and 2007 but a predominance of age-4 spawners in 2003 and 2006. This inconsistency likely in part reflects variation in cohort strength and ocean fishing mortality, whereas cohort reconstructions can extract maturation schedules after accounting for this variation (but must assume constant adult natural mortality and must be interpreted with caution when based on small sample sizes). With these caveats in mind, Grover et al. (Citation2004) reported age-3 maturation rates of 40% and 28% for natural-origin Butte Creek spring-run fish from brood years 1998 and 1999, respectively. Cramer and Demko (Citation1996) estimated age-3 female maturation rates of 15–30% for subyearling releases from Feather River Hatchery (compared to 63–72% for yearling releases), and Palmer-Zwahlen et al. (Citation2006) estimated Feather River Hatchery spring run age-3 maturation rates of 39% for brood year 1998 and 28% for brood year 1999, which were lower than maturation rates for fall-run fish of the same brood years.
Release Practices
Recovered late-fall-run and winter-run Chinook Salmon were released near January 1 on average (; ), with the peak release of late-fall-run fish coming two calendar years after the brood year. Spring and fall runs were released later in the calendar year, during late April–May, with the spring run released 14 d earlier on average than the fall run (; ). Sacramento River basin-sourced fall-run individuals were released nearly the same time on average as the spring run, while the San Joaquin River fall-run fish were released 20 d later on average. Late-fall-run fish were the largest and most developed at release, with the spring run and San Joaquin River fall run somewhat larger and more developed than the Sacramento River fall run ().
TABLE 5. Average (arithmetic mean) and SD of characteristics of hatchery releases for the various runs of Central Valley Chinook Salmon. Means were calculated by weighting the characteristics of different release groups by the number of fish from a release group recovered in the ocean fishery; therefore, these statistics do not summarize releases per se but instead summarize the characteristics of releases that contributed to the ocean harvest of coded-wire-tagged fish. The last two columns indicate the time from mean release until either the nominal peak of age-2 spawner returns (leaving the ocean; see ) or January 1 of the year after emergence (i.e., the reference time for ocean size comparisons). Sample sizes do not match total sample sizes for the size-at-age analysis because release characteristics were not reported for some releases.
The interaction between release time and assumed birth date has implications for how long fish of a given numeric ocean age have been in the ocean at any given time. Note that because the late-fall run spawns in January–March of the calendar year after the brood year (which is designated based on spawners returning in December), and then juveniles rear into the calendar year after that, late-fall-run fish are near or even past the point at which they would be assigned ocean age 2 when they enter the ocean (). Similarly, winter-run fish have an assumed March 1 birthday (O’Farrell et al. Citation2012), so the average winter-run release occurs two months before reaching ocean age 2 (). Fall-run fish have an assumed September 1 birthday (O’Farrell et al. Citation2010), so their typical release date is several months in advance of their first birthday. Thus, for a fixed “age” (where by convention, ocean age corresponds to the age a fish will be at its next spawning opportunity) and a fixed month, Sacramento River fall-run Chinook Salmon will have spent the longest time in the ocean, followed closely by the spring run and the San Joaquin River fall run, with the late-fall and winter runs having spent an average of 8–9 months less in the ocean. For example, for age-3 fish harvested in April, a winter-run fish will have spent approximately 14 months in the ocean, and a late-fall-run fish will have typically spent approximately 15 months in the ocean. In contrast, a Sacramento River fall-run fish will have typically spent approximately 23 months in the ocean, with a San Joaquin River fall-run or a spring-run fish having spent a few weeks less ().
Support for Each Hypothesis
Consistent with the juvenile head start hypothesis, runs with later release dates had smaller sizes at a common age or date (). Earlier ocean entry (relative to the time at which age-2 spawners returned for each run) also generally predicted a younger spawning age structure (i.e., a smaller proportion of age 3 or older; ). However, the proportion of age-2 spawners was much lower in the San Joaquin River fall run than in the Sacramento River fall run (), despite slightly later releases of San Joaquin River fish. Furthermore, although the winter run had very few age-2 spawners, it also had very few age-4 or older spawners, unlike the late-fall run ().
FIGURE 6. Graphical tests of hypotheses explaining variation among Chinook Salmon runs of the Central Valley (FR = fall run; LFR = late-fall run; SR = spring run; WR = winter run; Sac = Sacramento River basin; SJ = San Joaquin River basin). The juvenile head start hypothesis predicts that earlier entry to the ocean leads to (a) larger size at age/size at date and (b) earlier maturation of spawners. (c) The smolt size hypothesis predicts that larger smolts lead to earlier maturity. The premature adult migration hypothesis predicts (d) earlier return of stocks spawning further upstream but (e) no effect of spawning location on spawner age. (f) The adult holding time hypothesis predicts few large, old fish among stocks with extended holding time prior to spawning.
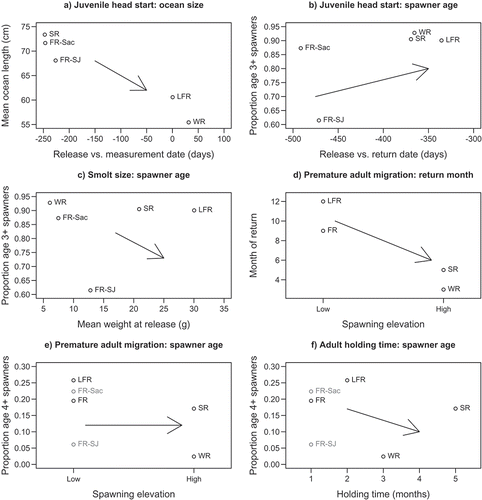
Across run timings, stocks with larger smolts did not mature earlier (), contrary to the smolt size hypothesis. However, larger smolt size might explain the greater frequency of age-2 spawners in the San Joaquin River compared to the Sacramento River fall run.
As predicted by the premature adult migration hypothesis, stocks spawning upstream returned earlier in the calendar year (). However, this hypothesis would not predict any relationship between spawning location and the frequency of age-4 or older spawners, but such a cline was evident among run timings in the Sacramento River basin ().
Although age-4 spawners were relatively rare among stocks with the longest adult holding times in advance of spawning (), there was no monotonic relationship observed between holding time and spawner age.
DISCUSSION
The four run timings of Central Valley Chinook Salmon show considerable diversity in their size at age/size at date, month of return, and spawner age distribution, along with previously documented differences in their ocean distribution (Satterthwaite et al. Citation2013). Our findings offer both equivocal support and some challenges to each of the four hypotheses for explaining the life history variation observed among and within the four seasonal runs (juvenile head start, smolt size, premature adult migration, and adult holding time). The juvenile head start hypothesis successfully explained variation across the runs in their ocean size at age/size at date, and the premature adult migration hypothesis successfully explained variation across runs in their month of return. However, no hypothesis could fully explain variation among runs in their spawner age structure, although the smolt size and holding time hypotheses might explain deviations from expectations under the head start hypothesis.
Further research is needed to identify drivers of variation in age structure, and the observed maturation rates likely reflect a complex interplay of multiple competing pressures. The juvenile head start hypothesis could explain much of the variation in age structure within Sacramento River basin runs, and the difference in release time for the Sacramento River versus San Joaquin River fall run is small and so should have only a weak effect on age structure compared to their difference in smolt size at release. Theory suggests that size-dependent maturation (Mangel Citation1994) could make early maturation more likely for stocks with a head start on ocean growth, yielding a higher probability of exceeding some threshold size for early maturation. Consistent with this prediction, Hankin (Citation1990) found that early release tends to promote earlier maturation, while Tattam et al. (Citation2015) found earlier maturation by fish that were larger as smolts. Although the size-dependent maturation hypothesis predicts that the winter run should delay maturation past age 3 due to their small size in the ocean, the energetic costs of holding upstream (i.e., adult holding time hypothesis) in the limited spring-fed habitats historically utilized by the winter run might select against the larger body size associated with age-4 spawners.
Beyond the explored hypotheses, other factors surely also contribute to the diversity observed. Variation among runs in the size at date and maturation schedules in the ocean suggest diversity in ocean foraging strategies and survival patterns among runs—diversity that we do not yet fully understand (see also Hilborn et al. Citation2003). For example, Satterthwaite et al. (Citation2013) noted that both the winter run and the late-fall run tend to be restricted to waters off central and southern California, whereas the fall and spring runs extend well into Oregon waters. Thus, they likely encounter substantially different prey, and this could be an additional mechanism behind differences in their size and maturation schedules. These differences in size and spatial distributions may have implications for competitive interactions among runs or the degree to which each run contributes to the marine food chain.
We should note that our results are driven by data on hatchery-origin fish. There is considerable concern in the salmon management arena about the suitability of tagged hatchery-origin fish as a proxy for natural-origin stocks (e.g., Hankin et al. Citation2005: finding 5). There have been few studies comparing natural stocks to hatchery proxies, but they have generally found similar spatial distributions of natural-origin stocks and their hatchery indicators (e.g., Weitkamp and Neely Citation2002; Weitkamp Citation2010; Satterthwaite et al. Citation2014b, Citation2015); however, studies have raised concern about the equivalency of maturation rates (Sharma and Quinn Citation2012). We did have limited data on natural-origin spring run from Butte Creek, and for the 3 months in which tag recoveries were adequate to estimate ocean sizes for these fish, they were almost identical to those of the Feather River spring run in the corresponding years (analysis not shown). It is also important to realize that current life histories reflect the evolutionary history of the stocks, including response to the selective pressures imposed by past habitat conditions that no longer exist, so it may be unrealistic to expect any hypothesis to fully predict the current life histories of the stocks based on their current distributions, ecology, and management.
Management Implications of Life History Diversity
Because the population sizes of the different Chinook Salmon runs in the Central Valley differ greatly, with the fall-run population vastly exceeding the sizes of the other runs, the potential contribution to the portfolio effect by a given run depends on the size of that run relative to the other runs (i.e., evenness in abundance). For example, the winter-run population is unlikely to ever match or exceed the size of the fall-run population, and while the spring run was once of comparable abundance to the fall run (Yoshiyama et al. Citation1998), this is unlikely to be the case again in the foreseeable future given the inaccessibility of most upstream habitat. However, management practices that maintain and enhance life history diversity within the smaller runs can still add to the life history diversity of the complex and buffer the individual runs, as evidenced by the differences between Sacramento River and San Joaquin River fall run demonstrated here and the reduced correlation across basins documented by Carlson and Satterthwaite (Citation2011).
As past studies point out, age structure is an important contributor to portfolio effects via risk spreading, both within and among stocks. Buffering arises both from the existence of age structure (Schindler et al. Citation2010) and differences in age structure across stocks (Greene et al. Citation2010). An important next step in such analyses is to identify the drivers of this variation. Our results suggest a complex interplay of factors in driving age structure, but emigration timing likely plays a major role. Satterthwaite et al. (Citation2014a) demonstrated that a greater diversity of release timing could enhance the stability of Central Valley Chinook Salmon by reducing variation in juvenile survival by integrating over the unpredictable timing of favorable ocean phenology, and this study suggests a further role of diverse emigration timing in fostering stability through the effects of release timing on age structure. Thus, an understanding of the factors that contribute to variation in size at age—and ultimately age structure—is critically important from a conservation perspective. Within Central Valley Chinook Salmon, for example, the run with the least diverse spawner age structure (i.e., the winter run) is listed as endangered under the U.S. Endangered Species Act and the second least diverse run (the spring run) is listed as threatened, whereas the other two runs are more diverse and are not federally listed.
An understanding of how and when runs vary in their ocean sizes may also inform fishing regulations for the region, which rely on a mix of time-, area-, and sector-specific minimum size limits that are often targeted to reduce impacts on specific stocks (O’Farrell et al. Citation2012; McHugh et al. Citation2015). The small size at age and early maturation of winter-run fish support the current use of increased minimum size limits to reduce fishery impacts on the endangered winter run (O’Farrell and Satterthwaite Citation2015), but the larger size of spring-run fish along with their somewhat delayed maturation (at least as implied by cohort reconstructions and the greater frequency of recovering older fish tags from the ocean) suggests that minimum size limits would be less effective in protecting the threatened spring run. Additionally, seasonal variation in the rate at which size at age increases (or not) throughout the year could confound predictions of the monthly proportion of legal-sized Chinook Salmon based on use of a single smooth growth curve (e.g., McHugh et al. Citation2015). Variation in within-year return time can also affect the relative vulnerability of stocks to terminal and freshwater fisheries (Hilborn et al. Citation2003).
Conclusions
Our results contribute to an enhanced understanding of Central Valley Chinook Salmon by quantifying patterns in ocean size at age, timing of return to freshwater, and spawner age distribution and by exploring how these patterns correlate with aspects of their juvenile life history. Differences in maturation schedules point to differences in ocean ecology (foraging and survival patterns) that we do not yet fully understand (see also Hilborn et al. Citation2003). Overall, our results emphasize that how stocks experience the ocean influences life history diversity within and among runs in ways that affect their vulnerability to the fishery and contribute to portfolio performance.
T2016-0152_1293562_REV_Supplement.pdf
Download PDF (258 KB)ACKNOWLEDGMENTS
We thank Rachel Johnson, Marc Mangel, Michael Mohr, Michael O’Farrell, and Brian Wells for helpful discussions and feedback on multiple aspects of this work. The manuscript was greatly improved by comments from the editors and two anonymous reviewers. This research was partially supported by the California Department of Fish and Game Ecosystem Restoration Program (Grant Agreement E1183014 to S.M.C. and W.H.S.) and by the U.S. Bureau of Reclamation (Interagency Agreement R11PG20213 supporting A.C.).
REFERENCES
- Baker, P. F., and J. E. Morhardt. 2001. Survival of Chinook Salmon smolts in the Sacramento–San Joaquin Delta and Pacific Ocean. U.S. National Marine Fisheries Service Fishery Bulletin 179:163–182.
- Bellinger, M. R., M. A. Banks, S. J. Bates, E. D. Crandall, J. C. Garza, G. Sylvia, and P. W. Lawson. 2015. Geo-referenced, abundance calibrated ocean distribution of Chinook Salmon (Oncorhynchus tshawytscha) stocks across the West Coast of North America. PLOS (Public Library of Science) ONE [online serial] 10(7):e0131276.
- Browne, M. W. 2000. Cross-validation methods. Journal of Mathematical Psychology 44:108–132.
- Burnham, K. P., and D. R. Anderson. 2002. Model selection and multimodel inference: a practical information-theoretic approach, 2nd edition. Springer-Verlag, New York.
- Carlson, S. M., and W. H. Satterthwaite. 2011. Weakened portfolio effect in a collapsed salmon population complex. Canadian Journal of Fisheries and Aquatic Sciences 68:1579–1589.
- Cramer, S. P., and D. B. Demko. 1996. The status of late-fall and spring Chinook Salmon in the Sacramento River basin regarding the Endangered Species Act. Special Report submitted to National Marine Fisheries Service on behalf of the Association of California Water Agencies and California Urban Water Agencies, Gresham, Oregon.
- Fisher, A. C., W. M. Hanemann, and A. G. Keeler. 1991. Integrating fishery and water resource management: a biological model of a California salmon fishery. Journal of Environmental Economics and Management 20:234–261.
- Fisher, F. W. 1994. Past and present status of Central Valley Chinook Salmon. Conservation Biology 8:870–873.
- Fry, D. H. 1961. King Salmon spawning stocks of the California Central Valley, 1940–1959. California Fish and Game 47:55–71.
- Garman, C. E., and T. R. McReynolds. 2008. Butte and Big Chico creeks spring-run Chinook Salmon, Oncorhynchus tshawytscha life history investigation 2006-2007. California Department of Fish and Game, Inland Fisheries Administrative Report 2008-1, Sacramento.
- Garman, C. E., and T. R. McReynolds. 2009. Butte and Big Chico creeks spring-run Chinook Salmon, Oncorhynchus tshawytscha life history investigation 2007-2008. California Department of Fish and Game, Inland Fisheries Administrative Report 2009-1, Sacramento.
- Goldwasser, L., M. S. Mohr, A. M. Grover, and M. L. Palmer-Zwahlen. 2001. The supporting databases and biological analyses for the revision of the Klamath ocean harvest model. National Marine Fisheries Service, Santa Cruz, California.
- Greene, C. M., J. E. Hall, K. R. Guilbault, and T. P. Quinn. 2010. Improved viability of populations with diverse life-history portfolios. Biology Letters 6:382–386.
- Grover, A., A. Low, P. Ward, J. Smith, M. Mohr, D. Viele, and C. Tracy. 2004. Recommendations for developing fishery management plan conservation objectives for Sacramento winter Chinook and Sacramento River spring Chinook. Interagency Workgroup Progress Report to the Pacific Fishery Management Council, Portland, Oregon.
- Hankin, D. G. 1990. Effects of month of release of hatchery-reared Chinook Salmon on size at age, maturation schedule, and fishery contribution. Oregon Department of Fish and Wildlife, Fish Division, Information Report 90-4, Portland.
- Hankin, D. G., J. H. Clark, R. B. Deriso, J. C. Garza, G. S. Morishima, B. E. Riddell, C. Schwarz, and J. B. Scott. 2005. Report of the expert panel on the future of the coded wire tag recovery program for Pacific salmon. Report to the Pacific Salmon Commission, Vancouver.
- Harvey, B. N., D. P. Jacobson, and M. A. Banks. 2014. Quantifying the uncertainty of a juvenile Chinook Salmon race identification method for a mixed-race stock. North American Journal of Fisheries Management 34:1177–1186.
- Healey, M. C. 1991. Life history of Chinook Salmon Oncorhynchus tshawytscha. Pages 311–391 in C. Groot and L. Margolis, editors. Pacific salmon life histories. University of British Columbia Press, Vancouver.
- Hilborn, R., T. P. Quinn, D. E. Schindler, and D. E. Rogers. 2003. Biocomplexity and fisheries sustainability. Proceedings of the National Academy of Sciences of the USA 100:6564–6568.
- Huber, E. R., and S. M. Carlson. 2015. Temporal trends in hatchery releases of fall-run Chinook Salmon in California’s Central Valley. San Francisco Estuary and Watershed Science [online serial] 13(2).
- Johnson, J. K. 1990. Regional overview of coded wire tagging of anadromous salmon and steelhead in northwest America. Pages 782–816 in N. C. Parker, A. E. Giorgi, R. C. Heidinger, D. B. Jester Jr., E. D. Prince, and G. A. Winans, editors. Fish-marking techniques. American Fisheries Society, Symposium 7, Bethesda, Maryland.
- Lapi, L., M. Hamer, and B. Johnson. 1990. Data organization and coding for a coastwide mark-recovery data system. Pages 720–724 in N. C. Parker, A. E. Giorgi, R. C. Heidinger, D. B. Jester Jr., E. D. Prince, and G. A. Winans, editors. Fish-marking techniques. American Fisheries Society, Symposium 7, Bethesda, Maryland.
- Lindley, S. T., R. S. Schick, E. Mora, P. B. Adams, J. J. Anderson, S. Greene, C. Hanson, B. P. May, D. R. McEwan, R. B. MacFarlane, C. Swanson, and J. G. Williams. 2007. Framework for assessing viability of threatened and endangered Chinook Salmon and steelhead in the Sacramento–San Joaquin basin. San Francisco Estuary and Watershed Science [online serial] 5:art 4.
- Mangel, M. 1994. Climate-change and salmonid life-history variation. Deep-Sea Research Part II Topical Studies in Oceanography 41:75–106.
- McHugh, P., G. Johnson, and J. Schaffler. 2015. Chinook FRAM base period documentation: growth functions. Report to Pacific Fishery Management Council. Available: www.pcouncil.org/wp-content/uploads/2015/10/D2_Att2_FRAM_Growth_Meth_Nov2015BB.pdf. ( March 2017).
- McReynolds, T. R., C. E. Garman, P. D. Ward, and S. L. Plemons. 2006. Butte and Big Chico creeks spring-run Chinook Salmon, Oncorhynchus tshawytscha life history investigation 2004–2005. California Department of Fish and Game, Inland Fisheries Administrative Report 2006-4, Sacramento.
- McReynolds, T. R., C. E. Garman, P. D. Ward, and S. L. Plemons. 2007. Butte and Big Chico creeks spring-run Chinook Salmon, Oncorhynchus tshawytscha life history investigation 2005–2006. California Department of Fish and Game, Inland Fisheries Administrative Report 2007-2, Sacramento.
- McReynolds, T. R., C. E. Garman, P. D. Ward, and M. C. Schommer. 2005. Butte and Big Chico creeks spring-run Chinook Salmon, Oncorhynchus tshawytscha life history investigation 2003–2004. California Department of Fish and Game, Inland Fisheries Administrative Report 2005-1, Sacramento.
- Michel, C. J., A. J. Amman, E. D. Chapman, P. T. Sandstron, H. E. Fish, M. J. Thomas, G. P. Singer, S. T. Lindley, A. P. Klimley, and R. B. MacFalane. 2013. The effects of environmental factors on the migratory movement patterns of Sacramento River yearling late-fall run Chinook Salmon (Oncorhynchus tshawytscha). Environmental Biology of Fishes 96:257–271.
- Mohr, M. S. 2006. Klamath River fall Chinook assessment: overview. National Marine Fisheries Service, Santa Cruz, California.
- Moore, J. W., M. McClure, L. A. Rogers, and D. E. Schindler. 2010. Synchronization and portfolio performance of threatened salmon. Conservation Letters 3:340–348.
- Moyle, P. B. 2002. Inland fishes of California. University of California Press, Berkeley.
- Myers, J. M., R. G. Kope, G. J. Bryant, D. Teel, L. J. Lierheimer, T. C. Wainwright, W. S. Grant, F. W. Waknitz, K. Neely, S. T. Lindley, and R. S. Waples. 1998. Status review of Chinook Salmon from Washington, Idaho, Oregon, and California. NOAA Technical Memorandum NMFS-NWFSC-35.
- Nandor, G. F., J. R. Longwill, and D. L. Webb. 2010. Overview of the coded wire tag program in the greater Pacific region of North America. Pages 5–46 in K. Wolf and J. O’Neal, editors. Tagging, telemetry and marking measures for monitoring fish populations—a compendium of new and recent science for use in informing technique and decision modalities. Pacific Northwest Aquatic Monitoring Partnership, Special Publication 2010-002. Available: https://www.pnamp.org/sites/default/files/pnamp_2010_002.pdf. (March 2017).
- Neillands, G. W. 1995. Documents submitted to the ESA administrative record for West Coast Chinook Salmon: responses to questionnaires for the Merced, San Joaquin, Stanislaus, and Tuolumne rivers. National Marine Fisheries Service, Environmental and Technical Services Division, Portland, Oregon.
- O’Farrell, M. R., M. S. Mohr, A. M. Grover, and W. H. Satterthwaite. 2012. Sacramento River winter Chinook cohort reconstruction: analysis of ocean fishery impacts. NOAA Technical Memorandum NMFS-SWFSC-491.
- O’Farrell, M. R., M. S. Mohr, M. L. Palmer-Zwahlen, and A. M. Grover. 2013. The Sacramento index (SI). NOAA Technical Memorandum NMFS-SWFSC-512.
- O’Farrell, M. R., M. L. Palmer-Zwahlen, and J. Simon. 2010. Is the September 1 river return date approximation appropriate for Klamath River fall Chinook? NOAA Technical Memorandum NMFS-SWFSC-468.
- O’Farrell, M. R., and W. H. Satterthwaite. 2015. Inferred historical fishing mortality rates for an endangered population of Chinook Salmon (Oncorhynchus tshawytscha). U.S. National Marine Fisheries Service Fishery Bulletin 113:341–351.
- Palmer-Zwahlen, M. L., A. M. Grover, and J. A. Duran. 2006. Feather River Chinook cohort reconstruction brood years 1998 and 1999 fall and spring runs. California Department of Fish and Game, Marine Region, Ocean Salmon Project Technical Report, Santa Rosa.
- Quinn, T. P., P. McGinnity, and T. E. Reed. 2016. The paradox of “premature migration” by adult anadromous salmonid fishes: patterns and hypotheses. Canadian Journal of Fisheries and Aquatic Sciences 73:1015–1030.
- Satterthwaite, W. H., and S. M. Carlson. 2015. Weakening portfolio effect strength in a hatchery-supplemented Chinook Salmon population complex. Canadian Journal of Fisheries and Aquatic Sciences 72:1860–1875.
- Satterthwaite, W. H., S. M. Carlson, S. D. Allen-Moran, S. Vincenzi, S. J. Bograd, and B. K. Wells. 2014a. Match-mismatch dynamics and the relationship between ocean-entry timing and relative ocean recoveries of Central Valley fall run Chinook Salmon. Marine Ecology Progress Series 511:237–248.
- Satterthwaite, W. H., J. Ciancio, E. Crandall, M. L. Palmer-Wahlen, A. M. Grover, M. R. O’Farrell, E. C. Anderson, M. S. Mohr, and J. C. Garza. 2015. Stock composition and ocean spatial distribution inference from California recreational Chinook Salmon fisheries using genetic stock identification. Fisheries Research 170:166–178.
- Satterthwaite, W. H., M. S. Mohr, M. R. O’Farrell, E. C. Anderson, M. A. Banks, S. J. Bates, M. R. Bellinger, L. A. Borgerson, E. D. Crandall, J. C. Garza, B. J. Kormos, P. W. Lawson, and M. L. Palmer-Zwahlen. 2014b. Use of genetic stock identification data for comparison of the ocean spatial distribution, size at age, and fishery exposure of an untagged stock and its indicator: California coastal versus Klamath River Chinook Salmon. Transactions of the American Fisheries Society 143:117–133.
- Satterthwaite, W. H., M. S. Mohr, M. R. O’Farrell, and B. K. Wells. 2012. A Bayesian hierarchical model of size-at-age in ocean-harvested stocks—quantifying effects of climate and temporal variability. Canadian Journal of Fisheries and Aquatic Sciences 69:942–954.
- Satterthwaite, W. H., M. S. Mohr, M. R. O’Farrell, and B. K. Wells. 2013. A comparison of temporal patterns in the ocean spatial distribution of California’s Central Valley Chinook Salmon runs. Canadian Journal of Fisheries and Aquatic Sciences 70:574–584.
- Schindler, D. E., R. Hilborn, B. Chasco, C. P. Boatright, T. P. Quinn, L. A. Rogers, and M. S. Webster. 2010. Population diversity and the portfolio effect in an exploited species. Nature 465:609–612.
- Shannon, C. E. 1948. A mathematical theory of communication. Bell System Technical Journal 27: 379–423, 623–656.
- Sharma, R., and T. P. Quinn. 2012. Linkages between life history type and migration pathways in freshwater and marine environments for Chinook Salmon, Oncorhynchus tshawytscha. Acta Oecologia 41:1–13.
- Tattam, I. A., J. R. Ruzycki, J. L. McCormick, and R. W. Carmichael. 2015. Length and condition of wild Chinook Salmon smolts influence age at maturity. Transactions of the American Fisheries Society 144:1237–1248.
- Vogel, D. A., and K. R. Marine. 1991. Guide to upper Sacramento River Chinook Salmon life history. Prepared by CH2M Hill for the U.S. Bureau of Reclamation, Central Valley Project, Redding, California.
- Ward, P. D., T. R. McReynolds, and C. E. Garman. 2003. Butte and Big Chico creeks spring-run Chinook Salmon, Oncorhynchus tshawytscha, life history investigation, 2001–2002. California Department of Fish and Game, Inland Fisheries Administrative Report 2003, Sacramento.
- Ward, P. D., T. R. McReynolds, and C. E. Garman. 2004. Butte and Big Chico creeks spring-run Chinook Salmon, Oncorhynchus tshawytscha, life history investigation, 2002–2003. California Department of Fish and Game, Inland Fisheries Administrative Report 2004-6, Sacramento.
- Weitkamp, L. A. 2010. Marine distributions of Chinook Salmon from the West Coast of North America determined by coded wire tag recoveries. Transactions of the American Fisheries Society 139:147–170.
- Weitkamp, L. A., and K. Neely. 2002. Coho Salmon (Oncorhynchus kisutch) ocean migration patterns: insight from marine coded-wire tag recoveries. Canadian Journal of Fisheries and Aquatic Sciences 59:1100–1115.
- Wells, B. K., C. B. Grimes, J. C. Field, and C. S. Reiss. 2006. Covariation between the average lengths of mature Coho (Oncorhynchus kisutch) and Chinook salmon (O. tshawytscha) and the ocean environment. Fisheries Oceanography 15:67–79.
- Williams, J. G. 2006. Central Valley salmon: a perspective on Chinook and steelhead in the Central Valley of California. San Francisco Estuary and Watershed Science [online serial] 4:art 2.
- Winship, A. J., M. R. O’Farrell, and M. S. Mohr. 2013. Management strategy evaluation applied to the conservation of an endangered population subject to incidental take. Biological Conservation 158:155–166.
- Yoshiyama, R., F. Fisher, and P. Moyle. 1998. Historical abundance and decline of Chinook Salmon in the Central Valley region of California. North American Journal of Fisheries Management 18:487–521.